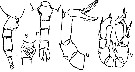|
|
 |
|
Calanoida ( Order ) |
|
|
|
Diaptomoidea ( Superfamily ) |
|
|
|
Pseudodiaptomidae ( Family ) |
|
|
|
Pseudodiaptomus ( Genus ) |
|
|
| |
Pseudodiaptomus marinus Sato, 1913 (F,M) | |
| | | | | | | Syn.: | no Pseudodiaptomus marinus : Grigg,1972; Greenwood, 1977 (p.65, figs.M, Rem.) | | | | Ref.: | | | Brodsky, 1950 (1967) (p.323, figs.F,M); Chiba, 1956 (p.51, figs.F,M); Shen & Lee, 1963 (p.593); Chen & Zhang, 1965 (p.80, figs.F,M); Tanaka,1966 (p.43, figs.F,M); Grindley & Grice, 1969 (p.125, figs.F, M, Rem); Pillai, 1976 (1980) (p.262, figs.F,M); Kos, 1976 (Vol. II, figs.F, M, Rem.); Dussart & Defaye, 1983 (p.31); Nishida, 1985 (p.131: figs.F,M, Rem.: p.132-133); Kos, 1984 (1985) (p.236, figs.F,M, Rem.); Walter, 1986 (p.133, 146-147, Rem. ); 1986 a (p.503); 1987 (p.367); Fleminger & Kramer, 1988 (p.535, figs. F,M, Rem., zoogeo.); Walter, 1989 (p.621: Rem., fig.18); Zheng Zhong & al., 1984 (1989) (p.246, figs.F,M); Chihara & Murano, 1997 (p.894, Pl.165: F,M); Bradford-Grieve,1999 b (p.153, figs.F,M, Rem., fig.181); Eyun & al., 2007 (p.265, molecular biology); Ferrari & Dahms, 2007 (p.22, 35, Rem. N); Brylinski & al. 2012 (p.577, figs.F,M, Rem.: alien species) | 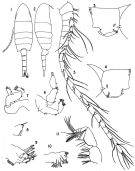 issued from : J.R. Grindley & G.D. Grice in Crustaceana, 1969, 16 (2). [p.126, Fig.1-11]. Female (from Port Louis harbor, Mauritius): 1, habitus (dorsal); 2, idem (lateral right side); 3, genital segment (lateral right side); 4, idem (lateral left side); 5, A1; 6, A2; 7, Md (mandibular palp); 8, Md (cutting edge); 9, Mx1; 10, Mx2; 11, Mxp. Nota: Head and 1st thoracic segment separate, 4th and 5th fused. - Posterior border of prosome produced into sharp outwardly directed spines. - Genital segment slightly asymmetrical with slight swellings laterally and a prominent ventral boss, a small patch of fine setae present on each side and a fringe of stiff setae runs across the ventral surface; Genital flaps prominent with 1 seta on each side near their distal ends. - The first three urosome segments furnished with rows of coarse teeth on their posterodorsal margins. - Caudal rami symmetrical, divergent and 4 times as long as wide. - A1 21-segmented, extending to posterior margin of genital segment. - A2 with basipod bearing 2 lateral and 2 terminal setae and 1 seta on a small medial protuberance; exopod 4-segmented bearing 7 terminal and 6 sub-terminal setae and a lateral fringe of fine hairs; endopod apparently 4-segmented, 3rd segment small and indistinct, bearing 1 seta on segment 1, 3 lateral and 2 distal on segment 2, 2 on segment 3 and 1 lateral and 3 terminal on segment 4. - Basipod of mandibular palp with 4 inner marginal setae; exopod apparently 1-segmented bearing 4 lateral and 7 terminal setae; there are some fine hairs on the medial surface of the exopod and the middle terminal seta is markedly crooked. - Mx1 with 1st inner lobe (gnathobase) bearing 10 strong spines, small 2nd inner lobe with 2 setae, 3rd inner lobe with 4 terminal setae; outer lobe (coxal epipodite) with 8 long setae; exopod with 9 marginal setae; 1 small marginal seta arises between outer lobe and exopod; 2nd basal segment with 5 setae; endopod 2-segmented with 2 setae medially on the 1st segment and 6 terminal setae on the 2nd. - Mx2 with 5 large medial lobes (endites) and 2 smaller terminal lobes; the terminal lobes bear 5 setae and 1 seta, the marginal lobes 4, 3, 3, 3 and 4 setae, and there is 1 separate proximal seta. - Mxp 6-segmented, 2 basal segments large, 4 distal segments small; 1st segment bearing on its madial border, 2 plumose setae, 3 plumose setae, and 4 fine setae and 1 spine; 3rd segment bearing 2 setae proximally and 2 peculiarly divided setae distally (each bear a short spatulate branch near their middle fringed with fine bristles.
|
 issued from : J.R. Grindley & G.D. Grice in Crustaceana, 1969, 16 (2). [p.128, Fig.12-17]. Female: 12, P1; 13, basipodal segments of P1 (anterior); 14, P2; 15, P3; 16, P4; 17, P5. Nota: Swimming legs 1 to 4 biramous, similar in both sexes. - P5 uniramous, 4-segmented, symmetrical; the 2nd basal segment bears 2 small spinules on the outer angle and 1 seta on the posterior surface.
|
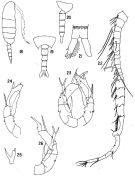 issued from : J.R. Grindley & G.D. Grice in Crustaceana, 1969, 16 (2). [p.126, Fig.18-26]. Male: 18, habitus (lateral right side); 19, posterior part cephalothorax and urosome (dorsal); 20, idem (lateral right side); 21, anal segment and caudal rami ( dorsal); 22, right A1; 23, P5 (posterior); 24, left P5 (anterior); 25, endopod of right P5 (medial view); 26, right P5 (anterior). Nota: Rostral filaments shorter than in female. - Urosome 5-segmented, half as long as prosome; posterior dorsal margins of urosome segments 1 to 4 fringed with rows of coarse teeth. - Caudal rami 3 times as long as wide. - Appendages similar to those of female except for right A1 and P5. - Right A1 geniculate, 21-segmented; 3 free segments distal to articulation; there is a hooked spine on segment 10 and a serrated margin to segment 18. - P5 biramous, basipods 2-segmented. Exopods 2-segmented, endopods 1-segmented. In the right leg 1st basal segment naked, 2nd basal segment larger, bears 3 small spinules on its outer margin, a posterior seta and a characteristic forked endopod.
|
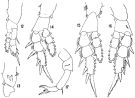
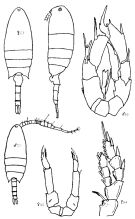 Issued from : K.A. Brodskii in Calanoida of the Far Eastern Seas and Polar Basin of the USSR. Opred. Fauna SSSR, 1950, 35 (Israel Program for Scientific Translations, Jerusalem, 1967) [p.323, Fig.225]. Female (from Sea of Japan): habitus (dorsal and lateral left side); S4, P4; S5, P5. Male: habitus (dorsal); S5, P5.
|
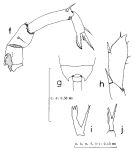 issued from : S. Nishida in Publ. Seto Mar. Biol. Lab., 1985, 30 (1/3). [p.131, Fig.4, f-j]. Female (from Hokkaido.): f, P5. Male: g, 5th thoracic segment (dorsal); h, left exopod of P5; i, right endopod of P5; j, P5 (Y-shaped spine on 1st segment of right exopod).
|
 issued from : H.Y. Soh, H.-L Suh, O.H. Yu & S. Ohtsuka in Hydrobiologia, 2001, 448. [p.213, Fig.8, B]. Female (from W and S Korea): scanning electron micrographs of the genital area.
