|
|
 |
|
Calanoida ( Order ) |
|
|
|
Calanoidea ( Superfamily ) |
|
|
|
Megacalanidae ( Family ) |
|
|
|
Megacalanus ( Genus ) |
|
|
| |
Megacalanus princeps Wolfenden, 1904 (F,M) | |
| | | | | | | Syn.: | Macrocalanus longicornis Sars, 1905 b (p.7);
Megacalanus longicornis Pearson, 1906 (p.6, Rem.); Van Breemen, 1908 a (p.13, fig.F); Farran, 1908 b (p.21); Sars, 1912; 1925 (p.11, figs.F,M); Sewell, 1929 (p.27, Rem.); Rose, 1929; 1933 a (p.64, figs.F,M); Jespersen, 1934 (p.45); Wilson, 1936 c (p.89); Wilson, 1942 a (p.193); Lysholm & al., 1945 (p.7); Vervoort, 1946 (p.49); C.B. 1950 (p.262); Tanaka, 1953 (p.129); Mazza, 1966 (p.69); Dowidar & El-Maghraby, 1970 (p.268); Shih & al., 1971 (p.37); Lee & al., 1971 (p.1151); Morioka, 1972 a (p.314); Vives & al., 1975 (p.35, tab.II); Vaissière & Séguin, 1980 (p.23, tab.2); Kovalev & Shmeleva, 1982 (p.82); Baessia de Aguiar, 1987 (p.40, fig.F, Rem.F,M); Lozano Soldevilla & al., 1988 (p.57); Baessa De Aguiar, 1991 (1993) (p.102); Non Miller, 2002 (p.129, figs.F,M, Rem.: variability);
Ref. compl.: Pearson, 1906 (p.6, Rem.); Van Breemen, 1908 a (p.13, fig.F); Farran, 1908 b (p.21); Sars, 1912; 1925 (p.11, figs.F,M); Sewell, 1929 (p.27, Rem.); Rose, 1929; 1933 a (p.64, figs.F,M); Jespersen, 1934 (p.45); Wilson, 1936 c (p.89); Wilson, 1942 a (p.193); Lysholm & al., 1945 (p.7); Vervoort, 1946 (p.49); C.B. 1950 (p.262); Tanaka, 1953 (p.129); Mazza, 1966 (p.69); Dowidar & El-Maghraby, 1970 (p.268); Shih & al., 1971 (p.37); Lee & al., 1971 (p.1151); Morioka, 1972 a (p.314); Macdonald & al., 1972 (p.213, fig.4, hydrostatic pressure effect); Vives & al., 1975 (p.35, tab.II); Vaissière & Séguin, 1980 (p.23, tab.2); Kovalev & Shmeleva, 1982 (p.82); Baessia de Aguiar, 1987 (p.40, fig.F, Rem.F,M); Lozano Soldevilla & al., 1988 (p.57); Baessa De Aguiar, 1991 (1993) (p.102); El-Sherif & Aboul Ezz, 2000 (p.61, Table 3: occurrence); Galbraith, 2009 (pers. comm.); Mazzocchi & Di Capua, 2010 (p.426);Fornshell & Ferrari, 23014 (p.113, fig.10: Von Vaupel Klein's organ)
Synonymies after Bradford-Grieve & al. (2017, p.30 ):
Macrocalanus longicornis Sars, 1905 (p.26);
Megacalanus longicornis : Farran, 1908 (p.21);
Megacalanus princeps : Wolfenden, 1911 (p.196-198, fig.1);
Megacalanus princeps : With, 1915 (p.41-44, pl.I, figs 3a-i, textfig.8a-d);
Megacalanus longicornis : Sars, 1924-25 (p.11-14, pls.I-II);
Megacalanus princeps var. inermis Sewell, 1947 (p.25-27, text-fig.2), considered by Bradford-Grieve & al. (2017, p.33) to be a deformed form;
Non Calanus princeps Brady, 1883 (p.36-37, pl.X, figs.3-7);
Non Megacalanus princeps Wolfenden, 1905b (p.1-3, pl.I, figs.1-6). | | | | Ref.: | | | Wolfenden, 1904 (p.112, Descr.F, figs.F); A. Scott, 1909 (p.13, figs.F, Rem.); Wolfenden, 1911 (p.196, figs.F,M); With, 1915 (p.41, figs.F, Rem.); Lysholm & Nordgaard, 1921 (p.8); Farran, 1926 (p.230); Jespersen, 1934 (p.45); Farran, 1939 (p.360); Jespersen, 1940 (p.9); Vervoort, 1946 (p.49, figs. juv., Rem.); Sewell, 1947 (p.25, Rem.: abnormality, fig.F); 1948 (p.555, Rem.); [Non Tanaka, 1956 (p.262, figs.F)]; Vervoort, 1957 (p.32, Rem.); 1963 b (p.86, Rem.); Paiva, 1963 (p.16, figs.F); Grice, 1963 a (p.495); Mazza, 1966 (p.69); Tanaka & Omori, 1967 (p.241); Owre & Foyo, 1967 (p.34, figs.F,M); Vinogradov, 1968 (1970) (p.268); Guérédrat, 1969 (p.65, Rem.); Lee & al., 1971 (p.1151); Silas, 1972 (p.645); [Non Brodsky & al., 1983 (p.191, figs.F, M, Rem.F)]; Roe, 1984 (p.356); Tremblay & Anderson, 1984 (p.4, Rem.); Guangshan & Honglin, 1984 (p.118, tab.); Fleminger, 1985 (p.276, 284, Table 1, fig.M)); Nishida, 1989 (p.173, table 2, 3, dorsal hump); Michel, 1994 (p.177, Rem.); [Non Bradfod-Grieve, 1994 (fig.18, Gigs. F,M)]; Chihara & Murano, 1997 (p.834, Pl.126: F,M); Bradford-Grieve & al., 1999 (p.877, 905, figs.F,M); Boxshall & Halsey, 2004 (p.141: figs.); Vives & Shmeleva, 2007 (p.912, figs.F,M, Rem.); Andronov, 2014 (p.33, fig.M); Bradford-Grieve & al., 2017 (p.30, Descr. F,M, NVI, copepodite II, figs. F,M, N6, juv.II ,Rem., phylogeny) |  issued from : Sars, 1924-25 [Pl. I]. As Megacalanus longicornis. Female: left picture, anterior surface of basis, exopodite 1 and endopodite 1 of P1, illustrating the position and form of the basis spine and seta, a macula cribrosa is located on the basis beside the articulation of exopodite 1 (little circle). Right picture, lateral view of P1 basis hook. Scale bars: left picture = 0.1 mm; right picture = 0.2 mm. Nota: The small, presumably sensory, organs termed laminae cribrosae, or maculae cribrosae, by With (1915) were found on the anterior surface of P1 at the positions (one each on basipodite 1, endopodite 2, exopodites 1 and 2) by Sewell (1947); the term translates as 'sieve plates' or 'sieve spots', but the actual structure is an oval neural nexus (clearly the swollen end of a nerve, visible proximally) in the epidermis, attached by thin tubules (axons, dendrites ?) to a ring of spherules embedded at the surface of the chitin (fig.5, b,-c); the patterns are not consistently different between crested and uncrested forms (maculae cribrosae are also reported for Bathycalanus by Sewell, 1947).
|
 issued from : Sars, 1924-25 [Pl. II]. As Megacalanus longicornis. Female: A, neural nexis that is the interior section of a macula cribrosa on the proximal surface of the mandibular gnathobase: B, surface spherules of the same macula cribrosa. Male: C, macula cribrosa with fewer spherules, one of them located centrally, on exopodite 3 of P5
|
 issued from : A. Fleminger in Mar. Biol., 1985, 88. [p.284, Fig.6, E ]. Male: Left A1 proximal segments (ventral view); Nota: see remarks in Calanus s.l. pacificus californicus (Fleminger, 1985, p.275) concerning the dimorphism in the female A1.
|
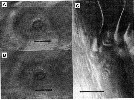 issued from : J. Mauchline in J. mar. biol. Ass, U.K., 1977, 57 (4). [p.979, Fig.3]. Female: A, General distribution of normal pores (dots) and groups of pores (small dots) in treated integuments; B, a group of glands openings, responsible for a group of pores in a treated integument, with the associated gland; C, the peculiar structure of the group of gland openings at the site indicated in the 5th thoracic segment; the heavy line is the posterior border of the segment.
|
 issued from : R.-M. Barthélémy in These Doct. Univ. Provence (Aix-Marseille I), 1999. [Fig.20, B]. As Megacalanus longicornis.Female (from 41°47'S, 175°01'E): B, external ventral view genital double-somite. go = genital operculum. Scale bar: 0.200 mm.
|
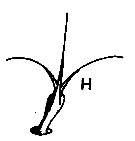
 issued from : J. Mauchline in J. mar. biol. Ass, U.K., 1977, 57 (4). [p.980, Fig.4, H]. Integumental organs. H, compound seta.
|
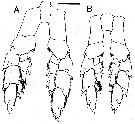
 Issued from : N.R. Wolfenden in J. mar. biol. Ass. U.K., n. ser. 7, 1904. [Pl. IX, Figs. 1, 2]. Female (from 59-60°N, 8°42'-80'W): 1, P1 (basal segments); 2, P3. Nota: Head and 1st pedigerous segment separate, 4th and 5th separate. A1 25-segmented, much longer than the body. P1 with an extraordinary double-hooked process on the dorsal surface of the basipod 2, an upper and lower hook placed vertically, the latter very strong and prominent. Genital segment well-developed symmetrical.
|
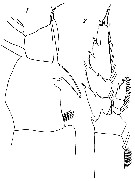
 issued from : G; Harding in Key to the adullt pelagic calanoid copepods found over the continental shelf of the Canadian Atlantic coast. Bedford Inst. Oceanogr., Dartmouth, Nova Scotia, 2004. [p.10]. Female (lateral view)
|
 issued from : G. Harding in Key to the adullt pelagic calanoid copepods found over the continental shelf of the Canadian Atlantic coast. Bedford Inst. Oceanogr., Dartmouth, Nova Scotia, 2004. [p.10]. Female: A, P1 (non P5 as indicated by error); B, Mx2.
|
 Issued from : J.M. Bradford-Grieve, L. Blanco-Bercial & G.A. Boxshall in Zootaxa, 2017, 4229 (1). [p.31, fig.7]. Female (from 34.996°N, 52.027°W): A-B, habitus (dorsal and lateral, respectively); C, anterior head (lateral); D, inner seta of Mx2 praecoxal endite 2; E, seta of Mx2 praecoxal endite 1 (and most of other setae on maxilla); F, A1 ancestral segments XIV-XV; G, A1 ancestral segments XVI-XVII; H, A1 ancestral segments I-IV (dorsal view); I, hair sensilla (hs) and macula cribrosa (mc) on ancestral segment III; J, P1. Scale bars: 1.0 mm (A, B, H, J); 0.1 mm on remaining figures.
|
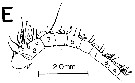 Issued from : J.M. Bradford-Grieve, L. Blanco-Bercial & G.A. Boxshall in Zootaxa, 2017, 4229 (1). [p.32, fig.8]. Male (from 11.683°N, 20.419°W): A-B, habitus (dorsal and lateral, respectively); C, forehead (lateral); D, caudal ramus (dorsal); E, P5 (anterior view); F, detail of inner distal specialised seta on left P5 exopod segment 2; G, A1 ancestral segments XV-XVI; H, A1 ancestral segments XIX-XXI; I, A1 ancestral segments XXI-XXIII. Scale bars: 1.0 mm (A, B); 0.1 mm on remaining figures.
|
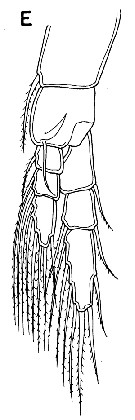 issued from : G. A. Boxshall & S. H. Halsey in An Introduction to Copepod Diversity. The Ray Society, 2004, N° 166. [p.141, Fig.29, E]. Female: E, P1. Nota: Large hook-like process present on anterior surface of basis of P1.
|
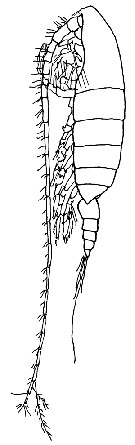 issued from : G; Harding in Key to the adullt pelagic calanoid copepods found over the continental shelf of the Canadian Atlantic coast. Bedford Inst. Oceanogr., Dartmouth, Nova Scotia, 2004. [p.10]. Female (lateral view)
|
 issued from : G. Harding in Key to the adullt pelagic calanoid copepods found over the continental shelf of the Canadian Atlantic coast. Bedford Inst. Oceanogr., Dartmouth, Nova Scotia, 2004. [p.10]. Female: A, P1 (non P5 as indicated by error); B, Mx2.
