|
|
 |
|
Calanoida ( Order ) |
|
|
|
Diaptomoidea ( Superfamily ) |
|
|
|
Acartiidae ( Family ) |
|
|
|
Acartia ( Genus ) |
|
|
|
Odontacartia ( Sub-Genus ) |
|
|
| |
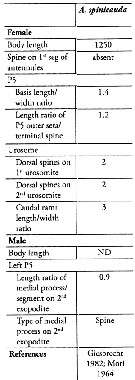
Acartia (Odontacartia) spinicauda Giesbrecht, 1889 (F,M) | |
| | | | | | | Syn.: | no Acartia spinicauda : Wellershaus, 1969 (p.275, figs.M); ? Mori, 1937 (1964) (p.103, figs.F); ? Chen & Zhang, 1965 (p.114, figs.F, Syn. part.)
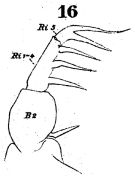
? Acartia (O.) pacifica female : Wellershaus, 1969 (p.275, figs.F) | | | | Ref.: | | | Giesbrecht, 1892 (p.508, 523, 770, figs.F,M); Giesbrecht & Schmeil, 1898 (p.155); A. Scott, 1909 (p.188, Rem.); Sewell, 1912 (p.354, 377); 1914 a (p.241); Steuer, 1923 (p.27, figs.F,M); Sewell, 1924 (p.789); 1932 (p.397); 1933 (p.28); 1934 (p.81); Kasturirangan, 1963 (p.61, 63, figs.F,M); Kos, 1972 (Vol.I, figs.F,M, Rem.); Goswami & Goswami, 1973 (p.242, fig.1, karyotype); Abraham, 1976 (p.77, 79, fig.M); Yoo & Hue, 1983? (p. 11, figs. F,M); Zheng Zhong & al., 1984 (1989) (p.258, figs.F,M; Srinui & al., 2019 (p.89, 90, Rem.: F,M); Lee S. & al., 2019 (p.86, Table 3). |  issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.43, Fig.11]. Male: 11, thoracic segment 5 and urosome (dorsal).
|
 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.30, Fig.16] Male: 16, Mxp (distal portion).
|
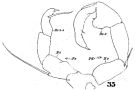
 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.30, Fig.21]. Female: 21, P5 (posterior view).
|
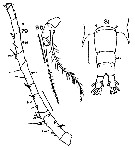
 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.30, Fig.35]. Male: 35, P5.
|
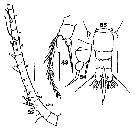
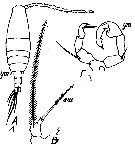
 issued from : T. Mori in The pelagic Copepoda from the neighbouring waters of Japan, 1937 (2nd edit., 1964). [Pl.50, Figs.5-7]. With doubt. Female: 5, habitus (dorsal); 6, P5; 7, urosome (dorsal). Nota: Rostal filaments present. lateral angles of the last thoracic segment produced into the pointed processes. Genital segment with 2 spines which are smaller than those of the following segment. Caudal rami about 3 times as long as wide. A1 with spinules on the proximal segments. Terminal segment of P5 filamentous, and swelled at the base
|
 issued from : S. Abraham in Crustaceana, 1976, 30 (1). [p.75, Fig.17]. Male: 17, right A1 (a portion showing spines on segment distal to geniculation)).
|
 Issued from : S. Wellershaus in Veröf. Inst. Meeresf. Bremerhaven, 1969, XI. [p. 274, Fig. 79-81]. Female (from Cochin Backwater and outlet, W Thevara): 79, right A1 (dorsal; hook like spine on the back, spines black, an additional small spine lirs on the distal posterior margin of segment 19, c = spine common in many species); 80, P5 (dorsal); 81, urosome (dorsal). Nota: - Ratio prosome : urosome 3.3. - Urosomal segment 1-3 bears son the ventral side groups of long hairs; urosomal segment 5 (= anal somite) shorter hairs. - A1 bears a small spine on the distal end of the posterior side of segment 19 (closely related species A. pacifica bears no such spines on A1. Remarks: The spines on urosomal;segment 4 are of different size in various specimens and can be considerably longer than in fig. 81. Also, A1 differs slightly from the specimens from China (Steuer, 1923).
|
 Issued from : S. Wellershaus in Veröf. Inst. Meeresf. Bremerhaven, 1969, XI. [p. 274, Fig. 82-85]. As Acartia (Odontacartia) pacifica Female. Doubtful. Female (from Cochin Backwater and outlet, W Thevara): 82, right A1 (c = as in A. spinicauda); 83-84, P5 (two aspects); 85, posterior part of thoracic segment 5 and urosome (dorsal) Nota: - Ratio prosome : urosome 3.3 (or more in other specimens). - Proportions of the urosomal segments + caudal rami 36 : 16 : 14 : 34 = 100. - Proportions of the caudal setae (in % of the urosome length: Si = 60; St1 = 111; St2 = 178; St3 = 109; St4 = 76; Se = 40. - A1 is stronger in the proximal portion yjan in other Acartia species; segment 19 is barev of spines; segment 2-6 to 13 seem not completely separated. - Rostral horns dagger-like in profile (as in A. centrura female).
|
 Remark: Erratum concerning the name: read Srinui and not Siruani. Female: 1 - Genital double-somite having paired posterodorsal processes. 2 - 2nd segment of A1 with strong curved processes posteriorly. 3 - 1st Segment of A1 lacking processes. Male: 1 - Urosomite 3 with large spine-like peocesses dorsally. 2 - Dorsal processes of urosomite 3 long, reaching half-length of anal somite. 3 - Urosomite 4 with 4 spine-like processes between pair of dorsal processes.
|
 Issued from : M.C. Kos in Field guide for plankton. Zool Institute USSR Acad., Vol. I, 1972. After Stueur, 1923. Female: 1, habitus (dorsal); 2, P5. Male: 3, P5.
|
 Issued from : S. Lee, H.Y. Soh & W. Lee in ZooKeys, 2019, 893. [p.84, Table 3]. Acartia (Odontacartia) spinicauda: Morphological characters. Compare with other Odontacartia species. Nota: 1 - Absence of spine on 1st to 2nd segments of female A1 .....2. 2 - Spine present on dorsal surface of female genital double-somite ..... 3. 3 - Dorsal surface of female genital bouble-somite and 2nd urosomite with 2 strong spines .....4. 4 - Female P5 outer seta longer than terminal spine ..... 5. 5 - Length-width ratio of female caudal rami as 3; medial process on 2nd exopodite male left P5 as spine.
| | | | | Compl. Ref.: | | | Carl, 1907 (p.17); Sewell, 1948 (p.324); Yamazi, 1958 (p.153, Rem.); Itoh, 1970 (tab.1); Subbaraju & Krishnamurphy, 1972 (p.25, 26 ); Patel, 1975 (p.660); Chen Q-c, 1980 (p.794); Stephen, 1984 (p.161, Distribution vs thermocline & geographic); Guangshan & Honglin, 1984 (p.118, tab.); Madhupratap & Haridas, 1986 (p.105, tab.2); Sarkar & al., 1986 (p.178); Mitra & al., 1990 (fig.3); Dai & al., 1991 (tab.1); Yoo & al., 1991 (p.261); Gajbhiye & al., 1991 (p.188); Gajbhiye & Abidi, 1993 (p.137); Godhantaraman, 1994 (tab.5, 6, 7); Shih & Young, 1995 (p.66); Ramaiah & al., 1996 (p.3); Marcus, 1996 (p.143, as spinacauda); Ramaiah & Nair, 1997 (tab.1); Park & Choi, 1997 (Appendix); Mauchline, 1998 (tab.8, 40); Nair & Ramaiah, 1998 (p.272, fig.4); Achuthankutty & al., 1998 (p.1, Table 2, fig.5, 6, seasonal abundance vs monsoon); Dalal & Goswami, 2001 (p.22, fig.2); Rainbow & Wang, 2001 (p.240); Rezai & al., 2004 (p.489, tab.2, p.495, tab.8); ? Zuo & al., 2006 (p.163: tab.1); Hwang & al., 2006 (p.943, tab.I); Dur & al., 2007 (p.197, Table IV); Madhu & al., 2007 (p.54, Table 4, abundance vs monsoon); Rakhesh & al., 2008 (p.154, abundance vs stations); Jerling, 2008 (p.55, Tabl.1); Fernandes, 2008 (p.465, Tabl.2); Perumal & al., 2008 (p.149, abundance vs hydrographic parameters); Tseng L.