|
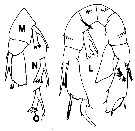 issued from : T.C. Walter in J. Plankton Res., 1986, 8 (1). [p.145, Fig.7, L-O]. Male (from Japan): L, P5 (anterior view); M-O, P5 variant forms of right endopod collected from different localities.
|
 issued from : P.P. Pillai in J. mar. biol. Ass. India, 1976 (1980), 18 (2). [p.263]. Comparison of some diagnostic characters of the Andaman Sea specimens with those of the previously published descriptions to draw attention to the variations observed in the species. After Grindley and Grice (1969) the variatiobns may be attributed to ecophenotypes or only geographical variations.
|
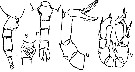 issued from : P.P. Pillai in J. mar. biol. Ass. India, 1976 (1980), 18 (2). [p.254, Fig.2, r-v]. Female (from Andaman Sea): r, urosome (dorsal); s, genital segment (ventral); t, urosome (lateral, right side); u, P5. Male: P5.
|
 issued from : O. Tanaka in Proceedings Symposium on Crustacea. Symp. Ser. mar. biol. Ass. India, 1966, (2) 1. [p.44, Fig.4]. Female (from coastal waters of Kyushu, Japan): a, habitus (dorsal; b, head (lateral); c, last thoracic segment and urosome (lateral); d, last thoracic segment and genital segment (lateral, right side); e, anal somite and caudal rami; f, A2; g, P1; h, P2; i, endopod of P3; j, P4; k, P5. Nota: proportional lengths prosome: urosome 62:38. Head and 1st thoracic segment separated, 4th and 5th fused. Rostrum with 2 fine filaments. A1 22-segmented. A2 exopod 4-segmented, 1.4 times as long as the endopod.Lateral distal corner of the last thoracic segment symmetrical with a strong spine directed outwards at the apex. urosomal segments and caudal rami in proportional lengths 26:18:20:14:22 = 100. Genital segment slightly asymmetrical, the left proximal margin is more inflated, the right lateral margin with rows of short hairs, the ventral surface is furnished with an acute triangular process just below the genital opening; the distal margin of the genital segment and 2nd and 3rd segments striated with small denticles. Caudal ramus more than 3 times as long as wide (11:3). P5 4-segmented, distal segment with 2 apical spines, of which the outer one carries a small subsidiary spine, the inner distal spine is coarsely serrated on the inner margin. Male: l, last thoracic segment and urosome (dorsal); m, right A1; n, P5. Nota: A1 21-segmented, 18th segment with a delicate ctenate process on the anterior margin. Proportional lengths prosome:urosome 66:34. Last thoracic segment symmetrical with a wing-like expansion on each side. Urosomal segments and caudal rami in the proportional lengths 13:18:18:20:11:20 = 100. Caudal rami about 3 times as long as wide (14:5). Left P5 exopod 2-segmented, endopod 1-segmented rudimentary; right P5 exopod 3-segmented, endopod absent.
|
 Issued from : J.-M. Brylinski, E. Antajan, T. Raud & D. Vincent in Aquatic Invasions, 2012, 7 (4). [p.580, Fig.3]. Female (from Calais harbour, France): A, habitus (dorsal); B, 4th abdominal segment and furca (dorsal); C-D, genital double-somite (ventral and left side, respectively); E, Right P5 (posterior). Male: E, P5 (posterior). Nota: Right A1 geniculated; 18th segment with serrated margin on its anterior face.
|
 Issued from : M.S. Kos in Issled. Fauny Morei, 1984 (1985), 30 (38). [p.237, Fig.6]. Female (from South Sakhalin Islands): 1, habitus (dorsal); 2, same (lateral) with eggs; 3, A2; 4, Mxp; 5, P1; 6, P4; 7, P2; 8, P3; 9, P5.
