|
|
 |
|
Calanoida ( Order ) |
|
|
|
Diaptomoidea ( Superfamily ) |
|
|
|
Acartiidae ( Family ) |
|
|
|
Paracartia ( Genus ) |
|
|
| |
Paracartia grani Sars, 1904 (F,M) | |
| | | | | | | Syn.: | Acartia (Paracartia) grani : Steuer, 1923 (p.17, figs.F,M); Rose, 1933 a (p.274, figs.F,M); Farran, 1948 a (n°12, p.3, figs.F); Sewell, 1948 (p.483); Marques, 1953 (p.121); Lance & Raymont, 1965 (p.619); Vilela, 1965 (p.10); Neto & Paiva, 1966 (p.28, Table III); Corral Estrada, 1970 (p.208, figs.F,M, Rem.); Corral & Alvarez-Ossorio, 1978 (p.138); Andronov & Maigret, 1980 (p.65, table 3); Castel & Courties, 1982 (p.417, Table II, fig.4, spatial & monthly distribution); Vives, 1982 (p.295); Alcaraz, 1983 (p.891); Rodriguez & Vives, 1984 (p.246); Diouf & Diallo, 1987 (p.260); Lozano Soldevilla & al., 1988 (p.60); Lakkis, 1990 (p.63);
Acartia grani : Lefèvre-Lehoërff, 1972 (p.1681); Corral Estrada & Pereiro Muñoz, 1974 (tab.I); Gallo, 1981 (p.847, 848); d'Elbée, 1984 (p.24, Fig.3); Jansa, 1985 (p.108, Tabl.I, II, III, IV); Rodriguez & Jiménez, 1990 (p.497); Saiz & Alcaraz, 1992 (tab.1); Sautour & Castel, 1993 (p.279); Lakkis, 1990 (p.63: Rem.); 1994 (p.481); Gurrrero & al., 1994 (p.95, temperature vs. embryogenic development); Rodriguez & al., 1995 (p.2233, egg production); Ban S, Burns C. & al., 1997 (p.287, Table 1, 2, feeding, reproduction); Guerrero & Rodriguez, 1997 (p.584, production); 1998 (p.305, resting eggs in sediments); Mauchline, 1998 (tab.17, 45, 47, 48); Siokou-Frangou, 1999 (p.478); d'Elbée, 2001 (tabl. 1); Zerouali & Melhaoui, 2002 (p.91, Tableau I); Daly Yahia & al., 2004 (p.366, fig.4); Henriksen & al., 2007 (p.119, feeding & swimming activity/ naiuplii); Guermazi & al., 2005 (p.280); Costa & al., 2005 (p.177, Rem.: feeding); Alcaraz & al., 2007 (p.298: tab.2); David & al., 2007 (p.59: tab.2); Khelifi-Touhami & al., 2007 (p.327, Table 1); Youn & Choi, 2007 (p.222: Table 1, egg production); Estrada & al., 2008 (p.1, Rem.: role of phosphorus); Drillet & al., 2012 (p.155, Table 1, culture); Costa & al., 2012 (p.75, ingestion, filtration rate vs toxic algae); Isari & al., 2013 (p.1, feeding vs food quality);
Acartia grani : Lidvanov & al., 2013 (p.290, Table 2, % composition); Barton & al., 2013 (p.522, Table 1: metabolism, poupulation dynamic, feeding mode);
Acartia granii : Kiørboe, 2008 (p.155, Table 2, swimming speed) | | | | Ref.: | | | Sars, 1904 b (p.4, figs.F,M); van Breemen, 1908 a (p.161, figs.F,M); Sars, 1919 (1921) (p.16, figs.F,M); Candeias, 1932 (p.6, figs.F); Bradford-Grieve, 1999 (n°181, p.14, figs.F,M); Barthélémy, 1999 (p.859, 867, figs.F); 1999 a (p.9, Fig.28, A-C); Avancini & al., 2006 (p.118, Pl. 86, figs.F,M, Rem.); Vives & Shmeleva, 2007 (p.433, figs.F,M, Rem.); Brugnano & al., 2009 (p.354, Rem.) | 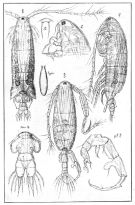 issued from : G.O. Sars in An Account of the Crustacea of Norway. Suppl. Vol. VII. Copepoda Calanoida. Published by the Bergen Museum, 1919 (1921). [Suppl. Pl. X]. Female & Male.