-C. & al., 2008 (p.153, Table 2, fig.5, occurrence vs geographic distribution, indicator species); Jiang Z.-B. & al., 2009 (p.196, Table 1, 2); W.-B. Chang & al., 2010 (p.735, Table 2, abundance); Shanthi & Ramanibai, 2011 (p.132, Table 1); Maiphae & Sa-ardrit, 2011 (p.641, Table 2); Chew & Chong, 2011 (p.127, fig.4, 5, Table 2, 3, abundance vs location); Zhang D. & al., 2011 (p.86, pH effects); Yoshida & al., 2012 (p.644, fig.1, 3, Table 1, egg development time and hatching vs temperature); Johan & al., 2012 (p.647, Table 1, 2, fig.2, salinity range); Jose & al., 2012 (p.20, fig.3 a,b,c: % vs monsoon); Beyrend-Dur, 2013 (p.771, fig.6: sex ratio, composition); Jagadeesan & al., 2013 (p.27, Table 3, 4, 6, fig.11, seasonal abundance); Anjusha & al., 2013 (p.40, Table 3, abundance & feeding behavior); Varadharajan & Soundarapandian, 2013 (p.2: occurrence vs stations); Rakhesh & al., 2013 (p.7, Table 1, 4, abundance vs stations); Trottet & al., 2017 (p. 7, Table 3: resting stage) | | | | NZ: | 6 | | |
|
Distribution map of Acartia (Odontacartia) spinicauda by geographical zones
|
| | | | | | | | |  Chart of 1996 Chart of 1996 | |
 issued from : C.T. Achuthankutty, N. Ramaiah & G. Padmavati in Pelagic biogeography ICoPB II. Proc. 2nd Intern. Conf. Final report of SCOR/IOC working group 93, 9-14 July 1995. Workshop Report No. 142, Unesco, 1998. [p.8, Fig.6]. issued from : C.T. Achuthankutty, N. Ramaiah & G. Padmavati in Pelagic biogeography ICoPB II. Proc. 2nd Intern. Conf. Final report of SCOR/IOC working group 93, 9-14 July 1995. Workshop Report No. 142, Unesco, 1998. [p.8, Fig.6].
Salinity ranges for A. spinicauda in coastal and estuarine waters of Goa (India).
Shaded area indicates the range of higher abundance. |
 Issued from : T. Yoshida, C.-F. Liong, A.M. Majid, T. Toda & B.H.R. Othman in Zoological Studies, 2012, 51 (5). [p.651, Fig.3]. Issued from : T. Yoshida, C.-F. Liong, A.M. Majid, T. Toda & B.H.R. Othman in Zoological Studies, 2012, 51 (5). [p.651, Fig.3].
Geographical records of A. pacifica, A. spinicauda, A. erythraea occurrences throughout the East Asian region. |
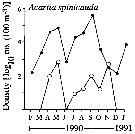 issued from : C.T. Achuthankutty, N. Ramaiah & G. Padmavati in Pelagic biogeography ICoPB II. Proc. 2nd Intern. Conf. Final report of SCOR/IOC working group 93, 9-14 July 1995. Workshop Report No. 142, Unesco, 1998. [p.6, Fig.5]. issued from : C.T. Achuthankutty, N. Ramaiah & G. Padmavati in Pelagic biogeography ICoPB II. Proc. 2nd Intern. Conf. Final report of SCOR/IOC working group 93, 9-14 July 1995. Workshop Report No. 142, Unesco, 1998. [p.6, Fig.5].
Monthly occurrence of Acartia spinicauda in coastal (black circle) in front of Goa and and the Mandovi estuary (clear circle). |
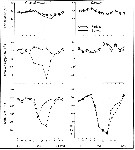 issued from : C.T. Achuthankutty, N. Ramaiah & G. Padmavati in Pelagic biogeography ICoPB II. Proc. 2nd Intern. Conf. Final report of SCOR/IOC working group 93, 9-14 July 1995. Workshop Report No. 142, Unesco, 1998. [p.3, Fig.2]. issued from : C.T. Achuthankutty, N. Ramaiah & G. Padmavati in Pelagic biogeography ICoPB II. Proc. 2nd Intern. Conf. Final report of SCOR/IOC working group 93, 9-14 July 1995. Workshop Report No. 142, Unesco, 1998. [p.3, Fig.2].
Hydrography of the coastal station in front of Goa and Mandovi estuarine station (W India).
Nota: The seasons are arbitrarily classified into the southwest monsoon (June-September), postmonsoon (October-January) and premonsoon (February-May). |
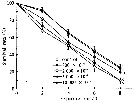 Issued from : D. Zhang, S. Li, G. Wang & D. Guo in Acta Oceanol. Sin., 2011, 30 (6). [p.89, Fig. 1 c]. Issued from : D. Zhang, S. Li, G. Wang & D. Guo in Acta Oceanol. Sin., 2011, 30 (6). [p.89, Fig. 1 c].
Influence of seawater acidification on survival rate in Acartia spinicauda.
Average pH of each pCO2 seawater culture. Control (380) 8.16-8.17; (800) : 7.84-7.85; (2000): 7.39-7.37; (5000): 7.19-7.24; (10 000): 6.92-6.94. |
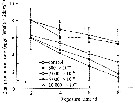 Issued from : D. Zhang, S. Li, G. Wang & D. Guo in Acta Oceanol. Sin., 2011, 30 (6). [p.90, Fig. 2 c]. Issued from : D. Zhang, S. Li, G. Wang & D. Guo in Acta Oceanol. Sin., 2011, 30 (6). [p.90, Fig. 2 c].
Influence of seawater acidification on egg producton rate in Acartia spinicauda.
Average pH of each pCO2 seawater culture. Control (380) 8.16-8.17; (800) : 7.84-7.85; (2000): 7.39-7.37; (5000): 7.19-7.24; (10 000): 6.92-6.94. |
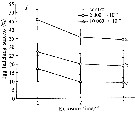 Issued from : D. Zhang, S. Li, G. Wang & D. Guo in Acta Oceanol. Sin., 2011, 30 (6). [p.91, Fig. 3 a]. Issued from : D. Zhang, S. Li, G. Wang & D. Guo in Acta Oceanol. Sin., 2011, 30 (6). [p.91, Fig. 3 a].
Influence of seawater acidification on egg hatching success in Acartia spinicauda.
Average pH of each pCO2 seawater culture. Control (380) 8.16-8.17; (2000): 7.39-7.37; (10 000): 6.92-6.94.
Data are described as mean ± SD (n = 3). Different letters indicate a significant difference among elevated pCO2 levels at p < 0.05. |
| | | | Loc: | | | S Korea, Japan, Tanabe Bay, Hong-Kong, Taiwan (N), Taiwan Strait (Amoy), Taiwan (S, Danshuei Estuary), Viet-Nam ( Cauda Bay), China Seas (Yellow Sea, East China Sea, South China Sea, Xiamen Harbour], Arabian Sea, Sri Lanka, India (Mangalore coast, G. of Manaar, Palk Bay, Pointcalimere-Manamelkudi, Saurashtra coast, W, Bombay, Mandovi-Zuari estuary, Kerala, Goa, S, Burhabalanga estuary,Porto Novo, Godavari region, Kakinada Bay, Chilka Lake, Hooghly estuary, Mandarmani, Parangipettai coast), S South Africa (Richard's Bay Harbour and Mhlathuze Estuary), Oman Sea, Nicobar Is., Bay of Bengal, Burma, Kurau Riv., Straits of Malacca, Sangga estuary, Singapore, Perai River Estuary, Indonesia-Malaysia, Ambon Bay (Baie d'Amboine), Pacif. (W equatorial) | | | | N: | 65 | | | | Lg.: | | | (46) F: 1,25; M: 1,17; ? (91) F: 1,25; (164) F: 1,25; M: 1,17; {F: 1,25; M: 1,17} | | | | Rem.: | ± brackish, estuary-neritic.
The locality records in the Chinese and Korean seas seem to be confirmed.
After Shanthi & Ramanibai (2011, p.135) the dominance of species in the near shore waters from Cooum and Adyar (SE India) can be considered as an indicator of pollution status.
For Itoh (1970 a, fig.2, from co-ordonates) the Itoh's index value from mandibular gnathobase = 840.
After Wellershaus (1969, p.275), samples from water between 10 and 31 p.1000.
A doubt subsists on the synonymy between A. pacifica female in Wellershaus (1969, p.276) and A. spinicauda female. | | | Last update : 03/12/2020 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2025. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed December 24, 2025] © copyright 2005-2025 Sorbonne University, CNRS
|
|
 |
 |


