|
 Issued from : M.S. Kos in Issled. Fauny Morei, 1984 (1985), 30 (38). [p.238, Fig.7]. Male (from South Sakhalin Islands): 1, habitus (dorsal); 2, A1; 3, P5.
| | | | | Compl. Ref.: | | | Yamazi, 1958 (p.149, Rem.); Peterson, 1975 (p.29, abundance); Uye & al., 1982 (p.25, annual cycle, egg production); Uye & al., 1983 (p.91, growth, production); Uye & Kasahara, 1983 (p.147, grazing); Nagasawa & Marumo, 1984 (p.277); Madhupratap & Haridas, 1986 (p.105, tab.2); Hirakawa & al., 1990 (tab.3); Uye & Takamatsu, 1990 (p.97, red-tide flagellate effects); Dai & al., 1991 (tab.1); Yoo, 1991 (tab.1) ; Orsi & Walter, 1991 (p.560, alien species); Huntley & Lopez, 1992 (p.201, Table A1, egg-adult weight, temperature-dependent production); Kim & al., 1993 (p.270); Kimmerer, 1993 (tab.2); Myung & al., 1994 (tab.1); Uye & Kaname, 1994 (p.43, length v.s. fecal pellet volume); Shih & Young, 1995 (p.73); Ohtsuka & al., 1995 (p.159); Kotani & al., 1996 (tab.2); Ajeel & Khalaf, 1997 (p.357); Liang & Uye, 1997 (p.409, egg production); 1997 a (p.415, population dynamics, production); Cordell & al., 1997 (p.2, 7, invasive species, % change 1980-1997); Mauchline, 1998 (tab.18, 25, 35, 45, 47, 48, 51, 78, p.506: Rem.); Logerwell & Ohman, 1999 (p.428, tab.1); Ueda & al., 2000 (tab.1); Shimode & Shirayama, 2004 (p.607, tab.1, 2); Chang & Fang, 2004 (p.456, tab.1); Yin & al., (p.3); Choi & al., 2005 (p.710: Tab.III); Humphrey, 2008 (p.64, 84: Appendix A); Ohtsuka & al., 2008 (p.115, Table 4, 5, Rem.: in Japan Harbors); Jiang Z.-B. & al., 2009 (p.196, Table 1, 2); Fazeli & al., 2010 (p.153, Table 1); W.-B. Chang & al., 2010 (p.735, Table 2, abundance); Hirst & al., 2010 (p.2193, sex ratio); Bollens & al., 2011 (p.1358, Table III, fig.7); De Olazabal & Tirelli, 2011 (p.1, Rem.); Itoh & al., 2011 (p.129, vertical distribution); Beltrao & al., 2011 (p.47, Table 1, density vs time); Rajakaruna & Strasser, 2012 (p.643, modellisation temperature-dependent); Delpy & al., 2012 (p.1921, Table 2); DiBacco & al., 2012 (p.483, Table S1, ballast water transport); Lee D.B. & al., 2012 (p.88, co-occurrence); Gusmao & al., 2013 (p.279, Table 4, sex ratio); Jha & al., 2013 (p.1 distribution); Tachibana & al., 2013 (p.545, Table 1, seasonal change 2006-2008); Suzuki, K.W. & al., 2013 (p.15, Table 2, 3, 4, estuaries, annual occurrence); Seo & al., 2013 (p.448, Table 1, occurrence); Oh H-J. & al., 2013 (p.192, Table 1, occurrence); Ma & al., 2013 (p.701, figs.1, 2, 3, UV radiation vs respiration, excretion, survival); Pansera & al., 2014 (p.221, Table 2, abundance); Sabia & al., 2014 (p.1, swimming behaviour, Rem.); Sabia & al., 2015 (p.460, European distribution); Uriarte & al., 2016 (p.718, fig.5: abundance vs years 1998-2011); Garbazey & al., 2016 (p.78, first occurrence); Ohtsuka & Nishida, 2017 (p.565, 578, 585, Table 22.1, Rem.); Belmonte, 2018 (p.273, Table I: Italian zones); Richirt & al., 2019 (p.3, Table 1, fig.2, 3, 4, 5, abundance changes vs years 1998-2014, table 2: diversity index) | | | | NZ: | 11 | | |
|
Distribution map of Pseudodiaptomus marinus by geographical zones
|
| | | | | | | | | | | | | | |  Chart of 1996 Chart of 1996 | |
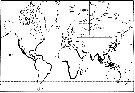 Issued from : J.-M. Brylinski, E. Antajan, T. Raud & D. Vincent in Aquatic Invasions, 2012, 7 (4). [p.578, Fig.1]. Issued from : J.-M. Brylinski, E. Antajan, T. Raud & D. Vincent in Aquatic Invasions, 2012, 7 (4). [p.578, Fig.1].