|
 issued from : G.O. Sars. in An Account of the Crustacea of Norway. Suppl. Vol. VII. Copepoda Calanoida. Published by the Bergen Museum, 1919 (1921). [Suppl. Pl.XI]. Female & Male. (continued)
|
 issued from : J. Corral Estrada in Tesis Doct., Univ. Madrid, A-129, Sec. Biologicas, 1970. [Lam.54, figs.4-7]. As Acartia (Paracartia) grani. Female (from Canarias Is.): 4, habitus (dorsal); 5, urosome (dorsal); 6, spermatophore; 7, P5. Male: distal part urosome (dorsal).
|
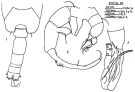 issued from : J. Corral Estrada in Tesis Doct., Univ. Madrid, A-129, Sec. Biologicas, 1970. [Lam.55, figs.1-3]. As Acartia (Paracartia) grani. Male: 1, posterior part cephalothorax and urosome (dorsal); 2, P5; 3, distal part of right A1.
|
 issued from : R.-M. Bathélémy in J. Mar. Biol. Ass. U.K., 1999, 79. [p.866, Fig.9, D-E]. Scanning electon miccrograph. Female: D, genital double-somite (ventral) showing lateroposterior position of the genital slits (arrows); E, an other view of the left genital slit. Scale bars: 0.020 mm (D); 0.010 mm (E). Nota: The species is characterized by a fragmented area but the internal area is provided with loop-shaped seminal ducts opening directly in the proximal zone of the egg-laying duct. This organization, at least for the external area with lateroposterior genital slits, is analogous to that observed in P. africana and P. latisetosa and could constitute an important characteristic of the genus.
|
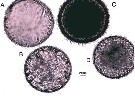 Issued from : S. Boyer & D. Bonnet in J. Plankton Res., 2013, 35 (3). [p.670, Fig.1]. Three types of Paracartia grani eggs (from Sète Channel in the Thau Lagoon, South France) just after spawning. (A) empty short-spined egg; (B) sghort-spined egg; (C) long-spined egg; (D) smooth egg. Nota: Three types corresponding to smooth eggs, eggs with short spines and eggs with long spines. These morphologies are similar those previously observed for Paracartia latisetosa with smooth and short-spined eggs defined as subitaneous eggs, and long-spined eggs as resting eggs (See Belmonte, 1992, and Balmonte & Pati, 2007)
|
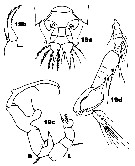 Issued from : J.M. Bradford-Grieve in ICES Identification Leaflets for Plankton N°181, 1999 [p.13, Figs.19 a-d]. Female: a, posterior part of last thoracic segment and urosome (dorsal); b, P5. Male: c, P5 (L = left leg; R = right leg); d, A1.
|
 Issued from : M.G. Mazzocchi in Guida al Riconoscimento del plancton dei Mari Italiani, Vol. II, 2006. [p.94, Tav. 86]. After A. Comaschi. Female: a, habitus (dorsal); b, urosome (dorsal); c, spermatophore; d, P5; e, left P5. Male: f, habitus (dorsal); g, right A1 (geniculate); h-i, P5 (various aspects).
| | | | | Compl. Ref.: | | | Belmonte & Potenza, 2001 (p.174); De Olazabal & al., 2006 (p.964); Calbet & al., 2007 (p.195, toxic effect); Selifonova & al., 2008 (p.305, Tabl. 2); Brylinski, 2009 (p.253: Tab.1, p.256: Rem.); Drira & al., 2010 (p.145, Tanl.2): Mazzocchi & Di Capua, 2010 (p.423); Zenetos & al., 2010 (p.397); Mazzocchi & al., 2011 (p.1163, fig.6, long-term time-series 1984-2006); Selifonova, 2011 a (p.77, Table 1, alien species in Black Sea); Boyer & al., 2012 (p.1, figs.1, 2, 3, geographic distribution, annual change); Zizah & al., 2012 (p.79, Tableau I); Annabi-Trabelsi & al., 2012 (p.445, egg production vs environmental conditions); Rekik & al., 2012 (p.336, Table 1, abundance); Mazzocchi & al., 2012 (p.135, annual abundance 1984-2006); Herrera & al., 2012 (p.101, nauplii growth, AARS activity vs temperature & food concentration); Boyer & Bonnet, 2013 (p.668, resting eggs); Lindeque & al., 2013 (p.737, molecular identification); Boyer & al., 2013 (p.125, egg production vs environmental parameters); Gubanova & al., 2013 (in press, p.4, Table 2); Ben Ltaief & al., 2017 (p.1, Table III, Summer relative abundance); El Arraj & al., 2017 (p.272, table 1, 2, spatial distribution); Belmonte, 2018 (p.273, Table I: Italian zones) | | | | NZ: | 4 | | |
|
Distribution map of Paracartia grani by geographical zones
|
| | | | | | | | |  Chart of 1996 Chart of 1996 | |
 issued from : F. Guerrero, J.M. Blanco & Rodriguez V. in J. Plankton Res., 1994, 16 (1). [p.99, Table I]. issued from : F. Guerrero, J.M. Blanco & Rodriguez V. in J. Plankton Res., 1994, 16 (1). [p.99, Table I].
Embryogenic development time (D) and rate (d) of A. (= Paracartia) grani eggs (from Malaga Bay) at six incubation temperatures (n = 2).
Nota: A comparative analysis was carried out on the several equations most commonly used to describe the dependance of the development of organisms on temperature, i-e Linear, Parabolic, Arrhenius, Arrenius modified, Belehradek, Heip and Tauti models. The subsequent application of the chosen equation to experimental data on eggs of P. grani suggested that to work with rates of development would give better results than times of development and revealed the superiority of Tauti's equation . |
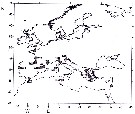 Issued from : S. Boyer, I. Arzul & D. Bonnet in Mar. Biodiv. Rec., 2012. [p.2, Fig.1]. Issued from : S. Boyer, I. Arzul & D. Bonnet in Mar. Biodiv. Rec., 2012. [p.2, Fig.1].
Biogeography (literature record) of Paracartia grani in Europe in 2011. |
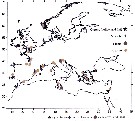 Issued from : S. Boyer, I. Arzul & D. Bonnet in Mar. Biodiv. Rec., 2012. [p.4, Fig.3]. Issued from : S. Boyer, I. Arzul & D. Bonnet in Mar. Biodiv. Rec., 2012. [p.4, Fig.3].
Records of Paracartia grani and several bivalves exploited in Europe.
Nota: Most distributional records of P; grani are correlated with bivalve farming sites. |
 Issued from : T. Kiørboe in Oecologia, 2008, 155. [p.185, Table 2]. Issued from : T. Kiørboe in Oecologia, 2008, 155. [p.185, Table 2].
Grand average swimming speeds (±SD) and cephalothorax lengths of males and females of Acartia granii (= Paracartia grani).
Nota: Experimental animals were reared in the laboratory at 22°C, this species probably feeds similarly to other Acartia species with feeding bouts alternating with sinking (see Tiselius & Jonsson, 1990). |
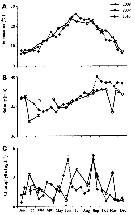
 Issued from : S. Boyer, M. Bouvy & D. Bonnet in Estuar, Coast. Shelf Sci., 2013, 117. [p.129, Fig.3 A, B]. Issued from : S. Boyer, M. Bouvy & D. Bonnet in Estuar, Coast. Shelf Sci., 2013, 117. [p.129, Fig.3 A, B].
Paracartia grani: A, Abundance (ind. 33) over a year at the monitoring station in 2008, 2009 and 2010. B, Egg production (EP) .
Bars: standards deviations.
Samples collected at a fixed station (43°25'N, 03°40'E) in the Thau lagoon, close to Sète channel, with a WP2 net (200 µm mesh aperture). Mesozooplankton collected by horizontal hauls at 1 m depth.
For Boyer & al. (2013, p.134) abundance of P. grani was clearly affected by the decrease of temperature occurring in autumn ; at this period, its abundance greatly decreased while egg production remained constant and hatching success increased. Furthermore, the egg production of a morphologically different type was observed together with the decrease of P. grani abundance. This type of eggs did not hatch during hatchuing experiments and have been described as resting eggs (see Guerrero & Rodriguez, 1998). Finally, none of the smooth eggs, considered as subitaneous eggs, did hatch even after a week of incubation , in late November or at the beginning of December when low temperature (below to 8°C) were observed. These environmental conditions could explain P. grani disappearance from the water column in January because no recruitment took place. |
 Issued from : S. Boyer, M. Bouvy & D. Bonnet in Estuar, Coast. Shelf Sci., 2013, 117. [p.128, Fig.2]. Issued from : S. Boyer, M. Bouvy & D. Bonnet in Estuar, Coast. Shelf Sci., 2013, 117. [p.128, Fig.2].
Environmental parameters at the sampling station in 2008, 2009, 2010, in the Thau lagoon, close to Sète channel.
Bars: Standards deviations.
A: temperature (°C); B, salinity (psu); c, chlorophyll a concentration (µg /L). |
 Issued from : S. Boyer, M. Bouvy & D. Bonnet in Estuar, Coast. Shelf Sci., 2013, 117. [p.132, Fig.6, A]. Issued from : S. Boyer, M. Bouvy & D. Bonnet in Estuar, Coast. Shelf Sci., 2013, 117. [p.132, Fig.6, A].
Paracartia grani: Relationship between egg production (EP) and prosome length (PL), in the Thau lagoon, close to Sète channel.
Black dotted lines represent the linear regression for all points. |
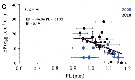
 Issued from : S. Boyer, M. Bouvy & D. Bonnet in Estuar, Coast. Shelf Sci., 2013, 117. [p.131, Fig.5, C]. Issued from : S. Boyer, M. Bouvy & D. Bonnet in Estuar, Coast. Shelf Sci., 2013, 117. [p.131, Fig.5, C].
Paracartia grani: Egg production (EP) in function of salinity (S) and temperature (T) in the Thau lagoon, close to Sète channel.
Blue circles: 2008; black circles: 2010. |
 Issued from : M.G. Mazzocchi, L. Dubroca, C. Garcia-Comas, I. Di Capua & M. Ribera d'Alcalà in Progr. Oceanogr., 2012, 97-100. [p.143, Fig.10]. Issued from : M.G. Mazzocchi, L. Dubroca, C. Garcia-Comas, I. Di Capua & M. Ribera d'Alcalà in Progr. Oceanogr., 2012, 97-100. [p.143, Fig.10].
Long-term variability of the abundance (Ind. m3) of rare copepod species that appeard in 1995 and occurred regularly starting from 1998 (autumn-winter) at Station MC (Gulf of Naples), by vertical tows in the upper 50 m. |
 Issued from : S. Ban, C. Burns, J. Castel, Y. Chaudron & al. in Mar. Ecol. Prog. Ser., 1997, 157. [p.289, Table 1]. Issued from : S. Ban, C. Burns, J. Castel, Y. Chaudron & al. in Mar. Ecol. Prog. Ser., 1997, 157. [p.289, Table 1].
Synopsis of feeding/reproduction experiments. A total of 17 diatom and 16 copepod species, representative of a variety of worldwide temperate and subarctic environments (E: estuarine, C: coastal ocean), were screened.
Data for fecundity and hatching success are average values measured at the start and end of the inciubations in a minimum of 3 replicate batches, showing the variation in time of the effects of diatoms on copepod reproduction.
Level of significance of the diatom effect between treatments and controls (e.g. non-diatom diets) in categories I to III was p < 0.01. No.. rank (for comparison with Table 2, p.290).
For the signification of the categories I to IV (fecundity vs. Hatching success vs. Duration of experiment days-1): See Calanus finmarchicus.
List of the 12 marine or estuarine species studied (fresh water excluded. CR): Acartia clausi, A. grani (= Paracartia grani), A. steueri, A. tonsa, Calanus finmarchicus, C. helgolandicus, C. pacificus, Centropages hamatus, C. typicus, Eurytemora affinis, Temora longicornis, T. stylifera. |
 Issued from : S. Ban, C. Burns, J. Castel, Y. Chaudron & al. in Mar. Ecol. Prog. Ser., 1997, 157. [p.290, Table 2]. Issued from : S. Ban, C. Burns, J. Castel, Y. Chaudron & al. in Mar. Ecol. Prog. Ser., 1997, 157. [p.290, Table 2].
Acartia grani (= Paracartia grani): Combinations of copepod and non-diatom diets in controls in concentrations ranging from 104 to 105 cells ml-1. Data for fecundity and hatching success are average values measured at the start and end of incubation in a minimum of 3 replicate batches.
In Category I. |
| | | | Loc: | | | Norway (Bergen), North Sea, off Gravelines, English Channel (Morlaix estuary, Dinard, Southampton, off Gravelines), Bay of Biscay (Mundaka estuary, St-Jean-de-Luz, Arcachon Bay, Oleron Is.: oysters parks), Angola, Baia Farta, Casamance, Dakar, Canary Is., Morocco-Mauritania, W Spain (Ria de Arosa), Portugal, Medit. (Ras Kebdana, Bay of Tunis : lagoon, Malaga (harbour), Baleares, Barcelona Harbour, Sitges beach & Masnou, Thau lagoon (Euzet, 1998, comm. pers.), Ligurian Sea, S Italian coast, G. of Napoli, G. of Manfredonia, G. of Trieste, Adriatic Sea (Koper Harbour), Gulf of Anaba, Sfax, G. of Gabès, G. of Saronikos, Lebanon, W Black Sea) | | | | N: | 69 | | | | Lg.: | | | (132) F: 1; M: ± 1; (164) F: 1,03; M: 1,03; (180) F: 1,2-1,16; M: 1,05; (373) F: 1,26-0,99; M: 1,12-0,95; (1047) F: 0,9; {F: 0,90-1,26; M: 0,95-1,12} | | | | Rem.: | Often observed in oister basins.
Exceptional in Norway where it could maintain itself thanks to the higher temperatures in the basins.
Most probably dispersed by human transport. Observed for the first time in the Barcelona Harbour in June 1975 by Alcaraz (comm. pers., 2008), absent during all the year 1974.
For Olazabal & al. (2006, p.967) the presence of this species in the Gulf of Trieste (Italia) may be due to the ballast waters discharged directed to the nearby Monfalcone Harbour.
Boyer & al. (2012, p.5) suggest that oyster transfers between the Atlantic and the mediterranean Sea might be responsible for its introduction into Mediterranea Sea. In addition, P. grani in Thau lagoon might be responsible for P. latisetosa drastic decrease.
See photographs of female and males (with the permission of Alessandra de Olazabal). | | | Last update : 28/10/2022 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2025. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed August 23, 2025] © copyright 2005-2025 Sorbonne University, CNRS
|
|
 |
 |















