|
|
 |
|
Calanoida ( Order ) |
|
|
|
Calanoidea ( Superfamily ) |
|
|
|
Paracalanidae ( Family ) |
|
|
|
Parvocalanus ( Genus ) |
|
|
| |
Parvocalanus crassirostris (F. Dahl, 1894) (F,M) | |
| | | | | | | Syn.: | Paracalanus crassirostris F. Dahl, 1894 c (p.21, figs.F); Giesbrecht & Schmeil, 1898 (p.24); Thompson & Scott, 1903 (p.233, 243); Früchtl, 1923 (p.456: Rem.); Sewell, 1924 (p.780, Rem.); Gurney, 1927 (p.144, figs.F,M, Rem.); Sewell, 1929 (p.38, 72, figs.F, Rem.: p.76); 1933 (p.25); Rose, 1956 (p.460); Deevey, 1956 (p.127, 134, tab. IV); Grice, 1956 (p.62, Rem.); Deevey, 1960 (p.5, Table II) ; Tanaka, 1960 (p.23, figs.F); Coker & Gonzalez, 1960 (p.18); Vervoort, 1962 a (p.394); Gaudy, 1963 (p.21, Rem.); Björnberg, 1963 (p.28, Rem.); Gonzalez & Bowman, 1965 (p.244, figs.F,M); Chen & Zhang, 1965 (p.41, figs.F,M); Faber, 1966 (p.191, 198, figs.N); 1966 a (p.419); Delalo, 1968 (p.137); Wellershaus, 1969 (p.247, 250, figs.F,M, f. cochinensis); Bowman, 1971 b (p.28); Shmeleva, 1973 a (p.538); Lawson & Grice, 1973 (p.43, figs. N, juv., F,M); Zismann & al., 1975 (p.59, prey); Ramirez, 1976 (p.22, figs.F, Rem.); Greenwood, 1976 (p.22, figs.F); Grindley, 1977 (p.341, Table 2, fig.2, 3, 13); Carter, 1977 (1978) (p.35); Chen Q-c, 1980 (p.795); Björnberg & al., 1981 (p.622, figs.F,M); Zheng & al., 1982 (p.22, figs.F); De Decker, 1984 (p.315); Kimmerer & al., 1985 (p.426); Moraitou-Apostolopoulou, 1985 (p.303, occurrence/abundance in E Mediterranean Sea, Rem.: p.310, 313); Kimmerer & McKinnon, 1985 (p.149); Nishida, 1985 (p.127, Rem.); Michel & al., 1986 (p.35); 1986 a (p.75); Dai & al., 1991 (tab.1); Yoo, 1991 (tab.1); Gajbhiye & al., 1991 (p.188); Mullin, 1991 (p.481); Kim al., 1993 (p.269); Hwang & Choi, 1993 (tab.3); Gajbhiye & Abidi, 1993 (p.137); Lopes, 1994 (tab.1); Webber & Roff, 1995 (tab.1); Shih & Young, 1995 (p.71); Eskinazi-Sant'Anna & Tundisi, 1996 (t.1,2); Roff & al., 1995 (p.165, Table 3: nauplii, copepodites growth rates); Webber & al., 1996 (tab.1); Park & Choi, 1997 (Appendix); Noda & al., 1998 (p.55, Table 3, occurrence); Suarez-Morales & Gasca, 1998 a (p.110); Hanafy & al., 1998 (p.465); Lopes & al., 1999 (p.215, tab.1); Neumann-Leitao & al., 1999 (p.153, tab.2); El-Serehy, 1999 (p.172, Table 1, occurrence); Ueda & al., 2000 (tab.1); Ara, 2001 b (p.121); El-Serehy & al., 2001 (p.119, tab.1, 3, 4, occurrence & abundance vs transect in Suez Canal); Lo & al., 2001 (1139, tab.I); Dunbar & Webber, 2003 (tab.1); Shimode & Shirayama, 2004 (p.607, tab.2); Wang & Zuo, 2004 (p.1, Table 2, dominance, origin); Lakkis & al., 2005 (p.152); Obuid Allah & al., 2005 (p.123, occurrence % vs metal contamination); Zenetos & al., 2005 (p.63, Rem.: p.82, casual occurrence); Fernandes, 2008 (p.465, Tabl.2); Lan Y.C. & al., 2008 (p.61, Table 1, % vs stations); Tseng L.-C. & al., 2008 (p.46, table 2, abundance vs moonsons); Ohtsuka & al., 2008 (p.115, Table 4, 5, Rem.: in Japan Harbors); Sun & al., 2010 (p.1006, Table 2); Fazeli & al., 2010 (p.153, Table 1); Maiphae & Sa-ardrit, 2011 (p.641, Table 2, 3, Rem.); Saitoh & al., 2011 (p.85, Table 5); Selifonova, 2011 (p.77, Table 1, alien species in Black Sea); El-Sherbiny & al., 2011 (p.837, seasonal abundance, Table 3: correlations), Zhang G.-T. & Wong, 2011 (p.277, fig.6, 7, 8, abundance, indicator); Huo & al., 2012 (p.1, Table 1: dominance); Johan & al., 2012 (p.647, Table 1, 2, fig.2, salinity range); Johan & al., 2012 (2013) (p.1, Table 1); Youn & al., 2012 (p.33, Table 1, 2: seasonal composition); Lee J. & al., 2012 (p.95, Table 2: abundance, annual & seasonal distribution);
P. crassirostris f. typica : Fruchtl, 1923 (p.456); 1924 b (p.36-39); De Decker, 1964 (p.16, 23, 30);
Paracalanus crassirostris var. nudus: Davis1944 (p.4, figs.F);
Paracalanus brevispinatus Shen & Lee, 1966 (p.213, 221, figs.F,M); Kesarkar & Anil, 2010 (Rem.: p.403);
No Paracalanus crassirostris : Unterüberbacher, 1964 (p.18, Rem.);