Worldwide distribution of the genus Pseudodiaptomus (grey lines) and of the species P. marinus (Adapted from Walter, 1986).
Black stars = P. marinus sensu stricto and white stars = Pseudodiaptomus cf P. marinus.
1: Brodsky, 1950; 2: Fleminger & Kramer, 1988; 3: Grindley & Grice, 1969; 4: Jiang & al., 2008; 5: Jimenez-Pérez & Castro-Longoria, 2006; 6: Jones, 1966: 7: Lawrence & Cordell, 2010; 8: De Olazabal & Tirelli, 2011; 9: Orsi & Walter, 1991: 10: Pillai, 1976; 11: Sato, 1913; 12: Shen & Lee, 1963; 13: Soh & al., 2001; 14: Tanaka, 1966; 15: Sautour & Dessier (pers. comm.); 16, Brylinski & al., 2012. (See Table 1 for details for dates, temperature, salinity and abundance).
Nota: Also in Berre Lagoon (G of Lion) by Felpy & al., 2012. |
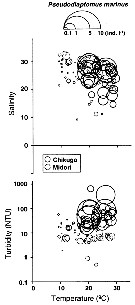 Issued from : K.W. Suzuki, K. Nakayama & M. Tanaka in J. Oceanogr., 2011, 69. [p.25, Fig.7]. Issued from : K.W. Suzuki, K. Nakayama & M. Tanaka in J. Oceanogr., 2011, 69. [p.25, Fig.7].
Bubble plots of densities of the non semi-endemic calanoid Pseudodiaptomus marinus in relation to temperature, salinity, and turbidity observed in the Chikugo and Midori River estuaries (south Japan) in 2005 and 2006. |
 Issued from : L. Sabia, G. Zagami, M.G. Mazzocchi, E. Zambianchi & M. Uttieri in Medit. Mar. Sci., 2015, 16 (2). [p.464, Fig.1]. Issued from : L. Sabia, G. Zagami, M.G. Mazzocchi, E. Zambianchi & M. Uttieri in Medit. Mar. Sci., 2015, 16 (2). [p.464, Fig.1].
European distribution of the invasive copepod Pseudodiaptomus marinus. |
 Issued from : H. Bi, K.A. Rose & M.C. Benfield in Mar. Ecol. Prog. Ser., 2011, 427. [p.157, Table 5]. Issued from : H. Bi, K.A. Rose & M.C. Benfield in Mar. Ecol. Prog. Ser., 2011, 427. [p.157, Table 5].
Instantaneous mortality rates (individuals per day) from litterature. |
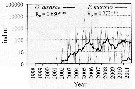 Issued from : I. Uriarte, F. Villate & A. Iriarte in J. Plankton Res., 2016, 38 (3). [p.724, Fig.5]. Issued from : I. Uriarte, F. Villate & A. Iriarte in J. Plankton Res., 2016, 38 (3). [p.724, Fig.5].
Monthly time-series from 1998 to 2011 of Pseudodiaptomus marinus (grey) and Oithona davisae (black) from inner Bilbao estuary (NW Spain). Thicker lines are moving averages. Ro is the Spearman's rank correlation coefficient (***P <0.001).
Nota: In the period 2010-1011 appears for the firdt time in 2010 the non-indigenous Pseudodiaptomus marinus. |
| | | | Loc: | | | Mauritius Is., Gulf of Oman (Chabahar Bay), Arabian Gulf, Bay of Bengal, Andaman, Australia (Moreton Bay), Taiwan (south), Hainan (Sanya Bay), China Seas (Bohai Sea, Yellow Sea, East China Sea, South China Sea, Xiamen Harbour, Taiwan (Kaohsiung Harbor), S & W Korea, Asan Bay, Geumo Is., Japan (Ariake Bay, Tokyo Bay, Fukuyama Harbor, Seto Inland Sea, Tanabe Bay, south estuaries: Chikugo, Midori & Kuma Rivers), Amur and Poseta Bays, E Siberia, Sakhaline Is., S Kuril Is., Vancouver, Friday Harbor, California (San Francisco Estuary, Sacramento Estuary), Tomales Bay, Hawaii, Kaneohe Bay, Medit. (Berre Lagoon, Po Valley, Lake Fusaro, Lake Faro (Sicily), Rimini, G. of Naples, Messina Strait, N Adriatic Sea: Trieste), Black Sea (Sebastopol) Bay of Biscay (Bilbao estuary, Gironde estuary), Pas de Calais (Calais Harbour, off Gravelines), S North Sea | | | | N: | 68 | | | | Lg.: | | | (22) F: 1,25; M: 0,97; (97) F: 1,32; M: 1,02; (290) F: 1,25-1,45; M: 0,95-1,2; (351) F: 1,21; M: 0,92; (643) F: 1,35-1,37; M: 1,05-1,07; (866) F: 1,25-1,6; M: 0,85-1,05; (1005) F: 1,21-1,26; M: 0,96-1,07; (1016) F: 1,08-1,31; M: 0,94-1,01; (1097) F: 1,3-1,8; M: 1,3-1,5; (1139) F: 1,3-1,8; M: 1,3-1,5; {F: 1,08-1,80; M: 0,85-1,50} | | | | Rem.: | Littoral, ± brackish.
In Ramosus species group (hickmani subgroup) after Walter & al. 2006, p.203.
A transport by the ballasts of ships from the Japanese waters to Hawwaii is not excluded (Jones, 1966, p. 317). The salinity in the Hawaii station was about 18 p.1000. Grindley & Grice (1969, p.131-132) come to the same conclusion regarding the Mauritius population. Jones dismisses the possibility of transport by ocean currents because the time required would exceed the life span of the individuals (because restricted to neritic waters usually of reduced salinity). A transport by Japanese fishing vessels to Mauritus by adhesion to algae or other fouling organisms attached to the hull of fishing vessels is also a possibility.
The specimens from Mauritius show a number of minor differences from specimens from Japan and from Hawaii, and from previously published sescriptions (Sato, 1913; Brodsky, 1950 and Chiba (1956) were no great as to prompt Grindley and Grice (p.132-133) to regard the Mauritius specimens as representing an undescribed species. Examination of Hawaiian specimens and comparison of this material with that from Japan and Mauritius and the previously publised descriptions and figures, indicate that there is considerable intraspecific variation by the sizes, P5 of the male and female. After careful comparison, these variations did not indicate even sub-specific differentiation of the Mauritius population, they might represent ecophenotypes, or minor geographical variations derived from different parts of the Japanese region.
According to Cordell & al., 2008 (p.753) the 1st record of this species in San Francisco estuary is 1986.
See remarks in P. sulawesiensis (Nishida & Rumengan, 2005, p.31).
