|
|
 |
|
Calanoida ( Order ) |
|
|
|
Calanoidea ( Superfamily ) |
|
|
|
Paracalanidae ( Family ) |
|
|
|
Acrocalanus ( Genus ) |
|
|
| |
Acrocalanus gibber Giesbrecht, 1888 (F,M) | |
| | | | | | | Syn.: | Acrocalanus pediger (F) Cleve, 1901 (part., p.33, figs.F)
Paracalanus Clevei Carl, 1907 (p.7, 16, Descr. M, Rem.). | | | | Ref.: | | | Cleve, 1901 (p.36, figs.F,M); Giesbrecht, 1892 (p.171, 175, 770, figs.F); Giesbrecht & Schmeil, 1898 (p.25, Rem. F); Thompson & Scott, 1903 (p.233, 243); Wolfenden, 1905 (1906) (p.1003, figs.F, Rem.F); A. Scott, 1909 (p.29, Rem.); Sewell, 1912 (p.353, 359); 1914 a (p.210, Rem.); Früchtl, 1924 b (p.42); Gurney, 1927 (p.147, figs.F,M, Rem.); Sewell, 1929 (p.80, figs.F, p.83: Rem., figs.M); 1933 (p.26); Farran, 1936 a (p.81); Mori, 1937 (1964) (p.32, figs.F, juv.M); Dakin & Colefax, 1940 (p.93, figs.F); Vervoort, 1946 (p.136, Rem.); Tanaka, 1956 c (p.372, Rem.F,M, p.376: Rem.M); Yamazi, 1958 (p.147, Rem.); Bowman, 1958 (p.121); Kasturirangan, 1963 (p.24, figs.F); Chen & Zhang, 1965 (p.46, figs.F,M); Tanaka, 1965 c (p.372, Rem.F,M); Marques, 1976 (p.989); Greenwood, 1976 (p.18); Zheng Zhong & al., 1984 (1989) (p.233, figs.F, M); Hiromi, 1987 (p.154); Nishida, 1989 (p.173, table 1, 2, 3, dorsal hump); Bradford-Grieve, 1994 (p.46, figs.F,M); Chihara & Murano, 1997 (p.845, Pl.136: F,M); Bradford-Grieve & al., 1999 (p.877, 909, figs.F,M); Al-Yamani & Prusova, 2003 (p.29, figs.F); Conway & al., 2003 (p.153, figs.F,M, Rem.); Mulyadi, 2004 (p.172, figs.M, Rem.); Ferrari & Dahms, 2007 (p.95, 96, Rem., fig.31: P1, Table XXIII, XXVI); Vives & Shmeleva, 2007 (p.917, figs.F,M, Rem.); Phukham, 2008 (p.116, figs.F,M); Al-Yamani & al., 2011 (p.16, figs.F,M) | 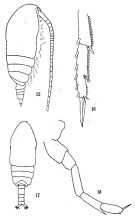 issued from: Q.-c Chen & S.-z. Zhang in Studia Marina Sinica, 1965, 7. [Pl.10, 15-18]. Female (from E China Sea): 15, habitus (lateral right side); 16, distal segments of exopod of P4 (posterior). Male: 17, habitus (dorsal); 18, P5 (posterior).
|
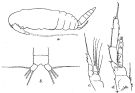 issued from : R.B.S. Sewell in Mem. Indian Mus., 1929, X. [p.85, Fig.34, a-d]. Male (from Nicobar Is.): a, habitus (lateral left side); b, anal segment and caudal rami (dorsal); c, P1; d, P4.
|
 issued from : R.B.S. Sewell in Mem. Indian Mus., 1929, X. [p.81, Fig.32]. Female (from N Indian Ocean): a, habitus (lateral left side); b, P1; c, P2; d, P3; e, P4.
|
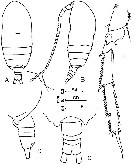 issued from : F.Y. Al-Yamani & I. Prusova in Common Copepods Northwestern Arabian Gulf : Identification Guide. Kuwait Institute for Scientific Research, 2003. [p.28, Fig.6]. Female: A, habitus (dorsal); B, idem (lateral left side); C, urosome (dorsal); D, idem (lateral left side); E, exopod of P4. Nota: Dorsal outline of cephalosome significantly humped in lateral view. Exopod 3 of P4: external edge teeth on distal end equal in size to those on proximal end.
|
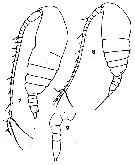 issued from : T. Mori inThe pelagic Copepoda from the neighbouring waters of Japan, 1937 (1964). [Pl.12, Figs.7-9]. Female: 8, hanitus (lateral). Male (immature): 7, habitus (lateral); 9, P5.