|
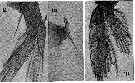 issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1967, 1, Part 1: Copepoda. [p.33, Figs.162-164]. Female (from Florida Current): 162, P1; 163, spine on basis of P1; 164, P5.
|
 Issued from : J.M. Bradford-Grieve, L. Blanco-Bercial & G.A. Boxshall in Zootaxa, 2017, 4229 (1). [p.31, fig.7]. Female (from 34.996°N, 52.027°W): A-B, habitus (dorsal and lateral, respectively); C, anterior head (lateral); D, inner seta of Mx2 praecoxal endite 2; E, seta of Mx2 praecoxal endite 1 (and most of other setae on maxilla); F, A1 ancestral segments XIV-XV; G, A1 ancestral segments XVI-XVII; H, A1 ancestral segments I-IV (dorsal view); I, hair sensilla (hs) and macula cribrosa (mc) on ancestral segment III; J, P1. Scale bars: 1.0 mm (A, B, H, J); 0.1 mm on remaining figures. Nota: - Anterior margin of head in dorsal view slightly produced into short rounded projection dorsal to base of rostrum. - Posterior borders pf pedigerous somite 5 extend into triangular lappets reaching 1/3 on 3rd of way along genital double-somite, in dorsal view lappets appear pointed. - A1 dorsal surface of ancestral segments I-V each with very small hair sensillum of which those on segments II and III accompanied by macula cribrosa; ancestral segments XIV-XVII 13/13, 20/21, 25/26, 24/26 distoventral teeth, respectively, distoposterior borders of segments XV and XVI with 26/32 and 29/33 minute blunt teeth, respectively; in some specimens, these blunt teeth appear to be cross-section of posterior border ridges that run at right angles to main axis of segment. - P1 outer spines on exopod segments 1 and 2 extend half way between bases of following 2 more distal spines; 1 macula cribrosa at base of outer spine on exopod segment 2. Remarks: Bradford-Grieve & al. (2017, p.17) use the term 'hair sensillum' because there is no adjacent pore to an integumental gland (see in Fleminger, 1973), this element is very small and difficult to see; these may or may not be accompanied by a macula cribrosa; maculae cribrosae were first mentioned by With, 1915, and interpreted as ''being a group of delicate filaments projecting through minute pores (?) The SEM of Miller (2002, p.136) showed that the dots represent small raised thickenings on the cuticle surface connected to the interior by a ''neural nexux'' (The ultrastructure and function of this organ is not known)
|
 Issued from : J.M. Bradford-Grieve, L. Blanco-Bercial & G.A. Boxshall in Zootaxa, 2017, 4229 (1). [p.32, fig.8]. Male (from 11.683°N, 20.419°W): A-B, habitus (dorsal and lateral, respectively); C, forehead (lateral); D, caudal ramus (dorsal); E, P5 (anterior view); F, detail of inner distal specialised seta on left P5 exopod segment 2; G, A1 ancestral segments XV-XVI; H, A1 ancestral segments XIX-XXI; I, A1 ancestral segments XXI-XXIII. Scale bars: 1.0 mm (A, B); 0.1 mm on remaining figures. Nota: - Anterior margin of head in dorsal view slightly produced into short rounded projection dorsal to base of rostrum which extends into 2 long, ventroposteriorly-directed, tapering points taht appear to be direct extensions of cuticule. - A1 right ancestral segment XIX without fused gripping element, instead 2 modified setae, 1 aesthetasc; XXI wiyj 1 short fused element (arising just proximal to midlength but fused to its segment only at its base), 1 modified seta, 1 aesthetasc; fused segments XV-XVI on right A1 each with longitudinal row of 16, 32 ventral teeth, respectively. - P5 inner distal border of basis with setules, specialised seta on inner distal border of exopod segment 2 on left with basal part longer than wide and lash longer than basal part. Right exopod segment 3 inner border completely lined with fine spinules to just short of inner articulated spine.