Paracalanus crassirostris s.l. : Brinton & al., 1986 (p.228, Table 1); | | | | Ref.: | | | De Decker, 1964 (p.16); Andronov, 1970 (p.984); Hiromi, 1981 (p.153, figs.F,M, Rem.); Kovalev & Shmeleva, 1982 (p.82); Turner & Dagg, 1983 (p.16, 22); Nishida, 1989 (p.173, table 2, no M dorsal hump); McKinnon, 1991 (p.471); Checkley & al., 1992 (p.11); Bradford-Grieve, 1994 (p.69, figs.F,M); Chihara & Murano, 1997 (p.844, Pl.134: F,M); Bradford-Grieve & al., 1999 (p.878, 911, figs.F,M); Al-Yamani & Prusova, 2003 (p.35, figs.F,M); Mulyadi, 2004 (p.176, figs.F,M, Rem.); Vives & Shmeleva, 2007 (p.975, figs.F,M, Rem.); Kesarkar & Anil, 2010 (Rem.: p.405); Al-Yamani & al., 2011 (p.26, figs.F,M); Jungbluth & al., 2013 (p.3125, PCR method vs naupliar abundances); S.Y. Moon & al., 2014 (p.43: Rem., p.45: F); Vidjak & al., 2016 (p.628, Descr: F, figs. F, Rem.) | 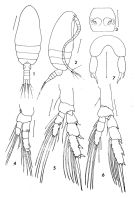 issued from: F.C. Ramirez in Neotropica, 22 (67). As Paracalanus crassirostris. Female: 1, habitus (dorsal view); 2, idem (right lateral view); 3, genital somite; 4, P1; 5, P2; 6, P3; 7, P5. lines 1 and 2: 0,1 mm; 3 to 7: 0,03 mm.
|
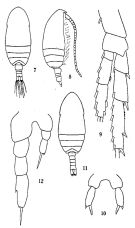 issued from: Q.-c Chen & S.-z. Zhang in Studia Marina Sinica, 1965, 7. [Pl.9, 7-12]. As Paracalanus crassirostris. Female (from E China Sea): 7, habitus (dorsal); 8, idem (lateral right side); 9, P4 (posterior); 10, P5 (anterior). Male: 11, habitus (dorsal); 12, P5 (posterior).
|
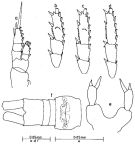 issued from : J.G. Greenwood in Proc. R. Soc. Qd, 1976, 87. [p.23, Fig.8]. As Paracalanus crassirostris forma typica. Female (from Moreton Bay, E Australia): a, P1; b, P2; c, P3; d, P4; e, P5; f, urosome (ventral). Nota: All specimens belonged to forma typica but with some variation from that form as described by Fruchtl (i.e., in number of spines on exopodal segment 3 proximal outer margin). These were (Fruchtl's figure in brackets) P1= 0 (0); P2 = 5-6 (8); P3 = 8-10 (10-12); P4 = 9-11 (10). In this respect material most closely resembles those taken by Gurney (1927), Gonzalez & Bowman (1965) and Tanaka (1960). In addition to leg spination, characterised by female P5 having 2 short terminal spines, inner only twice length of outer, without accessory spinules at base, and by shape of seminal receptacle.
|
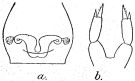 issued from : R.B.S. Sewell in Mem. Indian Mus., 1929, X. [p.73, Fig.27]. As Paracalanus crassirostris. Female (from N Indian Ocean): a, genital segment (ventral); b, P5.
|
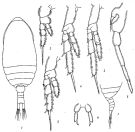 issued from : C.-j. Shen & F.-s. Lee in Acta zootaxon. sin., 1966, 3 (3). [p.215, Figs.1-8]. As Paracalanus brevipinatus. Female (from Chaikiang estuary): 1, habitus (dorsal); 2, P1; 3, P2; 4, P3; 5, P4; 6, P5. Male: 7, last thoracic segments and urosome (lateral left side; 8, P5 (anterior view).
|
 issued from : F.Y. Al-Yamani & I. Prusova in Common Copepods Northwestern Arabian Gulf : Identification Guide. Kuwait Institute for Scientific Research, 2003. [p.34, Fig.10]. Female: A, habitus (dorsal); B, idem (lateral right side); C, urosome (dorsal); D, forehead (lateral); E, rostrum (ventral); F, P1; G, P2; H, P3; I, P4; J, P5.
|
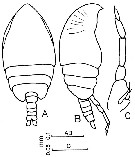 issued from : F.Y. Al-Yamani & I. Prusova in Common Copepods Northwestern Arabian Gulf : Identification Guide. Kuwait Institute for Scientific Research, 2003. [p.36, Fig.11]. Male: A, habitus (dorsal); B, idem (lateral right side); C, P5.
|
 Issued from : S. Wellershaus in Veröff. Inst. Meeresforsch. Bremerh., 1969, 11 (2). [p.249, Fig.3-9]. As Paracalanus crassirostris cochinensis nova forma Female (from Cochin Backwater, S India): 3, genital segment (ventral); 4-5, forehead (two aspects from right); 6, habitus (lateral right side); 9, P5.. Male: 7, habitus (lateral left side); 8, forehead (lateral).