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf. 10, Fig.37]. Female: 37, exopodite 3 of P4.
|
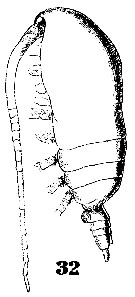 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf. 6, Fig.32]. Female: 32, habitus (lateral).
|
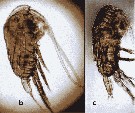 issued from : Y. Al-Yamani, V. Skryabin, A. Gubanova, S. khvorov & I. Prusova in Marine Zooplankton Practical Guide for the Northwestern Arabian Gulf, 2, 2011. [p.17, Fig.124, b-c]. Female (from Kuwait), b, habitus (lateral). Male: c, habitus (lateral).
|
 issued from : N. Phukham in Species diversity of calanoid copepods in Thai waters, Andaman Sea (Master of Science, Univ. Bangkok). 2008. [p.198, Fig.72]. Female (from W Malay Peninsula): a-b, habitus (dorsal and lateral, respectively); c, P4. Male: d-e, habitus (dorsal and lateral, respectively); f; P5. Body length after the drawings: F = 0.979 mm; M = 1.059 mm.
|
 issued from : Mulyadi in Published by Res. Center Biol., Indonesia Inst. Sci. Bogor, 2004. [p.173, Fig.96]. Male (from Indonesian Seas): a, habitus (dorsal); b, forehead (lateral); c, posterior thoracic segment with P5 andurosomites 1 and 2 (lateral); d, P3; e, P4; f, P5.
|
 Issued from : R. Gurney in Trans. zool. Soc. London, 1927, 22. [p.148, Fig. 18]. Female (from Gulf of Suez): D, P3. Male (from Gulf of Suez): B, habitus (lateral); C, P3; E, P4; F, P5. Nota: For Gurney (p.147), 5 species of Acrocalanus have been recorded from the Red Sea, but only one of them, A. gibber, from the Gulf of Suez and none from the Canal; in the abundant material examined from Suez and various points in the Canal, only one species is distinguishable, which, in spite of great variation in size and some variability in form, must certainly be regarded as A. gibber. All females are characterized by having a strikingly humped dorsal outline and a partial separation of the head from the 1st thoracic somite (2 characters which separate A. gibber from the others described (Wolfenden, 1908). Wolfenden states that the A1 of A. gibber does not reach the end of the abdomen; but Giesbrecht describes it as extending beyond by 1 to 2 segments, while in the Suez specimens it always exceeds the furca by 1 to 3 segments. In size the Suez Canal specimens are very variable, ranging from 0.74 mm to 0.98 mm. The smallest specimens are from station R.3, where the maximum size was 0.82 mm while at Port Taufik (near Suez Bay) it ranged from 0.85 to 0.98 mm. In the Bitter Lakes the length was about 0.86 mm. Adult males appear to be exceedingly rare in this genus, but not in the Suez specimens. After moult, in the adulut male, the dorsal outline becoming evenly rounded, the line of division between head and thorax disappearing, and the abdomen becoming 5-segmented. The A1 become modified, the basal segments fusing together into a thickened part provided with numerous aesthetes, only 18 segments being visible; they are shorter than the abdomen. The P3 differs from that of the female in having marginal spines on the distal part of the exopodal segment 3, and the spines on the distal part of the exopodal segment 3 of P4 are smaller and more numerous. The P5 on the left side is long and slender, 