|
 Issued from : J.M. Bradford-Grieve, L. Blanco-Bercial & G.A. Boxshall in Zootaxa, 2017, 4229 (1). [p.66, Table 7]. Morphological characters after identification key of Megacalanus females and males. Compare to other species of genus. Main characters identification after species key : 1 - Head without crest in males and females. 2 - Posterolateral lappets of pediger 5 triangular in females and males. 3 - Female A1 ancestral segments XV and XVI with posterior border blunt teeth; male right A1 segment XIX without distal fused gripping element.
|
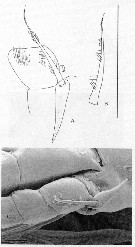 Issued from : J.A. Fornshell & F.D. Ferrari in Crustaceana, 2014, 87 (1). [p.105, Fig.1, A-C]. As Megacalanus longicornis Von Vaupel Klein's organ on left endopodal segment 1 and left basal seta of P1. A, anterior, distal up, central lumen of attenuation indicared with dotted lines; B, left basal seta; C, Von Vaupel Klein organ and middle segment on right leg of P1. Scale line; 270 µm (A, B); 50 µm (C). The Von Vaupel Klein organ is an association (srtucture) of the distal seta from basis and the proximal endopodal segment of P1. This structure shows significant variability among many gymnoplean copepods, in the shape of the distodorsal corner of the proximal endopodal segment, presence and location of denticles on the anterior face of the segment, presence and size of denticles along the distal margin of the segment , number of pores on the segment, shape of the seta that originates on the basis, and the morphology of the basis at the origin of the seta. The function of this complex structure is not known.
| | | | | Compl. Ref.: | | | Wickstead, 1962 (p.551, food & feeding); De Decker & Mombeck, 1964 (p.13); Mazza, 1966 (p.69); Grice & Hulsemann, 1967 (p.13); 1968 (tab.2); Gueredrat & Friess, 1971 (p.187, fig.2); Morris R.J., 1971 (p.275, Table 1, 2, lipids composition); Roe, 1972 (p.277, tabl.1, tabl.2); Björnberg, 1973 (p.303, ); Deevey & Brooks, 1977 (p.256, Table 2, Station "S"); Kovalev & Shmeleva, 1982 (p.82); Vives, 1982 (p.290); Guangshan & Honglin, 1984 (p.118, tab.); Petipa & Borichenko, 1985 (tab.2); Heinrich, 1990 (p.16); Suarez-Morales & Gasca, 1998 a (p.110); Barthélémy, 1999 a (p.10); Lapernat, 2000 (tabl.3, 4); Razouls & al., 2000 (p.343, tab. 5, Appendix); Holmes, 2001 (p.35); Hsiao & al., 2004 (p.326, tab.1); G. Harding, 2004 (p.10, figs.F); Ikeda & al., 2006 (p.1791,Table 2); Fernandes, 2008 (p.465, Tabl.2); Gaard & al., 2008 (p.59, Table 1, N Mid-Atlantic Ridge); Park & Ferrari, 2009 (p.143, Table 4, Appendix 1, biogeography); Drira & al., 2010 (p.145, Tanl.2); Teuber & al., 2013 (p.1, Table 1, 2, 3, fig.5, abundance vs oxygen minimum zone, respiration rates, enzyme activity); Bode & al., 2015 (p.268, Table 1, chemical components, trophic level, geographic zone); Almeida Alves-Jr. & al., 2017 (p.1, fig.F, Rem.); El Arraj & al., 2017 (p.272, table 2); Belmonte, 2018 (p.273, Table I: Italian zones); Bode & al., 2018 (p.840, Table 1, respiration & ingestion rates, depth); Bode & al., 2018 (p.66, fig.5, vertical distribution %, Rem). | | | | NZ: | 22 | | |
|
Distribution map of Megacalanus princeps by geographical zones
|
| | | | | | | | | | | | | | | 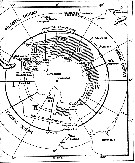 issued from : W. Vervoort in B.A.N.Z. Antarctic Reseach Expedition, Reports - Ser. B, Vol. III, 1957 [Fig.7]. issued from : W. Vervoort in B.A.N.Z. Antarctic Reseach Expedition, Reports - Ser. B, Vol. III, 1957 [Fig.7].
Chart showing the geographical distribution (white circle) in the seas surrounding the Antarctic continent.
Nota: In this chart the area frequented by whaling vessels has been hatched. The Antarctic circle (66°.5 S) has been drawn as a broken line. The numbers I to VI refer to the sectors into which the Antarctic seas are divided according to Mackintosh (1942) (after Vervoort, 1951).
Nota: After vervoort (1957, p.32) this species preferably inhabits the intermediate water layers, occasionally penetrating into very deep water. It may be carried to the North and South in the intermediate water mass; its occurrence at Antarctic localities is certainly accidental and may be explained as the result of transportation in Atlantic water masses. |
 Issued from : R. J. Morris in Comp. Biochem. Physiol., 1971, 40B. [p.277, Table 1]. Issued from : R. J. Morris in Comp. Biochem. Physiol., 1971, 40B. [p.277, Table 1].
Total lipid and saponification for Megacalanus princeps from Northeastern Atlantic. |
 Issued from : F. de Almeida Alves-Jr., E. Pinho Correira, L. Guedes Peirera Figueirédo, A. Galdino da Cunha, A. Bertrand & S. Neumann-Leitao in Nauplius, 2016, 25 [p.3, Fig. 2]. Issued from : F. de Almeida Alves-Jr., E. Pinho Correira, L. Guedes Peirera Figueirédo, A. Galdino da Cunha, A. Bertrand & S. Neumann-Leitao in Nauplius, 2016, 25 [p.3, Fig. 2].
Worldwide geographic distribution of Megacalanus princeps Wolfenden, 1904.
Nota: After F. de Almeida Alves-Jr & al. (2016), the proportional length of females urosomites and caudal rami are different from Tanaka (1956): 33 : 23 : 13 : 8 : 23, indicating a small morphological variation, when compared with specimens collected in the Pacific Ocean.
The Brasilian specimens presented a cephalic crest; this characteristic was only found before in specimens collected in deep waters off Southern California |
 Issued from : J.M. Bradford-Grieve, L. Blanco-Bercial & G.A. Boxshall in Zootaxa, 2017, 4229 (1). [p.34, fig.9]. Issued from : J.M. Bradford-Grieve, L. Blanco-Bercial & G.A. Boxshall in Zootaxa, 2017, 4229 (1). [p.34, fig.9].
Distribution of Megacalanus princeps (filled triangle), Megacalanus frosti (open triangle), Megacalanus ericae (filled square) and Megacalanus ohmani (open square). |
 Issued from : M. Bode, R. Koppelmann, L. Teuber, W. Hagen & H. Auel inGlobal Biogeochemical Cycles, 2018, 32. [p.844, Table 1). Issued from : M. Bode, R. Koppelmann, L. Teuber, W. Hagen & H. Auel inGlobal Biogeochemical Cycles, 2018, 32. [p.844, Table 1).
Cf. explanations of these measures in Calanoides natalis from the same authors. |
| | | | Loc: | | | Antarct. (Indian, SE Pacif.), sub-Antarct. (SW & SE Pacif.), South Africa (E), off S St. Helena Is., Angola, off E St. Paul Is., off E Sao Tomé Is., Cape Verde Is., off Mauritania-NW Cape Verde Is., Morocco-Mauritania, Canary Is., off Madeira, Azores, Bay of Biscay, Rocas Atoll (N Brazil), Caribbean Sea, G. of Mexico, Florida, Sargasso Sea, Station "S" (32°10'N, 64°30'W), off W Cape Finisterre, Woods Hole, off E Newfoundland, Greenland, W Iceland, Ireland (S & W), Faroe, Ibero-moroccan Bay, Medit. (Ligurian Sea, Tyrrhenian Sea, G. of Gabès, Ionian Sea, Alexandria), Red Sea (Sharm El-Sheikh), Arabian Sea, indian (equatorial), SW Indian, Bay of Bengal, Indonesia-Malaysia, Philippines, Pacif. (W equatorial), China Seas (East China Sea, South China Sea), E Taiwan, Japan, off SE Hokkaido, G. of Alaska, British Columbia, off California, New Zealand, Pacif. (tropical), Hawaii, off E Easter Is., off Peru, Pacif. (SE tropical), off Juan Fernandez Is., N & S Chile, Drake passage;
Type locality: 51-60° N, 6-13° W. | | | | N: | 80 | | | | Lg.: | | | (1) F: 10- 9,5; (5) F: 9,5; (7) F: 10,5; M: 10,6; (10) F: 10; (14) F: 11,5-8,7; M: 12-9; (28) F: 11,5-8,75; M: 10,15-7,9; (29) M: 9,5; (38) F: 9,2-9; (49) F: 10,3-9,8; (70) F: 11,9-10,2; M: 10,8-10; (73) F: 10,68-10; (140) F: 11-9,2; M: 9,5-9,3; (199) F: 9,92-9,44; (787) F: 13-10; M: 10; (865) F: 10,6; (904) M: 10,9-11,2; (1089) F: 12-13,5; M: 9,3-9,5; (1311) F: 9,21; M: 10,31-9,9; (1316) F: 8,5-10,5; M: 9,5-10,4; {F: 8,50-13,50; M: 7,90-12,00}
The mean female size is 10.149 mm (n = 29; SD = 2.0062), and the mean male size is 9.903 mm (n = 17; SD = 1.0984). The size ratio (male : female) is approximately 0.93. | | | | Rem.: | Meso-bathypelagic.
Sampling depth (Antarct., sub-Antarct.) : 2000 m. Sargasso Sea: 500-1500 m (Deevey & Brooks, 1977, Station "S").
For A. Scott (1909, p.14) there is no difference between the Indonesian-Malaysian forms and the original description given by Wolfenden (1904) from the North Atlantic specimen.
According to Bradford-Grieve (1994, p.18) the present specimens seem to differ from the descriptions of other authors in some respects. The Southwest Pacific females do not have the head produced as far anteriorly as that figured by Sars (1924-1925), the terminal spine on exopod segment 3 of at least legs 3 and 5 are long relative to the length of exopod segment 3. The inner-edge seta on exopod segment 2 of male left P5 is short and very bulbous at its base unlike that figured by Sars and Wolfenden (1905 b). A considerable amount of variability has been recorded in the reflex hook on P1 basis, ranging from complete absence, through atrophied, to well developed (See Gueredrat, 1969).
For Park & Ferrari (2009, p.175) Megacalanus princeps Wolfenden, 1904 (p.49, fig.4) and Vervoort (1957, p.47 & al., fig.7: Antarctic chart) is a valid species and no a synonym. This position is contested by Damkaer (2000), followed by Miller (2002, p.129), however the name of Megacalanus princeps is maintened by Boxshall & Halsey (2004, p.140).
The length of A1 can be in relation with the water salinity as showed by Gaudy (1971a, for Centropages typicus at Marseille), and for Oithona similis by Dvoretsky & Dvoretsky (2009) showing the relation with the temperature and salinity. After Bradford-Grieve & al; (2017, p.33) the males may be distinguished from males of all other species by the absence of a gripping element on ancestral segment XIX of the right A1.
R. Stephen, 2007 : Data sheets of NIO, Kochi, India (on line).
After us, this species is noted for the first time in the northern Red Sea by El-Sherif & Aboul Ezz (2000, p.72) as Megacalanus longicornis. | | | Last update : 19/06/2023 | |
| | | | Dear Editor, I would like to indicate a little update in topic distribution of Megacalanus princeps, with new record in Brazilian waters. and some aspects about the differentiation in ours specimens.
The paper was recently published with title: First report of deep-sea copepod Megacalanus princeps Wolfenden, 1904 (Calanoidea: Megacalanidae) from southwestern Atlantic,
Authors: Flavio de Almeida Alves-Júnior, Érika Pinho Correia, Lucas Guedes Pereira Figueirêdo, Aislan Galdino da Cunha, Arnaud Bertrand and Sigrid Neumann-Leitão.
Here the link >> http://www.scielo.br/pdf/nau/v25/2358-2936-nau-25-e2017007.pdf
www.scielo.br/pdf/nau/v25/2358-2936-nau-25-e2017007.pdf
Best wishes! | |
|
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2024. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed April 23, 2024] © copyright 2005-2024 Sorbonne University, CNRS
|
|
 |
 |