Scale : single line = 0.1 mm ; double line = 0.05 mm. Nota female: - Ratio Thorax : abdomen = 3.1. - Rostral horns short and blunt. - P1 with endopod 2-segmented. - Armature of the outer margins of P2-P4 exopodal segment 2 and exopodal segment 3: exopodal segment 2 of P2 without spines, exopodal segment 2 of P3-P4 with spines, P2-P4 proximal and distal part of exopodal segment 3 with spines. This form is distinguished by having marginal spines also in the distal part of rxopodal segment 3. - P5 closely resembles the P5 of P. crassirostrisf. sewelli Früchtl. However it bears less spines in the 'corona' at the end of the terminal segment. - The 'receptacula seminis' (fig.3) are typical for all animals of this species in the Cochin Backwater, and differ in the shape and in the armature of the swimming legs from sewelli form. The present form cochinchinis is not wholly uniform as regards the spinulation of the outer margins of the exopods of the females: in exopodal segment 3 of P2-P4 distal part, the marginal spines tend to be reduced in some samples. Nota male: - Ratio thorax : abdomen = 3.1. - As in P. crassirostris f. typica Früchtl from Suez Bay, the P2 differs from that of the female in having spines on the outer margin of exopodal segment 2 (Gurney, 1926). - The rostral horns are shorter than in the female, triangular in profile.
|
 Issued from : S. Wellershaus in Veröff. Inst. Meeresforsch. Bremerh., 1969, 11 (2). [p.248, Fig.1-2]. As Paracalanus crassirostris cochinensis nova forma.
Female: 1, P1-P4 (anterior).
Male: 2, P1-P4 (anterior; P5 (posterior).
Scale : single line = 0.1 mm ; double line = 0.05 mm.
|
 Issued from : S. Wellershaus in Veröff. Inst. Meeresforsch. Bremerh., 1969, 11 (2). [p.251, Table 1]. Paracalanus (= Parvocalanus) scotti and Paracalanus (= Parvocalanus) crassirostris, armature on the outer margin of the female swimming legs 2-4. After Früchtl (1924), with modifications. + = present; - = absent.
Scale : single line = 0.1 mm ; double line = 0.05 mm.
|
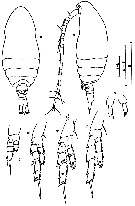 issued from : J. Hiromi in Bull. Plankton Soc. Japan, 1981, 28 (2). [p.156, Fig.1]. Female (from Kabira Bay & Omura Bay, Japan): a-b, habitus (dorsal and lateral, respectively); c-g, P1 to P5. Scale bars 0.100 mm.
|
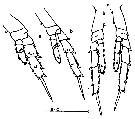 issued from : J. Hiromi in Bull. Plankton Soc. Japan, 1981, 28 (2). [p.157, Fig.2]. Female (other specimens): a-c, variations of serrations on the lateral borders of the exopods of the P2 to P4. Nota: The serrations are variable between individuals in the same localities.
|
 issued from : J. Hiromi in Bull. Plankton Soc. Japan, 1981, 28 (2). [p.158, Table 2]. Female: Extent and variability of serrations on the lateral margins of the exopods of the siwimming legs P2 to P4 in several localities of Japan. Re2-Re3: 2nd-3rd exopodal segments.
|
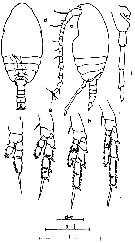 issued from : J. Hiromi in Bull. Plankton Soc. Japan, 1981, 28 (2). [p.156, Fig.2]. Male: d-e, habitus (dorsal and lateral, respectively); f-j, P1 to P5. Scale bars 0.100 mm.
|
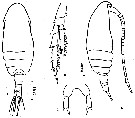 issued from : Z. Zheng, S. Li, S.J. Li & B. Chen in Marine planktonic copepods in Chinese waers. Shanghai Sc. Techn. Press, 1982 [p.23, Fig.10]. As Paracalanus crassirostris. Female: a-b, habitus (dorsal and lateral, respectively); c, P4; d, P5. Scale bars in mm.
|
 issued from : C.C. Davis in Publs Chesapeake biol. Lab., 1944, 61. [p.9, Pl.I, Figs.1-10]. As Paracalanus crassirostris var. nudus. Female (from Chesapeake Bay): 1, habitus (dorsal); 2, forehead (ventral); 3, right caudal ramus; 4, habitus (lateral); 5, A1; 6, forehead (lateral); 7, Md (masticatory blade); 8, Md (mandibular palp); 9, Mx2; 10, Mx1. Nota : A1 reach about the middle of urosome, 24-segmented, the last two segments equal in length (for Gurney they reach the caudal rami and the last segment longer than the penultimate in the ratio of 21 to 16)). A2 with rami approximately equal in length (in contrast to the specimens reported by Scott, in which the exopod only ½ the length of the endopod) ; endopod with 7 segments. Md with masticatory edge provided terminally and subterminally with numerous small teeth, arranged in a complicated manner ; on the inner corner tgere is a large double tooth, giving the whole masicatory portion a very different appearance than in that of P. parvus (Claus); palp small and rmi approximately equal in length. Mx1 with 9 heavy setae on the 1st inner lobe ; endopod with 5 setae. Mx2 6-segmented, 1st segment with 2 inner lobes, each of which has 2 heavy setae ; 2nd segment with 3 lobes, the 1st with 3, 2nd and 3rd with 2 setae ; the 3rd segment has 1 inner lobe an dit bears 3 setae, one of which is shorter and stouter than the others ; the other 3 segments very short and bear from 1 to 3 setae each.
|
 issued from : C.C. Davis in Publs Chesapeake biol. Lab., 1944, 61. [p.11, Pl.II, Figs.1-10]. As Paracalanus crassirostris var. nudus. Female: 1, A2; 2, Mxp; 3, P5; 4, endopod of P3; 5, endopod of P1; 6, P1 (endopod without its setae drawn); 7, P2; 8, 3rd segment of the exopod ot P4; 9, endopod of P4; 10, P4. Nota : P2 entirely naked on posteriot faces ofthe rami ; there are 4 serrations on the outer margin of the 3rd segment of the exopod (in Scott’s specimens, the 2nd exopodal segment bears similar serrations). P3 with a curved row of about 7 small spinules on the posterior face of the middle segment of the endopod, these constituting the only armature of this kind to be found on any of the legs (this differentiates the Chesapeake specimen from all other described specimens) ; in addition to the spinules, there is a row of fine hairs near, the outer distal corner of the same segment ; the 3rd exopod bears 6 to 7 fine serrations on the outer margin proximal to the 1st marginal spine (in Scott’s specimens, the 2nd exopod segment also bears such serrations). There is no armature on the posterior faces of the rami inP4, except that there is a row of fine hairs on the outer distal corner of the 2nd endopod segment ; the outer border of the 3rd exopod segment bears 8 to 9 fine serrations. P5 with the proximal of the two segments considerably swollen and bears no armature ; 2nd segment of P5 much longer in proportion to the 1st segment ; the distal segment is about 1 and ½ times the length of the other, and bears terminally a small spne on the outer distal corner and a longer spine on the inner distal corner (the proportional length of the two segments agrees with Scott, but differs from those of Dahl and Gurney).
|
 issued from : K.S. Kesarkar & A.C. Anil in J. mar. Biol. Ass. UK, 2010, 90 (2); [p.403, Table 2]. As Paracalanus brevispinatus after Shen & Lee,1966.. Characteristics of females. (X: figure not available).
|
 issued from : K.S. Kesarkar & A.C. Anil in J. mar. Biol. Ass. UK, 2010, 90 (2); [p.405, Table 2]. Characteristics of females.
|
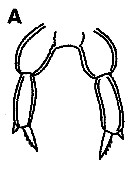 issued from : K.S. Kesarkar & A.C. Anil in J. mar. Biol. Ass. UK, 2010, 90 (2); [p.407, Fig.5, A]. As after Shen & Lee,1966.
Female: A, P5.
Nota: Inner terminal spine serrated with 4 teeth.
|
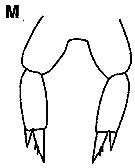 issued from : K.S. Kesarkar & A.C. Anil in J. mar. Biol. Ass. UK, 2010, 90 (2); [p.407, Fig.5, M]. Female: P5.
|
 issued from : Y. Al-Yamani, V. Skryabin, A. Gubanova, S. khvorov & I. Prusova in Marine Zooplankton Practical Guide for the Northwestern Arabian Gulf, 2, 2011. [p.27, Fig.140, c-d]. Female (from Kuwait): c, rostrum (vental view); d, forehead (right lateral view).
|
 issued from : Mulyadi in Published by Res. Center Biol., Indonesia Inst. Sci. Bogor, 2004. [p.177, Fig.99]. Female (from Indonesian Seas): a, habitus (dorsal); b-f, P1 to P5, respectively. Male: g, habitus (lateral); h, A1; i, P5. Nota female: Prosome 3.2 times as long as urosome. Proportional lengths of urosomal somites and caudal ramus 29 : 13 : 13 : 20 : 25. Caudal ramus about 2.4 times as long as wide, innermost seta more than twice as long as ramus. A1 nearly as long as body, segments 1 and 2 fused. The specimens present some variation from that as described by Fruchtl in number of spines on proximal outer margin. Such variation is also found in other forms (see Wellerhaus, 1969). In this respect the present specimens most closely resembles those taken by Gurney, 1927; Gonzales & Bowman, 1965; Tanaka, 1960; Greenwood, 1976.
|
 Issued from : O. Tanaka in Spec. Publs. Seto mar. biol. Lab., 10, 1960 [Pl. VI]. As Paracalanus crassirostris. Female (from off Cape of Good Hope): 1, habitus (dorsal); 2, head (lateral); 3, thoracic segments and abdomen (lateral); 4, P1; 5, P2; 6, P4; 7, P5 Same scale in P1 to P5. Nota: Cephalothorax and abdomen in the proportional lengths 76 to 24. Hrad and 1st thoracic segment fused; the last two thoracic segments incompletely separate. Rostrum short and thick without terminal filament. Abdomen 4-segmented. Segments and caudal rami in the proportional lengths 28 : 12.5 : 12.5 : 21 : 25 = 100. Genital segment wider than long. Caudal rami 2.4 times as long as wide. A1 24-segmented, extends about to the distal end of the caudal rami; segments 1 and 2 fused, segments 8 and 9 separate. P5 2-segmented; the distal segment has 2 apical spines of which yje inner one is about 2 times as long as the outer marginal.
|
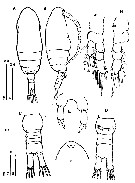 Issued from : O. Vidjak, N. Bojanic, Z. Nincevic Gladan, S. Skejic & B. Grbec in Medit. Mar. Sci., 2016, 17 (3) [p.629, Fig.2]. Female (from Sibenik harbour): A-B, habitus (dorsal and lateral, respectively); C, urosome (dorsal); D, urosome (ventral); E, P5; F, rostrum; G, P1 (anterior surface); H, P2 (posterior surface, endopod not shown). Scale bars in micrometers. Nota: Cephalosome slightly humped in lateral view. - Rostrum short and blunt. - Prosome about 2.5 times longer than wide and 3 times longer than urosome inclusing caudal rami. - 4th and 5th pedigerous somites partially separated. - A1 extending approximately to 2bd urosomite. - Urosome 4-segmented, urossomites 2 and 3 narrower than the preceding and successive somites. - Genital somite approximately 1.4 times wider than long with paired genital pores on the sides and operculum located midventrally. - Caudal rami short and cylindrical, twice as long as wide and with 5 caudal setae. - P5 symmetrical, 2-segmented, proximal segment unarmed, distal segment elongated, 2.3 times longer than wide, devoid of spinules along the distal edge, and apically armed with 2 unequal spines, the inner without serration and 2.25 times longer than outer spine.
|
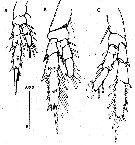 Issued from : O. Vidjak, N. Bojanic, Z. Nincevic Gladan, S. Skejic & B. Grbec in Medit. Mar. Sci., 2016, 17 (3) [p.629, Fig.3]. Female: A, P2; B, P3; C, P4. Posterior( surfaces. Scale bar in micrometers
|
 Issued from : O. Vidjak, N. Bojanic, Z. Nincevic Gladan, S. Skejic & B. Grbec in Medit. Mar. Sci., 2016, 17 (3) [p.629, Table 1]. Female : armature formula for swimming legs P1 to P4.
|
 Issued from : S. Y. Moon, S.-H. Youn & H.Y. Soh in ZooKeys, 2014, 456. [p.42, Table I]. Female : marphological characters. Compare with other species of Parvocalanus : ( arabiensis, leei, dubia, elegans, latus, serratipes, scotti).
|
 Issued from : R. Gurney in Trans. zool. Soc. London, 1927, 22. [p.146, Fig. 17]. As Paracalanus crassirostris. Female (from Suez Canal): A, habitus (lateral); B, P5; C, P2; D,P3. Male: E, P2. Nota female: - Cephalothorax scarcely tapering anteriorly, narrowest in mandibular region, its greatest breadth in region of P1, rather more than 1/3 of length (31-82). - In many specimens, a patch of purple colour between the somites of P2 and P3. - Abdomen of 4 somites, length (including furca) nearly 1/3 of cephalothorax (29-82). - Relative length of abdominal somites 13 : 4.7 : 4.7 : 10 : 1.12. - Rostral filaments short and thick, not filiform. - A1 with joints [= segments] somewhat indistinct, but apparently 24, reaching to base of furca [= caudal rami] or sometimes beyond it. Length of last three joints 16 : 16 : 21; last joint slender. - Posterior face of basal segment and exopod of P3, smooth. - P4 more slender and longer than P3 (exopod of P3 = 85, of P4 = 96). No spines on posterior face. Exopodal segment 3 proximal part with about 10 spines. - P5Proximal segment slightly longer than distal (18-16). Terminal segment with a short spine and a stout but short seta, about 5/8 of length of segment (10-16).
|
 Issued from : R. Gurney in Trans. zool. Soc. London, 1927, 22. [p.145, Fig. 16]. As Paracalanus crassirostris. Male: B, habitus (lateral; C, P3; D, P5. Nota male: - A1 modified as usual in Paracalanus, shorter than in female, extending about to 2nd abdominal somite. - Rostral filaments replaced by a small conical process. - P1 as in female.
- P2-P4 differ from those of female in having spines on the outer margin of 2nd exopodal segment and on the distal part of the 3rd exopodal segment.
- P5 on left side very large, 5-segmented, twisted between 1st and 2nd segments and reaching back to base of furca. Terminal segment with a short spinous process and a long stout spine.
| | | | | Compl. Ref.: | | | Ueda, 1985 (p.125); Madhupratap & Haridas, 1986 (p.105, tab.1); Othman & al., 1990 (p.561, 563, Table 1, Rem.: p.569); McKinnon & Ayukai, 1996 (p.595); Suarez-Morales & Gasca, 1997 (p.1525); Sharaf & Al-Ghais, 1997 (tab.1); Lopes & al., 1998 (p.195); McKinnon & Klumpp, 1998 (tab.1); Alvarez-Cadena & al., 1998 (tab.1,2,3); Hopcroft & al., 1998 (tab.2); Calbet & al., 2000 (p.75); Lopez-Salgado & al., 2000 (tab.1); McKinnon & Duggan, 2001 (p.121); Wang & al., 2002 (p.348); McKinnon & al., 2003 (p.101, tab.2, fig.11); Hsieh & al., 2004 (p.397, tab.1, p.399, tab.2); Rezai & al., 2004 (p.486, tab.2, 3, abundance, Rem.); Lo & al., 2004 (p.468, tab.2); Ara, 2004 (p.179, figs. 3,4,5); Lo al., 2004 (p.89, tab.1); Rezai & al., 2005 (p.157, Table 2, 5: spatial & temporal variations); Berasategui & al., 2005 (p.485, tab.1); Prusova & Smith, 2005 (p.75); Manning & Bucklin, 2005 (p.233, Table 1); Berasategui & al., 2006 (p.485: fig.2); Araujo, 2006 (p.173, Tab.3): Sterza & Fernades, 2006 (p.95, Table 1, occurrence); Hwang & al., 2006 (p.943, tabl. I); Mageed, 2006 (p.171, Table 4); Dur & al., 2007 (p.197, Table IV); Cornils & al., 2007 (p.278, Table 2); McKinnon & al., 2008 (p.844: Tab.1, fig.7); Humphrey, 2008 (p.84: Appendix A); Morales-Ramirez & Suarez-Morales, 2008 (p.521); Tseng L.-C. & al., 2008 (p.153, Table 2, fig.5, occurrence vs geographic distribution, indicator species); Selifonova & al., 2008 (p.305, Tabl. 2); Miyashita & al., 2009 (p.815, Tabl.II); Magalhaes & al., 2009 (p.187, Table 1, %); Cornils & al., 2010 (p.2076, Table 3, Fig.5); Hernandez-Trujillo & al., 2010 (p.913, Table 2); Zenetos & al., 2010 (p.397); Medellin-Mora & Navas S., 2010 (p.265, Tab. 2); Eleftheriou & al., 2011 (p.502); Sun X.-H. & al., 2011 (p.741, figs.5. 3, 4, tab.I, II, production); Magris & al., 2011 (p.260, abundance, interannual variability); Sakaguchi & al., 2011 (p.18, Table 1, occurrences); Kâ & Hwang, 2011 (p.155, Table 3: occurrence %); Chew & Chong, 2011 (p.127, fig.4, 5, Table 2, 3, abundance vs location); Ali & al., 2011 (p.601, fig.9, abundance vs tidal); Chen M. & al., 2011 (p.1075, Fig.8, annual abundance variation); Tseng L.-C. & al., 2011 (p.239, Table 3, abundance %); Hsiao & al., 2011 (p.317, Table 2, indicator of seasonal change); Selifonova, 2011 a (p.77, Table 1, alien species in Black Sea); Buskey & al., 2011 (p.57, Rem.: p.61: predation); Beltrao & al., 2011 (p.47, Table 1, fig.3, density vs time); Costa R.G. da & al., 2011 (p.364, Table 1, seasonal occurrence); Miyashita & al., 2012 (p.1557, Table 2: occurrence); Almeida LR. & al., 2012 (p.13, Table 1, abundance); Uysal & Shmeleva, 2012 (p.909, Table I); DiBacco & al., 2012 (p.483, Table S1, ballast water transport); Mulyadi & Rumengan, 2012 (p.202, Rem.: p.204); Moon & al., 2012 (p.1, Table 1); Gubanova & al., 2013 (in press, p.4, Table 2); Barton & al., 2013 (p.522, Table 1: metabolism, feeding mode, biogeo); Belmonte & al., 2013 (p.222, Table 2, abundance vs stations); Cornils & Blanco-Bercial, 2013 (p.861, Table 1, figs.3, 4, 5, molecular analysis); Tachibana & al., 2013 (p.545, Table 1, seasonal change 2006-2008); Tseng & al., 2013 (p.507, seasonal abundance); Garbosa da Costa & al., 2013 (p.756, Table 1, abundance vs tide); Jungbluth & Lenz, 2013 (p.630, Table I) ; Suzuki, K.W. & al., 2013 (p.15, Table 2, 3, 4, estuaries, annual occurrence); Seo & al., 2013 (p.448, Table 1, occurrence); Lidvanov & al., 2013 (p.290, Table 2, % composition); Chen M. & al., 2013 (p.47, clearance & ingestion rate); Bradley C.J. & al., 2013 (p.49, swimming behavior); Araujo & al., 2016 (p.1, Table 3, abundance, %) ; Marques-Rojas & Zoppi de Roa, 2017 (p.495, Table 1); Buskey & al., 2017 (p.754, myelinate fiber vs. escape response); Ohtsuka & Nishida, 2017 (p.565, 578, Table 22.1, Fig.22.3: as Paracalanus crassirostris) in central station-innermost station in Japanese waters); Atique & al., 2017 (p.1, Table 1); Belmonte, 2018 (p.273, Table I: Italian zones); Dias & al., 2018 a (p.189, Rem.: p.196); Palomares-Garcia & al., 2018 (p.178, Table 1: occurrence); Acha & al., 2020 (p.1, Table 3: occurrence % vs ecoregions). | | | | NZ: | 16 | | |
|
Distribution map of Parvocalanus crassirostris by geographical zones
|
| | | | | | | | | | | |  issued from : J. Hiromi in Bull. Plankton Soc. Japan, 1981, 28 (2). [p.158, Fig.3]. issued from : J. Hiromi in Bull. Plankton Soc. Japan, 1981, 28 (2). [p.158, Fig.3].
Distribution of Parvocalanus crassirostris, P. elegans and Delius nudus in the southwestern coast of Japan. |
 issued from : T.S.K. Björnberg in Bol. Inst. Oceanogr., Sao Paulo, 1963, XIII (1). [p.28, Fig.14]. issued from : T.S.K. Björnberg in Bol. Inst. Oceanogr., Sao Paulo, 1963, XIII (1). [p.28, Fig.14].
Probability of occurrence of Paracalanus ( = Parvocalanus) crassirostris in different environments.
T: Tropical waters (above 36.00 p.1000 salinity and above 20°C temperature); SST: Surface subtropical waters (salinity around 36 p.1000 and temperature 18°C or less); DST: Deeper shelf waters (salinity between 34 and 36, temperature under 20°C p.1000); SS: Surface shelf waters (same salinity and temperature above 20°C);
C: coastal waters with low salinity and variable temperature.
In each column no shading means no probability of finding the species in the samples from this environment; horizontal shading indicates the probability of finding the sepecies in percentages less than one in samples from this environment; cross shading indicates the probability of finding it in percentages higher than one and black shading represents the probability of finding it in the largest percentages of the total number of copepods.
Nota: In Brazilian waters it was observed only in coastal and shelf waters. |
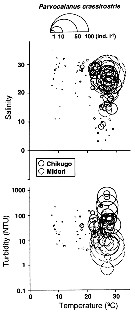 Issued from : K.W. Suzuki, K. Nakayama & M. Tanaka in J. Oceanogr., 2011, 69. [p.25, Fig.7]. Issued from : K.W. Suzuki, K. Nakayama & M. Tanaka in J. Oceanogr., 2011, 69. [p.25, Fig.7].
Bubble plots of densities of the non semi-endemic calanoid Parvocalanus crassirostris in relation to temperature, salinity, and turbidity observed in the Chikugo and Midori River estuaries (south Japan) in 2005 and 2006. |
| | | | Loc: | | | South Africa (E & W, off Cape of Good Hope), Saldanha Bay, Namibia, Angola, G. of Guinea, Dakar, Morocco-Mauritania, Argentina (coastal), Mar del Plata, Brazil (paranagua Bay, Cananeia Lagoon, Paranagua Estuary, Rio de Janeiro, Guanabara bay, Vitoria Bay, estuario do Pina, Mucuri estuary, Guarairas Lagoon, off Natal, Tocantins estuary, Curuça estuary, Ajuruteua Beach Bay, Caeté Estuary, Amazon estuary), Caribbean Colombia, Bahia de Mochima (Venezuela), Caribbean Sea, Yucatan, Yucatan (Ascension Bay), Jamaica, G. of Mexico, Porto Rico, Florida, North Carolina (Neuse River estuary), Delaware Bay (occasional throughout year), New York, Long Island Sound, G. of Maine, Cape Cod, Medit. (Marseille, Taranto, Adriatic Sea: Sibenik Bay, NE Aegean Sea, Iskenderoun Bay, Lebanon, Haifa Bay, Bardawill Lagoon, W Black Sea), Suez Canal, Lake Timsah, G. of Aqaba, Safâga, Red Sea, Gulf of Oman, Arabian Sea, Arabian Gulf (Kuwait, UAE coast), Natal, Bombay, N Indian, Chilka Lake, Bay of Bengal, Kurau Riv., Nicobar Is., Perai River estuary (Penang), Straits of Malacca, Sangga estuary, G. of Thailand, Indonesia (Cilacap Bay, off Labuan, Jakarta Bay-Seribu Islands, off Tegal, Lombok Sea, Ambon Bay, SW Celebes), Malaysia (Sarawak: Bintulu coast), Viet-Nam (Cauda Bay), Hong Kong, Hainan Is., 25°30'N-120°30'E, Taiwan Strait, Taiwan (W, SW, Tapong Bay, Mienhua Canyon, Danshuei Estuary, NE), China Seas (Bohai Sea, Yellow Sea, East China Sea, South China Sea, Xiamen Harbour, N Yangtze Riv. estuary, E Kuroshio Current), S & W Korea, Muan Bay, Chunsu Bay, Yongsan River estuary, Okinawa (Kabira Bay), Japan, Ishigaki Is., Kuchinoerabu Is., Ariake Bay, Tokyo Bay, south estuaries (Chikugo, Midori & Kuma Rivers), Honshu (Maizuru Bay, Seto Inland Sea), Palau Is., Australia (Melbourne, Great Barrier, Moreton Bay, Shark Bay, G. of Carpentaria, Haughton Riv., Exmouth Gulf), Hawaii, Kanehoe Bay, BaJa California (Bahia Magdalena, La Paz) , Gulf of California, W Costa Rica
Data from Cornils & Blanco-Bercial (2013): 38°16'N; 74°24'W and 21°26'N; 157°47'W | | | | N: | 191 | | | | Lg.: | | | (47) F: 0,5; (66) F: 0,505; (80) F: ± 0,5-0,415; M: ± 0,5-0,415; (148) F: 0,589-0,497; M: 0,422-0,374; (172) F: 0,48-0,76; M: 0,40-0,44; (178) F: 0,45-0,43; M: 0,34; (179) F: 0,5-0,47; M: 0,37-0,35; (237) F: 0,5; M: 0,5; (290) F: 0,56-0,62; M: 0,55-0,62; (431) F: 0,55-0,45; M: 0,43-0,38; (432) F: 0,82-0,55; (456) F: 0,567-0,476; (786) F: 0,66-0,57; (866) M: 0,4-0,6; (937) F: 0,54-0,61; M: 0,38-0,41; (1023) F: 0,52-0,54; (1047) F: 0,5; (1085) F: 0,48-0,65; M: 0,40 (1122) F: 0,5; M: 0,36; (1215) F: 0,52-0,48; {F: 0,415-0,820; M: 0,340-0,620}
For Sewell (1924) the sizes seem correlated with the sea-water denslties. | | | | Rem.: | Brackish, estuary and littoral. Salinity: 27 and 32 p. 1000.
According to Björnberg (1963, p.28) the species is extremely eurythermic and euryhaline, but stenoecius and then a good ''indicator'' species of coastal waters. It occurs in waters from salinities 55.00 p.1000 in the Suez Canal (Gurney, 1927) to 3.4 p.1000 in the Chilka Lake from India (Devasundaram & Roy, 1954).
After El-Sherbiny & al. (2011, p.852) this species in the Lake Timsah (Suez Canal) formed the main bulk of the copepods, in addition to 3 other species frequently captured (Centropages kroyeri, Euterpina acutifrons, Paracalanus parvus).
For Davis (1944, p.4) , other authors describing P. crassirostris have made no attempt to recognize distinct varieties. Chesapeake specimens differ from all others that have been described having a less well developed spiny armature on the posterior faces of the rami of the swimming legs, and hence the variety in Chesapeake Bay was named nudus. Dahl's (1894, p.21, figs.27-28) description of P. crassirostris is very short; Chesapeake Bay specimens differ from his in that the 2nd segment ofthe P5 is much longer in proportion to the 1st segment. Scott's (1894, p.27, pl.1, figs.1-8) specimens, which descibed as P. pygmaeus (Claus), are similar to those of Chesapeake Bay, but the exopod of A2 is only half the length of the endopod instead of the rami being sub-equal as in P. c. nudus. Cheasapeake Bay specimens differ from those described by Gurney (1927, p.144, figs.16B, 17) in several characteristics.
According to Mulyadi (2004, p.177) the species is widely distributed in Indonesian waters.
Früchtl (1923) was the first to suggest the division of the species into 3 forms, of which according to Vervoort (1963) the first has to be taken as an independant species: P. scotti Früchtl, 1923. In this species P1 bears endopod 1-segmented instead of 2 segments in P. crassirostris. Davis (1944) added one more forma nudus which has ''les well developed spiny armature on the posterior faces ofthe rami of the swimming legs'' (see Wellershaus, 1969, table 1, p.251).
After S.Y. Moon & al (2014, p.43) the species originally described by F. Dahl (as Paracalanus crassirostris) from the mouth of the river Tocantins (Brazil) , but the description was incomplete and based on the female only. The populations of this species from estuarine and shallow waters of Korea closely resemble the Brazilian population. The populations of P. crassirostris from Japanese waters are very similar to P. leei, but differ. Keserkar & Anil (2010) described a new species from the Mondovi and Zuari estuaries (Goa, West coast of India); however, these authors might have overlooked some previous morphological studies of P. crassirostris.
For Vidjalk & al. (2016) this species is probably introduced in the Sibenik harbour (Croatia) by the ballast's boats.
Ohtsuka & Nishida (2017, p.578) point to the Japanese coasts adjacent to the Kuroshio Current are distinguished by warm-water species Parvocalanus crassirostris, also the species is dominant in the Inley abd brackish waters in the main islands of Japan.
The feeding mode after Barton & al. (2013, Table 1) is 'feeding current' (hovering) (See definition in Rem. Centropages typicus). | | | Last update : 12/02/2021 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2025. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed August 17, 2025] © copyright 2005-2025 Sorbonne University, CNRS
|
|
 |
 |
