5-segmented, the basal segment swollen and the rest of the limb bending almost at right angles to it. The terminal segment has a single short seta. The length 0.85 to 1.06 mm. The differences between male and female are so great that it would be difficult if not impossible to attach them to their appropriate species if more than one species was present. For tghis reason A. gardineri Wolfenden cannot be maintened as a different species, since Wolfenden had males only, apparently taken from the same collections as contained other species of female, and also immature males of those species. A. pediger Cleve is a doubtful species. Carl (1907) has pointed out that the male described as belonging to A. pediger is actually the male of a Paracalanus to which he gives the name P. clevei, while Sewell (1914) claims it as the male of P. aculeatus. The female of A. pediger is actually the immature male, probably of A. gibber (Sewell, 1914)
| | | | | Compl. Ref.: | | | Cleve, 1904 a (p.184); Wilson, 1942 a (p.170); Sewell, 1948 (p.390, 395, 407, 413, 422, 433, 439); C.B. Wilson, 1950 (p.157); Krishnaswamy, 1953 (p.115); Chiba & al., 1957 (p.306); 1957 a (p.11); Bowman, 1958 (p.123: Rem.); Fagetti, 1962 (p.13); Ganapati & Shanthakumari, 1962 (p.7, 15); De Decker & Mombeck, 1964 (p.11); Fleminger, 1967 a (tabl.1); Delalo, 1968 (p.137); Deevey, 1971 (p.224); Patel, 1975 (p.659); Carter, 1977 (1978) (p.35); Kovalev & Schmeleva, 1982 (p.83); Guangshan & Honglin, 1984 (p.118, tab.); De Decker, 1984 (p.315, 328: chart); Stephen, 1984 (p.161, 169, Distribution vs thermocline & geographic); Greze & al., 1985 (p.7); Madhupratap & Haridas, 1986 (p.105, tab.1); Renon, 1987 (tab.2); Othman & al., 1990 (p.561, 563, Table 1); Hirakawa & al., 1990 (tab.3); Dai & al., 1991 (tab.1); Yoo, 1991 (tab.1); McKinnon, 1991 (p.471); Gajbhiye & al., 1991 (p.189); Gajbhiye & Abidi, 1993 (p.137); Shih & Young, 1995 (p.71); McKinnon, 1996 (p.1438, development); Sharaf & Al-Ghais, 1997 (tab.1); Go & al., 1997 (tab.1); Park & Choi, 1997 (Appendix); Wong & al, 1998 (tab.2); Noda & al., 1998 (p.55, Table 3, occurrence); Mauchline, 1998 (tab.45, 53); El-Serehy, 1999 (p.172, Table 1, occurrence); El-Serehy & al., 2001 (p.116, tab.1: abundance vs transect in Suez Canal); Lo & al., 2001 (1139, tab.I); Greenwood & al., 2002 (p.17, Table 2); Hsiao & al., 2004 (p.326, tab.1); Hsieh & al., 2004 (p.397, tab.1, p.399, tab.2); Rezai & al., 2004 (p.486, tab.2, 3, abundance, Rem.); Chang & Fang, 2004 (p.456, tab.1); Wang & Zuo, 2004 (p.1, Table 2, dominance, origin); Lan & al., 2004 (p.332, tab.1, tab.2); Gallienne & al., 2004 (p.5, tab.3); Satapoomin & al., 2004 (p. 102, 109, tab.2, 6); Shimode & Shirayama, 2004 (p.607, tab.1, 2); Yin & al., 2004 (p.3); Lo & al., 2004 (p.89, tab.1); Rezai & al., 2005 (p.157, Table 2, 5: spatial & temporal variations); Obuid Allah & al., 2005 (p.123, occurrence % vs metal contamination); Prusova & Smith, 2005 (p.75); Zuo & al., 2006 (p.159, tab.1, abundance, fig.8 stations group); Rakhesh & al., 2006 (p.93, Table 2, spatial distribution); Hwang & al., 2006 (p.943, tabl. I); Dur & al., 2007 (p.197, Table IV); Jitlang & al., 2008 (p.65, Table 1); Selifonova & al., 2008 (p.305, Tabl. 2); McKinnon & al., 2008 (p.844: Tab.1); Morales-Ramirez & Suarez-Morales, 2008 (p.521); Fernandes, 2008 (p.465, Tabl.2); Ohtsuka & al., 2008 (p.115, Table 5); Lan Y.C. & al., 2008 (p.61, Table 1, % vs stations); Tseng L.-C. & al., 2008 (p.153, Table 2, fig.5, occurrence vs geographic distribution, indicator species); Tseng L.-C. & al., 2008 (p.46, table 2, abundance vs moonsons, fig.8, table 3: indicator species); C.-Y. Lee & al., 2009 (p.151, Tab.2); Lan Y.-C. & al., 2009 (p.1, Table 2, % vs hydrogaphic conditions); Hwang & al., 2009 (p.49, fig.4, 5); Cornils & al., 2010 (p.2076, Table 3); Fazeli & al., 2010 (p.153, Table 1); Sun & al., 2010 (p.1006, Table 2); W.-B. Chang & al., 2010 (p.735, Table 2,, 3, 4, fig.5, abundance); Xu & Gao, 2011 (p.514, figs.3, 4, Table 2: optimal salinity); Hsiao S.H. & al., 2011 (p.475, Appendix I); Hsiao & al., 2011 (p.317, Table 2, 3, fig.6, indicator of seasonal change); Maiphae & Sa-ardrit, 2011 (p.641, Table 2, 3, Rem.); Chew & Chong, 2011 (p.127, Table 3, abundance vs location); Tseng L.-C. & al., 2011 (p.47, Table 2, occurrences vs mesh sizes); Guo & al., 2011 (p.567, fig.4, table 2, abundance vs spatial distribution; indicator); Beltrao & al., 2011 (p.47, Table 1, density vs time); Selifonova, 2011 a (p.77, Table 1, alien species in Black Sea); El-Sherbiny & al., 2011 (p.837, seasonal abundance), Johan & al., 2012 (2013) (p.1, Table 1); Uysal & Shmeleva, 2012 (p.909, Table I); Mulyadi & Rumengan, 2012 (p.202, Rem.: p.204); Tseng & al., 2012 (p.621, Table 3: abundance, indicator species); Johan & al., 2012 (p.647, Table 1, fig.2, salinity range); Dorgham & al., 2012 (p.473, Table 3: abundance %); Jang M.-C & al., 2012 (p.37, abundance and seasonal distribution); Gubanova & al., 2013 (in press, p.4, Table 2); Cornils & Blanco-Bercial, 2013 (p.861, Table 1, molecular analysis, figs.3, 4, 5); Varadharajan & Soundarapandian, 2013 (p.2: occurrence vs stations); in CalCOFI regional list (MDO, Nov. 2013; M. Ohman, pers. comm.); Tseng & al., 2013 (p.507, seasonal abundance); Suzuki, K.W. & al., 2013 (p.15, Table 2, 3, 4, estuaries, annual occurrence); El-Serehy & al., 2013 (p.2099, Rem.: p.2101); Terbiyik Kurt & Polat, 2013 (p.1163, Table 2, fig.9, seasonal distribution); Hwang & al., 2014 (p.43, Appendix A: seasonal abundance); Nakajima & al., 2015 (p.19, Table 3: abundance); Zakaria & al., 2016 (p.1, Table 1, Rem.); Ohtsuka & Nishida, 2017 (p.565, Table 22.1); Palomares-Garcia & al., 2018 (p.178, Table 1: occurrence); | | | | NZ: | 13 | | |
|
Distribution map of Acrocalanus gibber by geographical zones
|
| | | | | | | | | | | |  Issued from : D.-h. Guo, J.-q. Huang & S.-j. Li in Cont. Shelf Res., 2011, 31. [p.571, Fig.4]. Issued from : D.-h. Guo, J.-q. Huang & S.-j. Li in Cont. Shelf Res., 2011, 31. [p.571, Fig.4].
Distribution of Acrocalanus gibber abundance (ind/m3) during August 31 to September 3, 1988 (a); August 29 to September 8, 1994 (b); July 4 to 15, 2005 (c); and June 20 to July 2, 2006 (d).
Nota: The same sampling procedure was used for all cruises. Zooplankton samples were collected by vertical tows from 3 m of the bottom to the surface (plankton net : 505 µm mesh size).
The aim of the research is the relationship between copepods and associated water masses in the southern Taiwan Strait. |
 Issued from : S.-H. Hsiao, S. Kâ, T.-H. Fang & J.-S. Hwang inHydrobiologia, 2011, 666. [p.326, Fig.6]. Issued from : S.-H. Hsiao, S. Kâ, T.-H. Fang & J.-S. Hwang inHydrobiologia, 2011, 666. [p.326, Fig.6].
Variations in the most abundant copepod species (mean ± SE) along the transect in the boundary waters between the northern part Taiwan Strait and the East China Sea i March (black bar) and October (grey bar) 2005 (Mann-Whitney U test, sig. **P <0.01.
See drawings of Hydrological conditions and superficial marine currents in Calanus sinicus. |
| | | | Loc: | | | South Africa (E & W), off Bermuda, Medit. (Alboran Sea, W Egyptian coast, Iskenderun Bay, Lebanon Basin, W Black Sea), Suez Canal, G. of Suez, Lake Timsah, off Sharm El-Sheikh, Safâga, Red Sea, Gulf of Oman, G. of Aden, Arabian Sea, Arabian Gulf (UAE coast, Kuwait), E Africa (coast), Natal, Maldives Is., Sri Lanka (Pearl Banks), Madagascar, Rodrigues Is. - Seychelles, Mascarene Basin, Indian, India (Saurashtra coast, Bombay, Lawson's Bay, Pointcalimere, Madras), Bay of Bengal, S Burma (coast), Andaman Sea, W Malay Peninsula, off Phuket, Kurau Riv., Perai River estuary (Penang), Straits of Malacca, Sangga estuary (nearshore-offshore), Indonesia-Malaysia, Bintulu coast, Java (Cilacap Bay, off Labuan, Jakarta Bay, off Tegal), Ambon Bay (Ceram Is.), Tioman Is., SW Celebes, Philippines, Viet-Nam (Cauda Bay), Hainan (Sanya Bay), China Seas (Yellow Sea, East China Sea, South China Sea, Xiamen Harbour), Taiwan Strait, Taiwan (S, E, SW, W, Kaohsiung Harbor, NW, N, Mienhua Canyon, off Danshuei River, NE), Okinawa, S Korea, Korea Strait, Japan: Ariake Bay, Seto nInland Sea, Kuchinoerabu Is., south estuaries (Chikugo, Midori & Kuma Rivers) Pacif. (W equatorial), Australia (G. of Carpentaria, Great Barrier, Brisbane River estuary, Baie Moreton, off Port Jackson, Shark Bay, North West Cape), Pacif. (tropical), California, off Washington State, Hawaii, Clipperton Is., off Galapagos, La Paz (S Baja California), W Costa Rica, off Peru, Chile
Data from Cornils & Blanco-Bercial (2013) : 17°11'S; 150°25'W | | | | N: | 150 | | | | Lg.: | | | (28) F: 1,16-0,92; M: 1,07; (29) F: 0,81; M: 0,943; (34) F: 1,15-1,02; (47) F: 1-0,93; (55) F: 1,13; M: 1,4; (91) F: 1,2-0,9; M: ± 1; (104) F: 1; (178) F: 0,98-0,74; M: 1,06-0,85; (290) F: 1,25-1,15; M: 1; (333) F: 1,28-0,99; (334) F: 1-0,93; (795) F: 0,85; (937) F: 1,0-1,02; (991) F: 0,93-1,28; M: 0,94-1,24; (1047) F: 0,85; (1085) F: 0,9-1,2; M: 0,9-1,2; (1122) M: 0,9; {F: 0,74-1,28; M: 0,85-1,40} | | | | Rem.: | epipelagic. Coastal.
This species seems to be reported only by Deevey (1971) in the North Atlantic.
According to Mulyadi (2004, p.173) the species has a widely distributed in Indo-West Pacific, and widely recorded in Indonesian waters.
See in DVP Conway & al., 2003 (version 1)
R. Stephen: Data sheets of NIO, Kochi, India (on line). | | | Last update : 09/12/2020 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2025. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed December 31, 2025] © copyright 2005-2025 Sorbonne University, CNRS
|
|
 |
 |
