After S. Nishida (1985, p.133), Grindley & Grice (1969) noted marked difference among specimens of P. marinus collected from Mauritius, Japan and Hawaii, and the previous descriptions, especially in the right exopod and the forked spine on male P5, and the spine at the base of the forked spine ; they considered that these differences do not indicate even subspecific differenciation, and might represent ecophenotypes. For Nishida, the observation of P. marinus specimens from Nemuro Bay, Tokyo Bay, Seto Inland Sea and Ariake Bay, shows the differences between P. ishigakiensis and P. marinus with constant characters, including Grindley & Grice’s description of the specimens from Mauritius. The two species are well distinct.
According to De Olazabal & Tirelli (2011, p.2) the occurrence of this species in the Locavaz channel (Adriatic Sea) suggests two hypotheses: the species has arrived by ship in the port of Monfalcone and it has been spilled into the channel with the water cooling of the power plant or was accidentally imported with other organisms used in the aquaculture plant and has subsequently escaped into the channel. The presence of a female with eggs indicates the possibility that this species may find the conditions suitable in the Adriatic Sea to develop a population; like in a eutrophic inlet of the Inland Sea of Japan (Liang & Uye, 1997) where the species reproduced throughout the year, living at temperatures ranging from 8.9 to 28.2°C and salinity from 28.6 to 32.3, these environmental conditions are very similar to those observed at the Adriatic stations, except for the higher salinity measured near Rimini.
After Brylinski & al. (2012, p.577) the occurrence of this form in both Calais harbour and the coastal waters off Gravelines (southern bight of the North Sea) supports recent observations of other Asian species in the same area suggesting a passive transport via ship's ballast waters. Jha & al. (2013) extend the distribution across the Southern Bight between The Netherlands and British coasts as in German Bight.
After Sabia & al. (2014) is recorded for the first time in October 2008 in Lake Faro (Sicily, now a stable component of the zooplankton assemblage of the Lake. The environmental features of Lake Faro seem optimal for the establishment and successful proliferation of this invasive species in relation with the peculiar hydrological and bathymetric conditions. The species has evolved a fully pelagic attitude in correspondence to the deoxygenated part of Lake, avoiding the bottom and living in the water column all day (Sabia & al., 2012), though preserving its benthic behaviour along the shores. The plasticity in the behaviour of P. marinus can be considered as a major factor, enhancing its capacity of colonising new environments.
Ohtsuka & Nishida point to in the Inlet and brackish waters on the main islands of Japan, this species is dominant. For these authors (p.585), molecular analysis suggests that the P. marinus has most likely been introduced from Japan to San Francisco Bay via ballast water, such as East Asian copepods have also been reported from European waters, for example in the North Sea (see Brylinski & al., 2012) and Oithona davisae/em> from the Mediterranean Sea (see Saiz & al., 2003), The Black Sea (Temnykh & Nishida, 2012), and the North Sea (see Cornils & Wend-Heckmann, 2015). No introduction of ''alien'' planktonic and benthic copepods in Japan have a yet been reported partly because Japan is referred to as an exportater of ballast water based on foreign trade statistics (See Ohtsuka & al., 2008; Ohtsuka & Hiromi, 2009). The authors recall that the endoparsitic copepod Mytilicola orientalis has been introduced from Japan to Europe and rge Pacific Coast of North America via transplantation of the cultured oyster Ceassostrea gigas and has reinfected commercial and noncommercial native bivalves in Europe (See Stock, 1993; Steele & Mulcahy, 2007). In the same order of idea, Ueda & Ishida (1997) surveyed the freshwater copepod fauna in Okinawa from 1984 to 1991, compared with the resuts of a survey by F. Kiefer more than 50 years ago. Larger copepods such as Mesocyclops had disappeared, but smaller Thermocyclops crassus was dominant, which they suggestedmight have been a resukt of eutrophisation and/or the introduction of planktivorous African cichlids.
Garbazey & al.: (2016) note for the 1st time the occurrence of the species in the Black Sea at Sebastopol. | | | Last update : 02/01/2021 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed March 06, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |