|
|
 |
| Monstrillidae Dana, 1849 | | Ref.: | Giesbrecht, 1892 (p.80); Malaquin, 1901 (p.106); van Breemen, 1908 a (p.201, spp. Key); Sars, 1921 (p7); Rose, 1933 a (p.338); Sewell, 1949 (p.131, Rev., Key); Davis, 1949 a (p.245, Rev., genera & species Key); Razouls, 1972 (p.147); Davis & Green, 1974 (p.62); Isaac, 1974 (p.127); 1975 (n°144/145, p.2, genera key.); Kabata, 1979 (p.84); Razouls, 1982 (p.747); Bowman & Abele, 1982 (p.13); Zheng Zhong & al., 1984 (1989) (p.272); Huys & Boxshall, 1991 (p.154, 422, Rem.); Grygier, 1994 (p.235, 240-241); 1994 a (p.23); 1995 (p.245, 247, Rev.); 1995 a (p.1, Rev.); Chihara & Murano, 1997 (p.1002); Boxshall & Halsey, 2004 (p.16; 835: Def.; p.837: Genera key; Rem.); Grygier & Ohtsuka, 2008 (p.460, Rem.: New genus); Vives & Shmeleva, 2010 (p.156, Rem., Genera key); Suarez-Morales, 2011 (p.4, 8, Rem.: Rev.); Suarez-Morales & al., 2017 (p.1827, Rem.); Jeon & al., 2018 (p.45, 47: genus new, p.56-57: genetic divergences, phylogenetic trees), Rem. p.61 on Haemocera; hosts vs monstrilloid species) | | Rem.: | Originally 7 Genera are more or less well defined: Haemocera, Cymbasoma, Monstrilla, Monstrillopsis, Thaumaleus, Strilloma, Thaumatohessia.
Sewell (1949) gives the general characters of 4 genera (Table, p.132).
Davis (1949 a) synonymizes on the one hand Thaumaleus (as in Giesbrecht,1892, not Kröyer, 1849) and Cymbasoma, and on the other hand Monstrilla and Monstrillopsis. For Davis (p.246-247), the species have been distributed into 4 genera: Monstrilla, Monstrillopsis, Thaumaleus, Cymbasoma, but the discovery of recent new species casts doubt upon the validity of some of these genera. Monstrilla and Monstrillopsis have been separated from Cymbasoma and Thaumaleus (Wilson, 1932, p.604) on the basis of the number of urosomal segments posterior to the genital segment, the former genera having 2 segments in the female and 3 in the male, while the latter genera have only 1 and 2 segments respectively. In both of the species described there is a tendency towards a fusion of the segments, perhaps in the future this line of demarcation between the two categories will be eradicate. If this should occur, then all species of the family Monstrillidae would have to be placed in a single genus Monstrilla. Monstrilla has been separated from Monstrillopsis on the basis of the position of the oral papilla, on the presence or absence of male P5, and on the number of furcal setae.
Thus, in Monstrilla the oral papilla lies near the center of the cephalic segment, P5 are present in the male, and there are 5 or 6 setae. In Monstrillopsis, the oral papilla lies near the anterior margin of the segment, P5 are lacking in the male, and there are only 4 furcal setae (2 lateral and 2 apical). However, Monstrilla reticulata is intermediate in these respects, having the oral papilla intermediate in position, the male lacking P5, and having 5 furcal setae in the female and 4 in the male [if female and male are of the same species ? C.R]; also M. floridana has the oral papilla near the center of the cephalic segment, yet it has only 4 furcal setae (male). In tthe male M. rugosa Davis, 1947, the oral papilla is towards the center of the segment and there are 6 furcal setae. Therefore, it seems that the distinction between Monstrilla and Monstrillopsis can no longer be maintened.
Similarly, the distinction between Cymbasoma and Thaumaleus has been based upon position of the oral papilla at the anterior margin in the former and towards the center of the cephalic segment in the latter. Furthermore, in Cymbasoma the female has been said to have 3 furcal setae, and the male 4, while the female of Thaumaleus has 5 setae (males unknown); however, in the species described by Davis (1947) as Cymbasoma quadridens, the oral papilla is far from the anterior margin, and there are only 3 furcal setae (the specimen is a male and usually the males have more, rather than fewer, furcal setae than the females). Thus all species formerly described as belonging to the genera Cymbasoma and Thaumaleus will be listed as belonging to the genus Thaumaleus which has priority.
Key:
1 - Body cyclopoid. P4 and P5 rudimentary .......1.Thespesiopsyllus.
1' - Body elongated and P4 not rudimentary; P5 rudimentary or absent .....2.
2 - 2 abdominal segments in female posterior to genital segment, and3 such segments in the male.......Monstrilla.
2' - 1 abdominal segment in female and 2 in male.......Thaumaleus.
Isaac (1975), in the zooplankton identification cards (pages 144/145), classifies the species into 5 genera: Monstrilla, Monstrillopsis, Thaumaleus, Thaumatohessia, Strilloma.
Huys & Boxshall (1991, 153) give the main morphological characters of the family, and in 2004 (p.835 ) consider 4 genera plus 1 inquirendum: Cymbasoma Thompson, 1888; Monstrilla Dana, 1849; Monstrillopsis Sars, 1921; Strilloma Isaac, 1974; plus Guanabaraenia Oliviera, 1945.
Main morphological characters after Huys & Boxshall (1991, p.154, Table 3) :
- Female genital apertures : median, fused.
- Caudal ramus 6 setae.
- A1 females : 4 segments; male : 5 segments.
- Segments distal to geniculation : 1.
- Rostrum (adult) : absent or rudimentary.
- Oral structures : conical opening.
- Legs 1 to 3 : coxa/basis partly fused.
- Inner coxal seta : absent legs 1 to 4.
- P4 exopod : 3-segmented.
- P4 endopod : 3-segmented.
- P4 intercoxal sclerite : present.
- P5 female : biramous;
- P5 exopod female : fused to protopod.
- P6 : ovigerous spines or median operculum.
- Eggs : carried on ovigerous spines.
Grygier (1994) demonstrates the identity between Monstrilla Dana, 1849 and Thaumaleus Kröyer,1849; the precedence going back to Monstrilla.
Awaiting a more correct redefinition of the genera, in compiling the species by genus, I have only taken into consideration those genera that seem to me the best settled.
Grygier (1995 a) provides a bibliographic synthesis and an index of the species and their citations by various authors, with remarks on synonymies. The brief description of the type species Haemocera danae does not permit to keep this form. Furthermore, numerous species are poorly described hence synonymical confusions possible.
The keeping of the genus Strilloma is questionable taking into account the quality of the illustrations. Type : Strilloma longa Isaac, 1974. Suarez-Morales & Gasca (2004, p.292) confirm the non-validity of this genus.
The family comprises currently (2004) 3 genera: Cymbasoma, Monstrilla, Monstrillopsis, plus an unclassified form Thaumatohessia.
Grygier & Ohtsuka (2008, p.460), summarize the relationship of the Monstrillidae to other taxa within the Copepoda and listed the history of the genera and their validity: only four monstrillid genera can now be considered as clearly valid at this time: Cymbasoma, Maemonstrilla, Monstrilla, Monstrillopsis.
For Suarez-Morales (2011, p.4) 116 species are recognized, of which 56 belong to the genus Monstrilla, 41 to Cymbasoma, 12 to Monstrillopsis, and 7 to Maemonstrilla; the generic assignment or validity of about 10 other nominal species is uncertain.
Suarez-Morales & al. (2008, p.222) emphasize that one of the main problems related to the taxonomy of the Monstrilloida is the strong sexual dimorphism; it is difficult to match males to females because specimens of either sex are usually found randomly in plankton samples. Only a few species have been described based on both sexes. In some cases these can be linked when they are found in the same host (Gallien, 1934; McAlice, 1985; Grygier & Ohtsuka, 2008), or when some distinguishing morphological characters are present in both sexes (Suarez-Morales & Escamilla, 1997). A genetic matching may be another option, but in this case, genetic analysis of the formalin-preserved specimens was not possible. Finally, the co-occurrence in the same sampling site alone is unreliable when many species are present.; these areas include coral reefs and tropical and subtropical coastal systems.
Key of genera after Vives & Shmeleva (2010, p.157) :
Females :
1 - Abdominen 2-segmented ......... Cymbasoma.
1' - Abdomen 3-segmented ........2.
2 - Oral papilla located more than 1/4 on the ventral area of cephalosopme.; generalement in the middle. Eyes absent or small .......... Monstrilla.
2' - Oral papilla located approximately at 1/4 on the ventral length of the cephalosome. Eyes well-developed .......... Monstrillopsis.
Males :
1 - Abdomen 2-segmented .........Cymbasoma.
1' - Abdomen 4-segmented .......... 2
2 - Eyes very prominent ....... Monstrillopsis.
2' - Eyes not prominent. P5 absent or very small ........ Monstrilla.
Jeon & al. (2018, p.45-47, 61) create the new genus Caromiobenella in the Korean waters, bearing the number of 6 genera in this family in 2018. The phylogenetic trees established, based on the sequences of mtCOI derived from 5 genera and 11 species of monstrilloids including 3 outgroup taxa (see figs. 11 and 12). The number of caudal setae cannot be used to strictly distinguish genera.
For Suarez-Morales & al. (2006, p.104), it is very difficult to reliably link both sexes of monstrilloids unless they were collected from the same host or they share a clearly distinctive set of characters (see Suarez-Morales & Escamilla, 1997).
Total up to date 2019: 6 genera + 2 doubtful: Australomonstrillopsis, Caromiobenella, Cymbasoma, Maemonstrilla, Monstrilla, Monstrillopsis plus ? Haemocera Malaquin, 1896 (type species H. danae Claparède, 1863), and Thaumatohessia Giard, 1900 (type species Taumatohessia armoricana (Hesse, 1868). The following genera are not valid or in synonym: Thaumatoessa Krøyer, 1842; Thaumaleus Krøyer, 1849; Strilloma Isaac, 1974.
Grygier & Ohtsuka (2008, p.501) point to the presence of integumental organs suggests that monstrilloids have a rather limited arrangement of such structures (see in Huys & Boxshall, 1991, p.158 concerning Monstrilla grandis; Grygier, 1994, p.23 concerning Cymbasoma morii, and Grygier & Ohtsuka (1995, p.703). It would be premature to try to use these structures in systematics (see in Park, 1996, on the potential pore sites). It is natable that all species of Maemonstrilla lack dorsal pores on the genital compound somite where Monstrilla hamatapex has 2 pairs (see p.499: fig.29 in Grygier & Ohtsuka, 2008). Species of Maemonstrilla furthermore have pit setae posteriorly on the 4th free pediger, where Monstrilla hamatapex has pores.
Based on the monhstrillids from the Ryukyu Islands the authors list the integumental organs one might expect to find in any female monstrillid:
1 - Pair of hair-like sensilla arising from anterior pores on forehead..
2 - Up to 3 pairs of pores anterior to oral papilla and 2 pairs close behind, one anterior pair sometimes produced as conical tubes.
3 - Various paired arrangements of anteroventral scars.
4 - Various pore patterns anterodorsally on cephalothorax, sometimes including a medial cuticular pit of unknown function.
5 - Few pores dorsally along cephalothorax, perhaps concentrated in front of former anterior margin of incorporated first pediger.
6 - 4 or 5 pairs of dorsal and lateral pit setae at rear of cephalothorax, 1 dorsal and 2 lateral pairs at rear of 2nd free pediger, 2 dorsal pairs at rear of 3rd free pediger, 1 dorsal pair at rear of 4th free pediger.
7 - 1 or 2 pairs of pores near anterior margin of all four free pedigers, often hidden by overlapping rear margin of preceding somite or cephalthorax.
8 - Additional 1 or 2 dorsal pairs of pores, some simple and some valved, on some segments as far posteriorly as each half of genital compound segment.
- 9 - P1-P4, outer anterior pore or pair of pores near basis seta, and 1 or 2 distal pores anteriorly on 3rd segment of each ramus.
10 - Ventral pores on caudal ramus.
SEM micrographs show cuticular ornamentations, such as reticulate ridges and beds of spinules and denticles. Up to date we cannot yet judge the intraspecific variability, nor the utility of this kind of feature in systematics.
Grygier & Ohtsuka (2008, p.502) point to adult female copepods ordinarily release eggs freely into the water (some Calanoida) or they carry the eggs in 1 or 2 sacs hanging from the genital segment (remaining Calanoida, other orders except Monstrilloida). Female monstrilloids carry the eggs on a pair of ovigerous spines that arise from the ventral side of the genital compound somite and usually directed posteriorly. The unusual direction of the spines, forward, suggests that species of Maemonstrilla practise subthoracic brooding. A few eggs (about23 µm in diameter) were found attached to the anteriorly pointing spines in Maemonstrilla turgida. All specimens of Maemonstrilla polka bore very large egg masses held beneath, and matching in leng, the cephalothorax and metasome; each mass consisted of thousands of eggs. Maemonstrilla okameQ carrying eggs beneath the thorax. The egg mass is somewhat oval in lateral viuew with a more pointed anterior end, it is laterally compressed with flat sides, and it reaches ventrally further than the tips of the leg setae. In lateral view the egg mass appears bigger than the copepod, but it is a little les than half as wide as the cephalothorax, within which all the eggs were stored before deposition. In comparison with other copepods, P1-P4 of all species of Maemonstrilla are very widely separated across the midline, and the intercoxal sclerites are very low as well as very wide; these features are adaptations that make room for a large egg mass borne beneath the thorax. The loss of the inner seta of the 1st segment of each leg ramus in all species of the Maemonstrilla hyottoko species-group may be a further adaptation to avoid interference between the legs and the egg mass while swimming. Subthoracic brooding may avoid the problem of egg stripping from trailing spines, either by friction against some obstacle or by predators.
Specialized brooding in enclosed or semi-enclosed chambers formed from body somites and/or appendages has evolved independently many times in copepods. (see Grygier & Ohtsuka, in Table I, p.503). This tendency is particularly remarkable in symbiotic copepods. This may be due to the restricted space for the symbiont on the host and/or a need to avoid loss of eggs within the host. Numerous examples are podoplean copepods, which generally carry egg-sacs, as opposed to free release of eggs into water, characteristic of many gymnopleans copepods (except Euchaetidae, among others). Four types of special brooding can be recognized in copepods: some free-living hapacticoids and a commensal cyclopoid have foliaceous P5 that shield the developing eggs, which are attached to or held in a ventral pocket of the genital area. A brood pouch that is formed dorsally or dorsolaterally within one or more thoracic somites is present in parasitic or commensal notodelphyid cyclopoids and a parasitic poecilostomatoid.
Suarez-Morales (2011, p.7) points out the difficulty in the knowledge of the biogeography of numerous species, because the insufficient description and bad figures of species except ones from specialist of this group. The taxonomic problems have led to many unlikely records and improbable cosmopolitan distributions. For instance, Monstrilla anglica, first known from different parts of Europe was later recorded from Java, Vietnam, and Florida; Monstrilla danae has been reported from Helgoland and adjacent areas of cold temperature latitudes and also from Vietnam. Given such conditions, it seemed more correct and informative to start by analyzing the smaller genera as Monstrillopsis, Maemonstrilla. Maemonstrilla longipes (A. Scott, 1909) is known from Indonesia, as well as supposedly the Nicobars, Singapore, and the Red Sea, it tends to occur in the Indo-West Pacific. The genus Monstrilla appears to be highly speciose in the Caribbean Sea-Gulf of Mexico area (15 species), in European waters (14), and the Indonesian area (10). Cymbasoma appears to be more diverse in Europe than in the Caribbean-Gulf of Mexico (14 vs. 7). Monstrilla and Cymbasoma are almost equally species- rich in European waters (14-14), the mediterranean (9-8), Japanese waters (4-5), and in the Indian Ocean (7-7). The former genus is more speciose than the latter in the Caribbean-Gulf of Mexico (15-7) and in the UIndonesia-malaysia-Philippines region (10-5).
Up to date the family admits 6 genera: Monstrilla Dana, 1849; Cymbasoma Thompson, 1888; Monstrillopsis Sars, 1921; with the new genera Maemonstrilla Grygier & Ohtuska, 2008; Australomonstrillopsis Suarez-Morales & McKinnon, 2014; Caromiobenella Jeon, Lee & Soh, 2018. |  issued : A. Raibaut in Traité de Zoologie. Crustacés, VII (2). Edit. Masson, 1996. Copépodes. II. Les copépodes parasites. [p.691, Fig.245]. Biological cycle of Haemocera danae (Claparède, 1863) [drawing by the author (1985) after Malaquin, 1901]. A, free naulius; B, nauplius going acrross integument of the annelid host; C, naupliar cells mass; D-G, four stages of naupliar development; H, parasitic terminal stage female; I, free adult female; J, parasitic terminal stage male; K, free adult male with spermatophores. |
 Issued from : R. Huys & G.A. Boxshall in Copepod Evolution. The Ray Society, London, 1991. [p.154, Table 3]. Comparison of the Monstrillidae and Thespesiopsyllidae [= Thaumatopsyllidae]. Nota: The family Thespesiopsyllidae is transfered in the new order Thaumatopsylloida by Ho, Dojiri, Hendler & Deets, 2003. |
 Issued from : G.A. Boxshall & S.H. Halsey in An Introduction to Copepod Diversity. The Ray Society, 2004, No 166, Part. II. [p.835]. Armature formula of swimming legs P1 to P4. Main morphological characters in the family: - Rostrum absent. - Nauplius eye present, sometimes elaborate with large miror surfaces. - A1 anteriorly directed, indistinctly 4-segmented in female, sometimes annulate distally; setal nomenclature system devised by Grygier & Ohtsuka (1995: Fig.6). Malme A1 geniculate, 4 to 5-segmented with 1 segment distal to geniculation. - A2, Md, Mx1 and Mx2 all absent in adult of both sexes. - Oral opening conical, located on ventral surface of cephalothorax of adult. - Prosome comprising cephalothorax, incorporating first pedigerous somite, and 3 free pedigerous somites to 6 setae; 1 to 3 free abdominal somites. Swimming legs 1 to 4 biramous, with 3-segmented rami; Legs 1 to 4 with intercoxal sclerites; inner seta on basis of P1 absent, inner coxal setae absent. - Urosome 4 to 5-segmented in male, with 2 free abdominal somites. - Genital apertures fused into large median, ventral aperture in female; paired and ventral in male, sometimes carried near apex of ventral prominence. - Caudal rami with 3 to 6 setae. - |
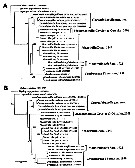 Issued from : D. Jeon, W. Lee & H.Y. Soh in J. Crustacean Biol., 2018, 38 (1). [p.59, Fig. 11]. Phylogenetic trees reconstructed based on the sequences of mtCOI derived from fixe genera and 11 species of monstrilloids including three outgroup taxa, Calanus sinicus (Calanoida), Tigriopus japonicus (Harpacticoida) and Lepeophtheirus salmoni (Siphonostomatoida).
A, Maximum likehood (ML) tree topology.
B, Bayesian inference (BI) tree topology.
Numbers above or below branches indicate bootstrpaping value (BP, in percentage, %) and Bayesian posterior probabilities (BPP, in probability, p) of ML and BI trees, respectively.
Each species name followed by the GenBank accession number (s); the numbers in brackets indicate the data from the other sources while the number for the sequences without brackets were preparated by the current authors. |
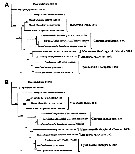 Issued from : D. Jeon, W. Lee & H.Y. Soh in J. Crustacean Biol., 2018, 38 (1). [p.60, Fig. 12]. Phylogenetic trees reconstructed based on the sequences of 28S rRNA derived from five genera and 11 species of monstrilloids including three outgroup taxa, Calanus sinicus, Tigrioipus japonicus, Lepeophtheirus salmonis.
A, A, Maximum likehood (ML) tree topology.
B, Bayesian inference (BI) tree topology.
Numbers above or below branches indicate bootstrpaping value (BP, in percentage, %) and Bayesian posterior probabilities (BPP, in probability, p) of ML and BI trees, respectively.
Each species name followed by the GenBank accession number (s); the numbers in brackets indicate the data from the other sources while the number for the sequences without brackets were preparated by the current authors. |
 Issued from : A. Malaquin in Arch. Zool. Exp. Gen., 1901, 3ème ser., IX. [Pl. II]. As Haemocera danae (= Monstrilla danae Malaquin, 1901 (non Claparède, 1863) ? = Cymbasoma rigidum female, and Monstrilla anglica male. Female (from Roscoff): 1, habitus (lateral); 2a, habitus (dorsal); 2b, forehead with eyes and oral aperture (b) (ventral view); 3, habitus (lateral) with ovigerous spines and egg masses; 6, parasite into his sheath, extracts of the host (annelid: Salmacyna dysteri) 7, nauplius before hatching. an 1 : Antennule; Moc : ocular muscle; cer : brain; St : stomodeum; Ovd : oviduct; Pth 5; ab ; Oel : lateral eye; oem : median eye; Ov : oovary.
Nota:
- Number of abdominal segments between the genital segment and the caudal rami : 2.
- Number of caudal setae : 4.
- Number of antennular segments : 5.
- Localization of the mouth : foremost.
Male (from Roscoff): 4, habitus (lateral); 5, same (ventral).
Sph : spermatophore; an1 : antennule; b : mouth aperture; Oel : lateral eye; Oem : median eye; a-b1-2 : urosomal somites 1 and 2; Th5 : 5th thoracic somite; Th2 : 2nd thoracic somite; F : caudal rami.
Nota:
- Number of abdominal segments between the genital somite and the caudal rami : 3.
- 4 setae on the caudal ramus.
- P5 absent.
- A1 with 4 segments
- Prominence well-developed on the genital segment.
Localization of the mouth cone : foremost.
Rem. Considered as the type of Cymbasoma danae sensu Malaqin, 1901.
8, Nauplius; 9, nauplius piercing in the thoracic cuticle. |
 Issued from : A. Malaquin in Arch. Zool. Exp. Gen., 1901, 3ème ser., IX. [Pl. III, figs. 10, 11, 12, 13, 14]. As Haemocera danae (= Monstrilla danae Malaquin, 1901 (non Claparède, 1863). 10, Salmacyna dysteri Huxley, infested by two different sexes of parasit's monstrillid. The first, anterior most, male (Pl. V, fig.35) (about 1 mm), the second, female (Pl. V, fig.34); 11, Salmacyna dysteri , in dorsal view, with three parasites (giving males) (Pl. IV, figs. 23, 32); 12, the same host infested by female in the ending development, dorsal view (asterisk indicates the way out of parasite; 13, S. dysteri dorsal view; 14, S. dysteri in lateral view with three paraites; 15, S. dysteri parasited by six parasites, the five first have approximatery the same age, the last introduced more recently or backward development. |
 Issued from : A. Malaquin in Arch. Zool. Exp. Gen., 1901, 3ème ser., IX. [Pl. IV, Figs.18 to 32]. As Haemocera danae (= Monstrilla danae Malaquin, 1901 (non Claparède, 1863). 18: Salmacyna dysteri: abdominal anterior portion showing two monjstrillid embryo (each: length ± 100µm) (like in fig.22), located in the ventral blood-vessel. 19: anterior part of cephalic and thoracic of S. Dysteri showing two very young embryo (20, 46), located in the blood-vessel. 20 a, b: embryo from n°20 in the fig.19; length 55 and 50 µm for a living embryonic. 21 a, b: a, embryo length 75 µm showing vitellus elements (vit), migrating in tentacles an 2 t and vestigial naupius eye pigment.; b, length 80 µm showing meso-endodermics (appearing clear on the preceeding. 23: embryo body length 100 µm in dorsal view; sheath apparent at the posterior part (F.c). 24: same, body length 180 µm. 25 and 26, Two embryos, with outline of antennule (an 1) and well-developed antennular tentacle (an 2t) ; the second tentacle (fig.26) shows the mandibule (Md.t). 27: Sagital cut from embryo stage nauplius. 28: embryo body length 300 µm from the apex of sheath to the distal extremity. appearance of the 1st pair of thoracic limb. 29: embryo body length 380 µm., lateral view. 30: approximately the same, ventral view.31: embryo body length 400 µm corresponding to Haemocera filogranarum (= Cymbasoma filogranarum), ventral view showing outline thoracic limbs. 32: embryo male; sheath length = 0.850 mm, whereas the body measure only0.400 mm at the rostral base to the abdominal curvature; the anterior part of the sheathis partially inhabited by the rostrum; the posterior parti inhabited by the extended tail (Caud), which is not the end of the abdomen and disappears afterwards. |
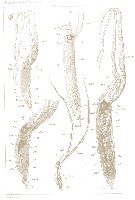 Issued from : A. Malaquin in Arch. Zool. Exp. Gen., 1901, 3ème ser., IX. [Pl. V, Figs.33 to 37]. As Haemocera danae (= Monstrilla danae Malaquin, 1901 (non Claparède, 1863). Embryo female extracted from vascular system from the Salmacyne: 33: famale body length 0.400 mm. 34: female body length 0.650 mm (Pl. III, fig.10). 35: embryo male, body length about 0.650 mm, sheath long 1 mm. 36: the same (different scale), showing very long tentacles antennular (an 2t) and blood lacunae (L.t, L); located in the host (Hae male in Pl.III, figs.10 and 11). 37: Male almost adult, length 1 mm (antennular base to curvature thoraco-abdominal), sheath length 1.450 mm. (corresponding fig.14, Pl. III, Hae 2. |
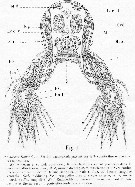 Issued from : A. Malaquin in Arch. Zool. Exp. Gen., 1901, 3ème ser., IX. [p. 93, Fig.1]. As Haemocera danae (= Monstrilla danae Malaquin, 1901 (non Claparède, 1863). Transversal section through the 3rd thoracic somite with the swimming legs. |
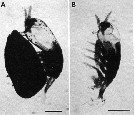 Issued from : M.J. Grygier & S. Ohtsuka in Zool. J. Lin. Soc., 2008, 152 [p.477, Fig.3]. Ovigerous females of Maemonstrilla gen. nov., south coast of Ishigaki Island, 1994, photomicrographs of preserved specimens. A, Maemonstrilla polka female (holotype), with large egg mass; B, Maemonstrilla turgida (A. Scott, 1909) comb. nov., with a few eggs attached to the anteriorly pointing ovigerous spines directed forward (subthoracic brooding). Scale bars = 0.5 mm. |
 Issued from : D. Jeon, W. Lee & H.Y. Soh in J. Crustacean Biol., 2018, 38 (1). [p.62, Table 3]. Polychaete hosts of monstrilloid copepods. | | | | | | (1) Australomonstrillopsis Suarez-Morales & McKinnon, 2014 | |
| | Ref.: | Suarez-Morales & McKinnon, 2014 (p.315) | | Rem.: | The new genus is erected by Suarez-Morales & McKinnon (2014, p.315) to accomodate a male specimen with a remarkable combination of important genus level characters not present in any other genus of the Monstrilloida.
It has a 5-segmented geniculate A1 that resembles that of Monstrillopsis in its general structure and armature, particularly in the presence of an inner protuberance of the last segment and the structure of the apical elenents. Setal element 2 (sensu Huys & al., 2007) forms a distinctive sabre-like structure in males of Monstrillopsis (Suarez-Morales & al., 2006) and element 1 is relatively short (as in M. fosshageni, M. chathamensis, M. cahuitae). In the new genus element 2 is short, curved and only slightly longer than element 1. This character is not present in males of Monstrillopsis. Another unique character present is the peculiar cuticular processes of the cephalothorax, forming sac-like protuberances on the anteroventral surface but also dorsal folds.
An important apomorphy found in this genus is the absence of an inner seta on the 1st segment of the exopods of P1-P4. The absence of such a seta is shared only with Maemonstrilla (Grygier & Ohtsuka, 1008).
Type species: Australomonstrillopsis crassicauda.
Total 1 sp. | | | | (2) Caromiobenella Jeon, Lee & Soh, 2018 | |
| | Ref.: | Jeon & al., 2018 (p.47, Def.); Suarez-Morales & Castellanos-Osorio, 219 (p.118: Rem) | | Rem.: | Type species: Caromiobenella castorea. 3 sp. + 8 sp. (comb. nov.).
Diagnosis male from Jeon & al. (2018,: p.47) :
- Cephalothorax not exceeding half of total body length (caidal rami excluding).
- Forehead margin generally round, lacking 2 usual short sensilla.
- Body segmented: cephalothorax with incorporated 1st pedigerous somit, free pedigers 1-3, 1st urosomal somite, genital somite, postgenital somite, penultimate somite, anal somite.
- A1 5-segmented with modified 5th segment (inner distal margin formed into several comb-like rows of spinules).
- Oral papilla on anterior ventral surface of cephalothorax, low, somewhat inconspicuous.
- Genital apparatus consisting of robust genital shaft pmus 2 short, subtriangular genital lappets diverging from distal posterior end of shaft.
- Branced setae of distal A1 segment replaced by unbranched, wello-developed simple setae in most species (branched setae reportedly in Caromiobenella arctica comb. nov.).
- Spine 2d, on 2nd segment elongated, biserially plumose, or both depending on species.
- Distal end of genital shaft with deep notch or medial protrusion.
- 3 or 6 caudal setae on each caudal ramus, depending on species.
- Two pairs of prominent crater-like depression on anterior dorsum of cephalothorax.
- Posterior dorsum of cephalothorax (i.e. incorporated 1st pediger) with 2 longitudinal rows of 4 pores each, arranged in pairs across midline.
Cruz Lopes da Rosa J. da & al (2021) new zooplankton collections in coastal waters and intertidal rocky pools of the SWA yielded several male and female monstrilloid copepods tentatively identified as Monstrilla brasiliensis. their results of both morphologic and molecular (mtCOI) analyses allowed them to confirm that these males and females were conspecific. They also found evidence suggesting that Caromiobenella is not a monophyletic taxon. Their male specimens are morphologically assignable to Caromiobenella, therefore, females of the nominal species Monstrilla brasiliensis, are matched here with the aforementioned males and, thus, the species should be known as C. brasiliensis comb. nov. (Dias and Suárez-Morales, 2000).
After these authors the species included in this new genus will be (comb. nov.): C. helgolandica (Claus, 1863); C. serricornis (Sars, 1921); C. arctica (Davis & Green, 1974); C. hamatapex (Grygier & Ohtsuka, 1995); C. pygmaea (Suarez-Morales, 2000); C. patagonica (Suarez-Morales, Ramirez & Derisio, 2008); C. humesi (Suarez-Morales, 2001); C. brasiliensis Dias & Suarez-Morales, 2000 and Caromiobenella sp. [= Monstrilla sp. in Huys & Boxshall, 1991].
The new genus displays a unique set of characters, but some ambiguity is present in the generic assignment of all species Caromiobenella mentioned, including the three new species C. castorea, C. ohtsukai and C. polluxea. For example, the numbers of urosomal somites and caudal setae match those of Monstrilla, whereas the modifications of setal element 2d 2 involving elongation and plumosity, are more like those in some species of Cymbasoma.
Molecular analysis provides an alternative means of compensating for uncertainties and defects caused by insufficient morphological information. The molecular evidence strongly supports the separation of caromiobenella from Monstrilla, with an about two-fold difference between the within-genus and between-genera divergences for genes mtCOI and for genes 28S rRNA (see in Table 2) | | | | | (3) Cymbasoma Thompson, 1888 | |
| | Syn.: | Thaumaleus : Giesbrecht, 1892 (p.80, 578), non Kröyer, 1849; Malaquin, 1901 (p.106); van Breemen, 1908 a (p.201); Isaac, 1974 (p.128); 1975 (p.2) | | Ref.: | I.C. Thompson, 1888 b (p.154); Sars, 1921 (p.19); Sewell, 1949 (p.132); Wilson, 1932 a (p.395); Rose, 1933 a (p.346); Threlkeld, 1977 (p.227); Razouls,1982 (p.758); Grygier, 1994 (p.241); 1994 a (p.24); Chihara & Murano, 1997 (p.1002); Suarez-Morales & Riccardi, 1997 (p.105, key spp. M); Suarez-Morales, 1999 (p.71: key of M. in Mediterranean Sea); Suarez-Morales, 2000 a (p.209, key of M); Suarez-Morales, 2000 d (p.144, Rem.); Boxshall & Halsey, 2004 (p.837); Grygier & Ohtsuka, 2008 (p.460, Rem.); Vives & Shmeleva, 2010 (p.157, Rem., spp. key); Chang, C.Y, 2012 (p.131); Suarez-Morales & McKinnon, 2016 (p., 125: Key to the Australian species) | | Rem.: | type: Cymbasoma rigidum Thompson,1888. 41 species in Suarez-Morales, 2011 (p.4) + 25 species in Suarez-Morales & McKinnon, 2016 (p.3). Total: 71 species + 1 undet.
Diagnosis after Vives & Shmeleva (2010, Vol. 33, p.157) :
Female :
- Abdomen 2-segmented.
Male :
- Abdomen 2-segmented.
For Jeo,g & al. (2018, p.61) the number of caudal setae 3 or 4 occurs in Cymbasoma including between two sexes of C. rigidum, C. longispinosum, C. tumorifrons, C. quintanarooensis, C. chelemense. | | Remarks on dimensions and sex ratio: | | For the body lengths from individuals measured from the anterior part of the head to the end of the caudal rami, the mean female size is 2.255 mm (n = 32; SD = 1.419), and the mean male size is 1.278 mm (n = 26; SD = 0.499). The size ratio (M:F) is 0.567. If one excludes the three species of which the lengths are exceptional (numbered 13, 16 and 30), the mean size females measure 1.962 mm (n = 26; SD = 0.8141), and a size ratio (M:F) 0.651. .
For the body lengths measured from the anterior part of the head to the end of the anal somite (without caudal rami), the mean female size is 2.012 mm (n = 24; SD = 1.431), and the mean male size is 1.350 mm (n = 9; SD = 0.420). The size ratio (M: F) is 0.671. | | | | | Guanabaraenia Oliveira, 1945 | |
| | Ref.: | Oliveira, 1945 a (p.468); Razouls, 1982 (p.770) | | Rem.: | non Monstrillide | | | | | Ref.: | Malaquin, 1901 (p.108); van Breemen, 1908 a (p.202); Razouls, 1982 (p.770); Grygier, 1994 (p.240, Rem.); 1995 a (p.) | | Rem.: | Sars (1921, p.9) considers this genus as a synonym of Cymbasoma. Isaac (1974, 1975) does not recognize this genus and does not retain the generic criteria in Sewell, 1949 (p. 132). 1 species (unidentifiable). | | | | (4) Maemonstrilla Grygier & Ohtsuka, 2008 | |
| | Ref.: | Grygier & Ohtsuka, 2008 (p.462, Key to the Rtukyu species); Ohtsuka & Nishida, 2017 (p.574, Rem.) | | Rem.: | Type: Maemonstrilla hyottoko. With 2 species-groups: kyottoko Group (Grygier & Ohtsuka, 2008, p.463) and turgida Group Grygier & Ohtsuka, 2008, p.492). Total: 11 species.
Diagnosis for females after Grygier & Ohtsuka (2008, p.462) :
- Ovigerous spines pointing forward between thoracopods.
- P1 to P4 widely separated across midline; intercoxal of legs 1-4 low and approximately as wide as legs themselves.
- Cephalothorax bulbous, about half of body length, with often very prominent oral papilla in anterior third of length and 2-4 (usually 3) small scars clustered behind base of each A1.
- Naupliar eye well developed; hyaline bodies absent in front of the widely separated lateral cups.
- Dorsal surface of metasomal pedigers and all urosomal segments except telson (for the so-called 'anal somite') occupied by extensive, more or less rectangular patches of denticles or spinules.
- A1 with branched outer distal b-setae.
- Single pore on anterior face of 3rd segment of each ramus in P1-P4.
- P5 either a long, narrow rod with 2 distal setae and at most a tiny, unarmed endopodal lobe, or bilobed with 3 setae on exopodal lobe and 1 (or supposedly 2 in one instance) on endopodal lobe.
- Urosome 4-segmented.
- Genital compound somite usually with obvious dorsal suture, lacking dorsal pores.
- Caudal rami with 6 setae and ventral pore.
This genus represents the only example of subthoracic brooding among planktonic copepods: Unusual direction of the ovigerous spines (foreward), the shape of the intercoxal sclerite, very low and very wide, and probably the loss of the inner seta of the 1st segment of each leg ramus (see remarks in Monstrillidae concerning the subthoracic egg brooding in copepods).
Males unknown (or unrecognized).
In the samples examined, monstrillid specimens and species of various genera were usually abundant, and up to three species of Maemonstrilla females co-occurred in samples; matching sexes by co-occurrence cannot be done in these circumstances.
Ohtsuka & Nishida (2017, p.574) underline a peculiar characteristic in this genus, which bears anteriorly directed genital spines on the genital compound somite of the female, which are posteriorly directed in other monstrilloids. Because egg masses attached to the spines are positioned in a space surrounded by the right and left legs, Grygier & Ohtsuka (2007) regarded it as a kind of brood chamber.
Maemonstrilla hyottoko species-Group :
- Cephalothorax with reticulate pattern of cuticular ridges.
- A1, lateral sides of metasomal somites and urosomal segments, dorsum of telson, and caudal rami ornamented (except in M. simplex).
- Dorsal surface of metasomal pedigers and first three urosomal segments, as well as outer face of thoracods (coxa and exopod), densely denticulate or spinulose (except in M. simplex).
- No spine-like scales posteriorly near dorsal midline on 1st and 2nd free pedigers.
- Outer basis seta of P3 at least as long as exopod.
- No inner seta of 1st exopodal segment of P1-P4; inner seta of 1st endopodal segment of these legs absent or represented by socket-like or button-like structure;
- P5 a long, thin rod with 2 distal setae, 1 apical and 1 slightly subapical; endopodal lobe absent (or reportedly tiny and unarmed in one instance).
- Posterior part of genital compound somite with ventral protrusion.
5 species: M. hyottoko, M. simplex, M. okame, M. polka, M. spinicoxa.
Maemonstrilla turgida species-Group : Monotypic. See diagnosis to the species Maemonstrilla turgida (A. Scott, 1909) comb. nov. from Grygier & Ohtsuka, 2008 (p.492); distinguished from the former species-Group by the cephalothorax non-reticulated. | | Remarks on dimensions and sex ratio: | | The mean female size is 1.685 mm (n = 13; SD = 0.352). It seems to note three groups: The little forms (species numbered 5 and 8) with the mean 0.627 mm (n = 3; SD = 0.172); the intermediate forms species numbered 1, 2, 3, 4, 6, 9, 10 with the mean 1.589 mm (n = 11; SD = 0.2738); and the large forms (species numbered 7 and 11) with the mean 2.140 mm (n = 4; SD = 0.2905). | | | | | (5) Monstrilla Dana, 1849 | |
| | Syn.: | Monstrilla Dana,1849; Thaumatoëssa Kröyer, 1845 (= Thaumatoessia); Thaumaleus Kröyer, 1849 (in Damkaer & Damkaer, 1979, p.43); MonstrillopsisStrilloma : Isaac, 1974 a (p.134); 1975 (n°144/145, p.2, 6, 9); Razouls, 1982 (p.769) | | Ref.: | Claus, 1863 (p.164); Bourne, 1890 b (p.574); Giesbrecht, 1892 (p.80, 585); van Breemen, 1908 a (p.202); A. Scott, 1909 (p.234); Pesta, 1920 (p.635); Sars, 1921 (p.10); Wilson, 1932 a (p.393); Rose, 1933 a (p.339); Davis, 1949 a (p.246); Sewell, 1949 (p.131, 132, Rev.); Ramirez, 1971 b (p.379); Davis & Green, 1974 (p.62, Rem.); Isaac, 1974 (p.128); 1975 (n°144/145, p.2, 7, spp. key); 1975 b (p.163); Razouls, 1982 (p.761); Huys & Boxshall,1991 (p.154, 464); Grygier, 1994 (p.241, Rem.); 1995 (B.Z.N., 52 (3), p.245); B.Z.N., 1997, 54 (2) (p.131); Boxshall & Halsey, 2004 (p.837); Grygier & Ohtsuka, 2008 (p.460, Rem.); Vives & Shmeleva, 2010 (p.172, Rem., spp. key); Suarez-Morales & Castellanos-Osorio, 219 (p.120, Key of 5 females & males of the Mexican Caribbean). | | Rem.: | Type: Monstrilla viridis Dana, 1849, but the genus concept needs to be clarified by designation and detailed description of a neotype. Total: 52 species + 2 uncertained (of which several doubtful). Provisional number, the species of the genus Monstrillopsis should no doubt be incorporated in this genus (Davis, 1949 a; Davis & Green, 1974).
Diagnosis after Vives & Shmeleva (2010, Vol. 33, p.157) :
Female :
- Abdomen 3-segmented.
- Oral papilla located approximately more than 1/4 of the ventral length of the cephalothorax (mainly at half length).
- Eyes absent or small.
Male : Abdomen 4-segmented
- Eyes not prominent.
- P5 absent or very small.
With the creation of the new genus Caromiobenella Jeon, Lee & Soh, 2018) the number of the species is at the present time 50. | | Remarks on dimensions and sex ratio: | | For the body lengths from individuals measured from the anterior part of the head to the end of the caudal rami, the mean female size is 2.789 mm (n = 44; SD = 1.0872), and the mean male size is 1.808 mm (n = 32; SD = 0.7938). The size ratio (M:F) is 0.6483.
For the body lengths measured from the anterior part of the head to the end of the anal somite (without caudal rami), the mean female size is 2.799 mm (n = 16; SD = 0.7070), and the mean male size is 1.783 mm (n = 4; SD = 1.0529). The size ratio (M: F) is 0.6370. | | | | (6) Monstrillopsis Sars, 1921 | |
| | Ref.: | Sars, 1921 (p.25, Rem.); Davis, 1949 a (p.246, Rem.); Sewell, 1949 (p.132, Rem.); Davis & Green, 1974 (p.62, Rem.); Isaac, 1974 (p.127); 1975 (n°144/145, p.2, spp. Key); Razouls, 1982 (p.768); Huys & Boxshall, 1991 (p.154,162, 165, 465); Grygier, 1994 (p.240, Rem.); Boxshall & Halsey, 2004 (p.837); Suarez-Morales & al., 2006 (p.101-105, tab.1, key females); Grygier & Ohtsuka, 2008 (p.460, Rem.); Vives & Shmeleva, 2010 (p.186, Rem., spp. key); Suarez-Morales & al., 2013 (p.619); Delaforge & al., 2017 (p.11: Rem.); Jeon & al., 2018 (p.62, Rem.: Haemocera Malaquin, 1901, synonym. | | Rem.: | type: Monstrilla dubia T. Scott,1904. The authors do not agree on the validity of this genus, in spite of the arguments of Davis (1949 a) : ? Cf. Monstrilla.
Total: 20 species (some probably doubtful).
Suarez-Morales & al. (2006, 2013) included the following nominal ten species in the genus Monstrillopsis: M. dubia (Scott, 1904) from Scotland (60°N), M. zernowi from the Black Sea (43°N), M. sarsi Isaac, 1974 from England (54°N), M. fosshageni from Brazil (20°S), M. dubioides Suarez-Morales, 2004 from Norway (62°N), M. ferrarii Suarez-Morales & Ivanenko from the White Sea (66°N), M. chilensis from off Chle (33°S), M. igniterra from the Southern Ocean (55°S), M. chathamensis from the Eastern Pacific, M. cahuitae from the Costa Rica (Caribbean Sea (9°45'N).
For Delaforge & al. (2017, p.11), several authors have questioned the validity of the genus Monstrillopsis Sars (1921) whereas others have accepted ir (Huys & Boxshall, 1991; Boxshall & Halsey, 2004; Suarez-Morales & al., 2006). The argumentation against it has relied on the presumed mixed characters shown by males of the Arctic species M. bernardensis (Willey, 1920); as in Monstrillopsis, these specimens have 4 caudal setae and the oral papilla is located anteriorly on the cephalothorax, but the specimens examined by Davis & Green (1974) from Resolute Bay have a rudimentary P5 as in Monstrilla; they explicitly state that the differ from Willey's population. The specimens of M. bernardensis from Resolute Bay should be redescribed. For instance, the number of caudal rami should be confirmed, some species of Monstrilla have a small, inconspicuous caudal seta V (i.e., M. elongata Suarez-Morales, 1994; M. gracilicauda Giesbrecht (1892). Also, the A1 segmentation and armature, particularly of the distal segment, should be examined to determine if it has the characters relatable to Monstrillopsis. Huys & Boxshall (1991) strengthened the genus concept by assigning to the males of Monstrillopsis a particular antennular type, different from those recognized in Cymbasoma and Monstrilla. With regard to the females of species of Monstrillopsis, little evidence contrary to the validity of the genus has been presented. The other Arctic species described by Davis & Green (1974), M. arctica and M. nasuta are clearly species of Monstrilla.
The distribution of the genus seems to be largely restricted to temperate and cold latitudes.
Diagnosis of the the genus Monstrillopsis owing to its possession of the combination of characters noted by Sars (1921) :
- 2 free somites posterior to the genital double-somite;
- Eyes fully developed;
- 4 segmented A1 in the female;
- Oral papilla occurring near the anteriormost part of the cephalothorax (< 20 % of way back along cephalothorax);
- P5 bilobed in female , outer lobe armed with 3 setae;
- Caudal rami with 4 setae.
For Delaforge & al. (2017, p.8), some species can have more caudal setae (i.e. M. reticulata (Davis, 1949), M. zernowi Dolgopolskaya, 1948), but except for the aberrant M. zernowi with 5 caudal setae, also present in the females.
Diagnosis after Vives & Shmeleva (2010, Vol. 33, p.157) :
Female :
- Abdomen 3-segmented.
- Eyes well developped.
- Oral papilla located 1/4 length of the ventral cephalothorax.
Male : Abdomen 4-segmented
eyes very prominent.
lee J. & al. (2016, p.421), in Monstrillopsis the number of caudal setae is generally 4 in both sexes; however, some congeneric species have more caudal setae (see Suarez-Morales & al., 2006), and are distinguished from the Korean species: in the male, 6 setae in M. cohuitae and 5 setae in M. reticulata and M. zernowi.
After Jeon (2018, p.62) the genus Haemocera Malaquin, 1901, should be a senior synonym of Monstrillopsis.
Remarks from Suarez-Morales & al. (2006, p.101)combination of characters defined by Sars (1921) in his diagnosis of this genus concerning females:
- 1: A1 4-segmented.
- 2: Oral papilla occurring near anteriormost part of cephalothorax.
- 3: zyzs fully developed.
- 4: Urosome with 3 somites including genital double-somite.
- 5: P5 of female bilobed, and outer lobe armed with 3 setae.
- 6: Caudal rami with 4 setae. Sars (1921) mentioned also unusually produced caudal rami; this character is not constant in the members of the genus.
- Additional characters proposed later for this genus are the absence or armament on the inner lobe of the female P5; a modified distal segment and a curved terminal spine in the male A1, and absent or reducted P5 in the male (character shared with Cymbasoma (Suarez-Morales, pers. obs.). | | Remarks on dimensions and sex ratio: | | For the body lengths from individuals measured from the anterior part of the head to the end of the caudal rami, the mean female size is 1.984 mm (n = 5; SD = 1.123), and the mean male size is 0.870 mm (n = 3; SD = 0.3205). The size ratio (M:F) is 0.4385.
For the body lengths measured from the anterior part of the head to the end of the anal somite (without caudal rami), the mean female size is 2.325 mm (n = 8; SD = 0.823), and the mean male size is 0.940 mm (n = 7; SD = 0.5377). The size ratio (M: F) is 0.4043. | | | | | | Ref.: | Isaac, 1974 a (p.134); 1975 (n°144/145, p.2, 6, 9); Razouls, 1982 (p.769); Huys & Boxshall, 1991 (p.154, Rem.); Boxshall & Halsey, 2004 (p.837) | | Rem.: | The maintenance of this genus is questionable considering the quality of the illustrations. Type : Strilloma longa Isaac,1974. Suarez-Morales & Gasca (2004, p.292) confirm the non-validity of this genus. | | | | | Syn.: | Thaumatoëssa Kröyer, 1842-45; non Thaumaleus : Giesbrecht, 1892 (p.578) | | Ref.: | Malaquin, 1901 (p.85, 108); A. Scott, 1909 (p.239); Pesta, 1920 (p.632); Isaac, 1974 (p.128); 1975 (n°144/145, p.2, 3, 8, clé spp.); Threlkeld, 1977 (p.227); Razouls, 1982 (p.754); Huys & Boxshall, 1991 (p.154); Gryier, 1994 (p.235 & suiv., Rem.); 1995 (B.Z.N., 52 (3) (p.245); B.Z.N., 1997, 54, (2) (p.131) | | Rem.: | Type: Thaumaleus typicus Kröyer, 1849. Cf. Cymbasoma et Monstrilla | | | | Thaumatoessa Kröyer in Gaimard, 1842-1845 ? | |
| | Syn.: | Thaumaleus Kröyer,1849; Monstrilla Dana,1849 | | Ref.: | Grygier,1994 (p.241, Rem.) | | Rem.: | In spite of the priority rule, Grygier (1994) proposes the maintenance of Monstrilla over Thaumatoessa and over Thaumaleus (this genus being considered as later established). | | | | (0) Thaumatohessia Giard, 1900 (? Monstrillidae ) | |
| | Ref.: | Giard, 1900 (p.396); Isaac, 1975 (n°144/145, p.2); Razouls, 1982 (p.769); Huys & Boxshall, 1991 (p.154, Rem.) | | Rem.: | Total : 1 sp.
This genus, created to understand an entirely original species (Thaumatohessia armoricana (Hesse,1868) does not correspond to the diagnosis of the family, but may belong to a neotenic form of a species of the preceding genera. This atypic form, presenting rudimentary mouth parts, has never been re-encountered in Brest (among the corals: Griffithsia corallina) | | | | | | History :
Among the pelagic copepods, the Monstrillidae constitute a particular case because of the characteristics of their meroplanctonic biological cycle and their protelian way of parasitism. They are under-represented in samples that are generally not adapted to their search. They distinguish themselves easily from the other copepods by their large size and their morphology.
Since the work of Malaquin (1901) on the biology of the Monstrillidae comprising a first revision of the group, we do not dispose of new studies concerning the totality of the group except numerous descriptions of new species.
A certain confusion exists concerning the synonymies following some proposed revisions: Van Breemen (1908 a), Sars (1921), Sars (1921), Wilson (1932 a), Sewell (1949), Davis (1949).
For Malaquin, the Monstrillidae family comprises two genera reexamined by Giesbrecht (1892), plus a third created by this author in 1896, of which the taxonomical characters are the following:
G1: Thaumaleus Kröyer,1849
Syn.: Monstrilla : Claparède, 1863; Möbius, 1884; Bourne, 1890; Cymbasoma : Thompson, 1888.
Ur : (F) : 2 Sgts; (M) : 3
F : (F) : 3 setae; (M) : 3 or 4
A1 : (F) : 3 or 4 Sgts; (M) : 5
Mouth at the front of the cephalothorax.
G2: Monstrilla Dana,1848
Syn.: Cymbasoma : Thompson,1887
Ur : (F) : 4 Sgts; (M) : 4
F : (F & M ): 5 or 6 setae
A1 : (F) : 4 Sgts; (M) : 5
Mouth in the middle of the cephalothorax
G3: Haemocera Malaquin,1896
Syn.: Monstrilla : Claparède, 1863; Bourne, 1890; Thompson, 1893
Ur: (F) : 3 Sgts; (M) : 4
F: (F) : 3 or 4 setae; (M) : 4
A1: (F) : 4 Sgts; (M) : 5
Mouth at the front of the cephalothorax.
In 1921, Sars divides the suborder of the Monstrillids in two well defined sections, each comprising one family:
Section 1: Monstrilloida cyclopimorpha with the family of the
Thaumatopsyllidae.
G: Thaumatopsyllus with the type species Thaumatopsyllus paradoxus Sars,1913.
The copepods belonging to this family are easily identifiable by the cyclopoid form of their body, the presence of three well-developed thoracic pairs of legs, the articulation of the body between the thoracic segments 3 and 4, the anal segment very elongated.
Section 2: Monstrilloida genuina with the family of the Monstrillidae
This family comprises the two genera previously defined: Monstrilla Dana,1848 which would be synonymous with Thaumaleus (Kröyer) no Giesbrecht. The genus Thaumaleus used by Giesbrecht (1892) would be synonymous with Cymbasoma Thompson (1888) to which one should rally the genus Haemocera created by Malaquin (1896). The principal criterium of Sars rests on the number of the furcal setae in the family. Besides, Sars creates the genus Monstrillopsisin which to include the species described by Scott
(1904): Monstrilla dubia.
The characters of these three genera are the following:
G1: Monstrilla Dana,1848
Ur: (F) : 3 Sgts; (M) : 4
F: (F) : 5 or 6 setae; (M) : 4 or 5 setae
Mouth generally far from forehead
P5 à 2 or 3 setae
G2: Cymbasoma Thompson,1888
Ur: (F) : 2 Sgts; (M) : 3
F: (F) : 3 setae; (M) : 4 (sometimes 3 setae ?)
A1: (F) : 4 Sgts; (M) : 5
Mouth closer to forehead
P5 (F) : 2 lobes
G3: Monstrillopsis Sars,1921
Ur: (F) : 3 Sgts; (M) : 4
F: (F & M) : 4 setae
A1: (F) : 4 Sgts; (M) : 5
Mouth close to forehead
P5 bilobed (F); absent in the male
G4: Thaumaleus Kröyer,1849
Ur: (F) : 2 Sgts, (M) : 3
F: linear, (F) : 5 setae
Mouth distant from anterior margin of the head
P4 female as long as the other leg pairs.
In the "Faune de France", Rose (1933 a) takes up the classification of Sars. This author describes in short nine species of the genus Monstrilla , nine species of the Cymbasoma and the unique species of the genus Monstrillopsis.
Monstrilla anglica Lubbock, 1857; M clavata Sars, 1921; M. gracilicauda Giesbrecht, 1892; M. grandis Giesbrecht, 1891; M. helgolandica Claus, 1863; M. longicornis Thompson, 1890; M. longiremis Giesbrecht, 1892; M. leucopis Sars, 1921; M. serricornis Sars, 1921.
Cymbasoma filogranarum (Malaquin, 1896) ; C. longispinosum (Bourne, 1890); C. malaquini (Caullery & Mesnil, 1914); C. reticulatum (Giesbrecht, 1892); C rigidum Thompson, 1888; C. roscovita (Malaquin, 1901); C. rostrata (T. Scott, 1904); C. thompsoni (Giesbrecht, 1892); C. zetlandica (T. Scott, 1904).
Monstrillopsis dubia (T. Scott, 1904).
In 1949, two revisions of the monstrillids have been realised separately by Sewell and Davis. These two authors do not acknowledge the genera defined by Sars, but diverge in their conclusion. The sections created by Sars were maintained as well as the genus Thesiopsyllus of Wilson (1932 a); Sewell adds a new genus to the section of the Cyclopimorphes: Orientopsyllus.
The family of the Monstrillidae would comprise the three genera of Sars, but also the maintenance of the genus Haemocera of Malaquin (not retained by Rose who considers H. danae cas a synonym of Cymbasoma rigidum ) and eventually the genus Thaumaleus de Kröyer (1849) of which the type would be T. typicus. Al Kholy (1963) studied some species of the Monstrillids from the Red Sea and describes two new species and one variety. This author exposes in short the criteria of the classification by Sewell which are summarized in the table below.
The number of segments of the urosome and the number of the furcal setae permits to separate the genera Cymbasoma and Monstrilla. The other characters do not authorize the creation of other genera. The family of the Monstrillidae comprises thus only two genera: Monstrilla and Cymbasoma (Syn.: Monstrillopsis Sars and Haemocera Malaquin).
|
Monstrilla |
Monstrillopsis |
Haemocera |
Cymbasoma |
Urs: Sgts Female
Male |
3 or 4
4 |
3
4 |
3
4 |
2
3 |
F: Setae Female
Male |
5 or 6
4 or 6 |
4
4 |
3 or 4
3 or 4 |
3 or 4
3 or 4
|
Mouth |
in the middle |
forward |
forward |
forward |
A1 : Sgts Female
Male |
4 ou 5 (pls 2)
5 |
4
5 |
4
5 |
3 ou 4
5 |
P5 Male |
2 setae |
0 |
0 |
0 |
Eyes |
undeveloped |
developed |
developed |
developed |
In 1949 Davis produces a revision of the Monstrillids, speaking of the description of two new species, accompanied by a key based on the descriptions of various authors. The separation of the genera according to the number of urosome segments was not a good criterium, certain species could only belong to one single genus: Monstrilla. This author proposes provisionally to classify the species in the two genera: Monstrilla (Syn.: Monstrillopsis ) and Thaumaleus (Syn.: Cymbasoma ).
The known species divide then in the following way:
G.1: Monstrilla
M. anglica Lubbock, 1857; M. clavata Sars, 1921; M. canadensis McMurrich, 1917; M. conjunctiva Giesbrecht, 1902; M. cymbula A. Scott, 1909; M. dakinensis Davis, 1949; M. dubia T. Scott, 1904; M. floridana Davis, 1949; M. gracilicauda Giesbrecht, 1892; M. grandis Giesbrecht, 1891; M. helgolandica Claus, 1863; M. inserta A. Scott, 1909; M. leucopis Sars, 1921; M. longicornis Thompson, 1890; M. longipes A. Scott, 1909; M. longiremis Giesbrecht, 1892; M. mixta T. Scott, 1914; M. nicholsii Davis, 1949; M. orcula A. Scott, 1909; M. ostroumowi Karavaev, 1894; M. reticulata Davis, 1949; M. rugosa Davis, 1947; M. serricornis Sars, 1921; M. turgida A. Scott, 1909; M. wandelii Stephensen, 1913.
G.2: Thaumaleus
T. bullatus A. Scott, 1909; T. danae (Claparède, 1863); T. gigas A. Scott, 1909); T. gracile (Gurney, 1927); T. longispinosus (Bourne, 1890); T. quadridens (Davis, 1947); T. rigidum (Thompson, 1888); T. thompsoni Giesbrecht, 1892.
Remarks: Monstrilla viridis Dana,1849 from the Sulu Sea is omitted because of its insufficient description. The description of the four species by Davis (1947 and 1949 a): Monstrilla floridana , M. reticulata , M. rugosa and Cymbasoma quadridens urges the author to consider the two genera Monstrilla and Monstrillopsis as synonyms, as well as Thaumaleus and Cymbasoma, what he confirms in 1974.
The genus Haemocera seems to have been maintained because of the questionable synonymy between Monstrilla danae Claparède, 1863 and Haemocera danae Malaquin, 1901 ( in Sewell, 1949, remark: p.145), although Grygier (1995 a, p. 62) considers these as synonyms.
The definition of these different genera would be the following:
|
Monstrilla |
Haemocera |
Cymbasoma
|
Thaumaleus |
Ur : Sgts Female
Male |
3 or 4
4 |
3
4 |
2
3 |
2
3 |
F (sertae): Female
Male |
4 to 6
4 to 6 |
3
4 |
wide, 3
" , 4 |
narrow, 3
" , 4 |
Grygier (1994) reexamines the type species Thaumatoessa typica previously illustrated ( in Atlas de Zoologie de Gaimard, 1842-1845 ?) by Kröyer and redescribed by this author as Thaumaleus typicus in 1849 ( in Damkaer & Damkaer, 1979) from a female juvenile stage 5 found in the entrance of the Trondheimsfjorden (Norway). In spite of the precedence of the genus Thaumatoessa, the genus Thaumaleus should be maintained (Bulletin of Zoological Nomenclature, 52 (3), p.245). However, T. typica could be a senior synonym of Monstrilla longicornis Thompson,1890 and of Monstrilla clavata Sars,1921. The genus Monstrilla, being defined by the type species Monstrilla viridis Dana, 1849 (non 1848), type of the Monstrillidae family (Grygier, 1995, p.247) has become of common use. In addition, numerous species have been attributed to the genus Thaumaleus while identifiable to the genus Cymbasoma with as type species Cymbasoma rigidum Thompson,1888.
In 1974 Isaac creates the genus Strilloma with as type species Strilloma longa found in Florida (Dry Tortugas), and taken up in the plankton identification leaflets of the ICES-CIEM (1975, no. 144/145, p. 2, 6, 9), but contested by Huys & Boxshall, 1991 (p.154, Rem.). The author includes Strilloma lata (Desai & Bal,1963 as Monstrilla lata) from the Indian Ocean (Bombay) and from Eastern Scotland (Firth of Forth). The maintenance of this genus is questionable taking into account the quality of the illustrations.
An important contribution to the knowledge of numerous new species is due to Suarez-Morales and coll. during this last period (1992-2004).
This short history of the various revisions of the Monstrillidae family and the inventory of the species, which I compiled from publications currently in my possession, permits me to draw the following conclusions:
Numerous insufficiently described species need new observations in the type localities in order to permit a clarification of the synonymies.
Breeding will prove to be necessary if one follows the opinion of Malaquin on the repercussions of the degeneration of these organisms on the criteria used for their classification.
The parasitic specificity, through examination and breeding of Polychaeta and of Gasteropoda would be a useful complement.
actual consensus seems only to consider two genera in the Monstrillidae family: Cymbasoma and Monstrilla, plus possibly the atypical case of the Thaumatohessia Giard,1900.
Various identifications, without comments, in the faunistic lists are questionable. Under these conditions the geographical distribution of the species remains very uncertain.
The detailed works of Suarez-Morales these last years and in progress should clarify the assemblage of this order. | | | | | | | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed February 08, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |














