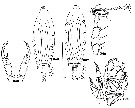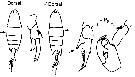|
|
 |
|
Calanoida ( Order ) |
|
|
|
Diaptomoidea ( Superfamily ) |
|
|
|
Pontellidae ( Family ) |
|
|
|
Labidocera ( Genus ) |
|
|
| |
Labidocera aestiva Wheeler, 1901 (F,M) | |
| | | | | | | Syn.: | Labidocera insolita C.B. Wilson, 1950 (p.245, figs.F,M); no L. aestiva : Oliveira, 1946 (p.470, figs.M); Carvalho, 1952 a (p.148, figs.M); Andronov, 2014 (p.32, fig.M) | | | | Ref.: | | | Wheeler, 1901 (p.178, figs.F,M); Willey, 1918 (1919) (p.203, fig.F); Wilson, 1932 a (p.147, figs.F,M); Anraku & Omori, 1963 (p.119, figs.); Fleminger, 1965 (p.127, Rem.); Faber, 1966 (p.191, 195, figs.N); Owre & Foyo, 1967 (p.98, figs.F,M); Fleminger, 1975 (p.401 & suiv.); Silas & Pillai, 1973 (1976) (p.773); Gibson & Grice, 1977 (p.7, figs.F,M, N-juv., Rem.); Bucklin & Marcus, 1985 (p.219, genetic); Blades-Eckelbarger, 1991 (p.247, figs.M); Bundy & Paffenhöfer, 1993 (p.3, figs.F); Chen & Marcus, 1997 (p.587, Rem: egg); G. Harding, 2004 (p.16, figs.F,M); Vives & Shmeleva, 2007 (p.504, figs.F,M, Rem.) | 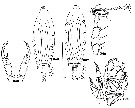 issued from : C.B. Wilson in Bull.U.S. natn Mus., 1950, 100, 14 (4). [Pl.24, Figs.346-350]. As Labidocera insolita. Female (from Caldera Bay anchorage, W coast Mindanao, Philippines): 346, habitus (dorsal); 348, P5. Male: 347, habitus (dorsal); 349, right A1; 350, P5.
|
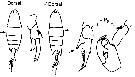 issued from : G. Harding in Key to the adullt pelagic calanoid copepods found over the continental shelf of the Canadian Atlantic coast. Bedford Inst. Oceanogr., Dartmouth, Nova Scotia, 2004. [p.16]. Female & Male. L = left leg; R = right leg.
|
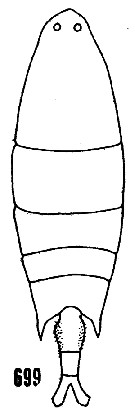 issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1, Crustacea, 1: Copepoda. Copepods of the Florida Current. 1967. [p.97, Fig.699]. After Wheeler, 1901. Female (feom Miami area): 699, habitus (dorsal).
|
 issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1, Crustacea, 1: Copepoda. Copepods of the Florida Current. 1967. [p.97, Fig.698]. After Wheeler, 1901. Female: 698, P5.
|
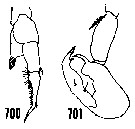 issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1, Crustacea, 1: Copepoda. Copepods of the Florida Current. 1967. [p.97, Figs.700, 701]. After Wheeler, 1901. Male: 700, left P5; 701, right P5.
|
 issued from : G.D. Grice & T.J. Lawson in Crustaceana, 1976, 30 (1). [Pl. I] Scanning electron micrographs of eggs of labidocera aestiva from Vineyard Sound near Woods Hole) in laboratory cultures. a-b: winter eggs; c-d: summer eggs. a, egg with debris on surface; b, fracture edge; c, ''hinge region'' of hatched egg; d, juncture of suture (right) and fracture (left) edges. Scale = 0.010 mm (a, c); 0.001 mm (b, d). Nota: The summer eggs lack surface ornamentation and are about the same diameter as winter eggs. The shell structure along the suture edge where the two halves separate during hatching appears to be differently constructed from that the rest of the egg.In section the suture edge (fig.1d) is relatively smooth as if overlain with a thin membrane, while the fracture edge is finely granular. A small hinge-like region (fig.1c) is present on the equator and this apparently prevents complete separation of the two halves and explains why most hatched eggs are found with the two halves reunited.
The ability to produce resting eggs is especially adaptative for these neritic copepods in temperate-boreal areas where biological and/or hydrographic conditions are unfavorable for year-round occurrence. The hatching of resting eggs upon the return of favorable conditions explains the sudden reappearance of them without postulating that they were reintroduced with incursions of water or that they persisted in a juvenile stage
|
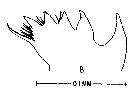 issued from : M. Anraku & M. Omori in Limnol. Oceanogr., 1963, 8. [p.122, Fig. 7, B] Labidocera aestiva (from Woods Hole): Cutting edge of Md. Nota; The cutting edge has 8 teeth with fine hairs in the ''valley'' between the 3rd and 4th and the 4th and 5th teeth. The three on the inner side are reduced to tiny points with fine hairs protruding toward the spine.
|
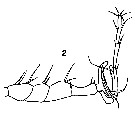 Issued from : V.N. Andronov in Russian Acad. Sci. P.P. Shirshov Inst. Oceanol. Atlantic Branch, Kaliningrad, 2014. [p.32, Fig.5, 2]. As Labidocera insolita After C.B. Wilson, 1950. 2, distal part of geniculate A1 male.
| | | | | Compl. Ref.: | | | Wilson, 1932 (p.24); Deevey, 1956 (p.127, tab. IV); Grice, 1956 (p.58); Deevey, 1960 (p.5, Table II, fig.7, 8, 15: annual abundance, Rem.: p.29, fig.18, 19) ; Grice & Hart, 1962 (p.287, table 3); Anraku, 1964 a (p.221, predation); 1964 b (p.195, respiration-grazing v.s. temperature); ; Faber, 1966 a (p.418, 419); Dawson, 1966 (p.176); Itoh, 1970 a (p.8: tab.2); Shih & al., 1971 (p.43); Björnberg, 1973 (p.352, 387); Grice & Gibson, 1975 (p.335, resting eggs); Childress, 1975 (p.787, respiratory rate); Grice & Lawson, 1976 (p.9, Rem., figs. eggs); Knatz, 1978 (p.68, Table 1, abundance, %); Blades & Youngbluth, 1979 (p.339, mating behaviour); Marcus, 1979 (p.297, life history, diapause);1980 (p.311, diapause vs photoperiod); Turner & al., 1979 (p.289, 292, Tab.3, Rem.); Turner & Collard, 1980 (p.527, Tab.1); Grice & Marcus, 1981 (p.125, Dormant eggs, Rem.: p.134); Blades & Youngbluth, 1981 (p.1, figs.M: ultrastructure); Marcus, 1982 (p.39, subitaneous & diapause egg production); 1982 a (p.45, egg diapause vs photoperiod & temperature); Vives, 1982 (p.295); Blades-Eckelbarger & Youngbluth, 1982 (p.1, figs.M: ultrastructure); Citarella, 1982 (p.791, 798: listing, frequency, Tableau II, V); Jacoby & Youngbluth, 1983 (p.84, Table 4, Rem: mating); Turner & Dagg, 1983 (p.16); Blades-Eckelbarger & Youngbluth, 1984 (p.33, figs.F: ultrastructure); Tremblay & Anderson, 1984 (p.5); Turner, 1984 b (p.265, Table 1, fig.8-10: pellet contents); Marcus, 1984 (p.47, diapause eggs); 1984 a (p.127, diapause eggs v.s. latitudes); Conley & Turner, 1985 (p.113, omnivory nutrition); Hargrave & al., 1985 (p.221, annual abundance); Marcus, 1985 (p.263, spawning v.s. endogenous control); 1985 a (p.687, genetic variation vs. environmental conditions, subitaneous eggs & diapuase eggs); Marcus & Fuller, 1986 (p.247, Rem.: subitaneous and diapause eggs); 1989 (p.319, Rem.: eggs); Tester & Turner, 1990 (p.169, egg production); Citarella, 1989 (p.123, abundance); Suarez & Gasca, 1991 (tab.2); Suarez, 1992 (App.1); Lutz & al., 1994 (p.119, hatching/O2 effect); Snell & Carmona, 1994 (p.255); Alvarez-Silva & Gomez-Aguirre, 1994 (p.265); Marcus & Lutz, 1994 (p.83, hatching success vs anoxia); Madhupratap & al., 1996 (p.77, Table 2: resting eggs); Marcus, 1996 (p.144, resting eggs); Marcus & al., 1997 (p.291, hatching success vs anoxia); Suarez-Morales & Gasca, 1997 (p.1525); Hopcroft & al., 1998 (tab.2); Alvarez-Cadena & al., 1998 (tab.2,3,4); Suarez-Morales, 1998 (p.345, Table 1); Suarez-Morales & Gasca, 1998 a (p.110); Mauchline, 1998 (tab.21, 40, fig.53); Cohen & Forward, 2002 (p.307); Alvarez-Silva & Gomez-Aguirre, 2000 (p.163: tab.2); Morales-Ramirez & Suarez-Morales, 2008 (p.514); Medellin-Mora & Navas S., 2010 (p.265, Tab. 2); Gemmell & al., 2012 (p.1, fig.3, behaviour predator-prey, jumping over sea surface); Peijnenburg & Goetze, 2013 (p.2765, genetic data); Dunlap & al., 2013 (p.1375, mortality vs viral infestion); Ortega & al., 2014 (p.495, Table I, %); Ortega & al., 2017 (p.123, fig. 1, 6, table 2, abundance, %); Baumgartner & Tarrant, 2017 (p.387, resting eggs, Rem.)
| | | | NZ: | 5 | | |
|
Distribution map of Labidocera aestiva by geographical zones
|
| | |  Chart of 1996 Chart of 1996 | |
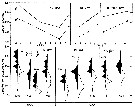 issued from : G.B. Deevey in Bull. Bingham Oceanogr. Coll., 1960 (2). [p.30, Fig.15]. issued from : G.B. Deevey in Bull. Bingham Oceanogr. Coll., 1960 (2). [p.30, Fig.15].
Variations in length (from the top of the head to the base of the caudal rami) of Labidocera aestiva in the Delaware Bay (NW Atlantic Ocean).
Top: Mean lengths of females and males. Bottom: Length distributions of females and males on the same dates. |
 issued from : B.T. Hargrave, G.C. Harding, K.F. Drinkwater, T.C. Lambert & W.G. Harrison in Mar. Ecol. Prog. Ser., 1985, 20. [p.227, Fig.7]. issued from : B.T. Hargrave, G.C. Harding, K.F. Drinkwater, T.C. Lambert & W.G. Harrison in Mar. Ecol. Prog. Ser., 1985, 20. [p.227, Fig.7].
Major species of zooplankton present at the central station in St. Georges Bay (45°45'N, 61°45'W) during 1977.
Nota: All zooplankton collections were made after sunset. The net towed obliquely throughout the water column (± 34 m in depth). |
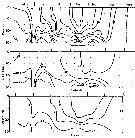 issued from : B.T. Hargrave, G.C. Harding, K.F. Drinkwater, T.C. Lambert & W.G. Harrison in Mar. Ecol. Prog. Ser., 1985, 20. [p.223, Fig.2]. issued from : B.T. Hargrave, G.C. Harding, K.F. Drinkwater, T.C. Lambert & W.G. Harrison in Mar. Ecol. Prog. Ser., 1985, 20. [p.223, Fig.2].
Seasonal profiles of water temperature and salinity in St. Georges Bay (45°45'N, 61°45'W) near the central station during 1977. |
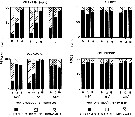 issued from : N.H. Marcus in Biol. Bull., 1984 a. [p.135, Fig.2]. issued from : N.H. Marcus in Biol. Bull., 1984 a. [p.135, Fig.2].
Subitaneous, diapause, and non-viable egg production (percent) by females Labidocera aestiva reared with dinoflagellates at 14.5°C, 18.0°C or 24.0°C (± 1.0°C) from different latitudes along the Atlantic coast of the United States (cf. chart of locations).
See Marcus, 1980 : Photoperiodic control of diapause; 1982: The reversibility of subitaneous and diapause egg production).
Nota: The results indicate that species is not genetically homogeneous throughout its range. Differences in the percentage of subitaneous and diapause eggs produced by laboratory-reared and field-collected populations reflected the unique combination of environmental conditions mainly length of photoperiods and temperature) occurring in the geographic regions from which the animals originated.
In the Virginian province where planktonic stages of the species are seasonally present, diapause egg production is triggered by short day length photoperiods and cool temperatures. In the Carolinian province where the planktonic stages tend to occur year-round diapause eggs are rarely produced by either field-collected females on their laboratory-reared offspring; this suggest that most of these animals lack the genetic capacity to produce diapause eggs.
Diapause may lead to the reproductive isolation of populations if gene flow is reduced or preventing owing to their separation on a temporal scale. |
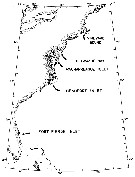 issued from : N.H. Marcus in Biol. Bull., 1984 a. [p.131, Fig.1]. issued from : N.H. Marcus in Biol. Bull., 1984 a. [p.131, Fig.1].
Location of study sites concerning subitaneous, diapause, and non-viable egg production by females Labidocera aestiva. |
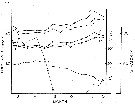 issued from : N.H. Marcus in Biol. Bull., 1979, 157. [p.298, Fig.1]. issued from : N.H. Marcus in Biol. Bull., 1979, 157. [p.298, Fig.1].
Trends in body size (cephalothorax: solid square; total length: solid circle), percent hatch (open square), and surface water temperature (open circle) for Labidocera aestiva .
Sampling a 1-2 m at weekly intervals in Vineyard Sound (Massachusetts) from July 7, 1978 to December 6, 1978.
Nota: The data document the seasonal variation in subitaneous and resting egg production during the summer-fall period in 1978. It is shown that the proportion of subutaneous eggs produced in greater during the summer, decreases markedly in September, and persists as a small percent throughout the fall. Moreover, the major change from subitaneous to resting egg production precedes the initiation of invreasing adult body size and decreasing surface water temperatures by 2 weeks. It is demonstrated that resting eggs which are chilled at 5°C for a minimum period of 30 days, will hatch synchronously within 2 days when warmed to 21 to 23°C. Comparable eggs kept continuously at 19° to 23°C will hatch over a longer period of time and not synchronously.
The resting eggs appear to be in a state of diapause similar to many insects, and it is suggested that photoperiod is the primary cue inducing the production of resting eggs. |
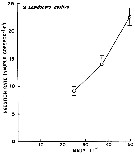 issued from : W.J. Conley & J.T. Turner in Mar. Ecol. Progr. Ser., 1985, 21. [p.117, Fig.3]. issued from : W.J. Conley & J.T. Turner in Mar. Ecol. Progr. Ser., 1985, 21. [p.117, Fig.3].
Labidocera aestiva. Predation (ingestion) rates of adults (males and females combined) feeding upon copepod nauplii (number of nauplii ingested by copepod and by day) over a range of natural concentrations. Means of 14 to 19 replicates; error bars: ± standard error.
Nota: Experimental animals collected in the Westport River estuary (Massachusetts).
Nota: Although L. aestiva was considered to be a carnivore (Anraku & Omori, 1963), this species does ingest phytoplankton. Animals were often collected with green guts, indicating that herbivory may be more intense in the field than in the experiments. There is little doubt that animal prey is preferred by this species. At natural food concentrations, rates of carbon ingestion of animal prey (mean = 3.35 µgC per copepod and by day) were about 507 % higher than those on phytoplankton (mean = 0.66 Compare with the same experimental method in Centropages hamatus. |
| | | | Loc: | | | off Mauritania, N Brazil, Barbada Is., Caribbean Colombia, Caribbean Sea (Yucatan), Venezuela, E Costa Rica, Gulf of Mexico, W Mississipi River mouth, Jamaica, Louisiana (off Grand Isle), Florida, Bayboro Harbor, Turkey Point, Georgia, Navesink River estuary, Delaware Bay, Chesapeake Bay, New York, Long Island Sound, Vineyard Sound, Woods Hole, Buzzards Bay, G. of Maine (rare), Georges Bank, St. Georges Bay Shédiac Bay, Northumberland Strait, Shediac Bay, G. of St. Lawrence, ? Philippines (in C. B. Wilson, 1950), Chile (N-S) (in Björnberg, 1973) | | | | N: | 57 | | | | Lg.: | | | (45) F: 2-1,75; M: 2,2-1,8; (137) F: 3-2,54; M: 2,45-2,3; (188) ?F,M: 2-1,75; (803) F: 2,22-1,82; M: 2,44-2,27; F: 2,64-2,26; M: 2,44-2,27; M: ; {F: 1,75-3,00; M: 1,80-2,45} | | | | Rem.: | epipelagic. Sometimes inshore and in brackish water. Neritic.
After Grice & Gibson (1975, p.335) the eggs that are laid in fall and incubated for 6 months in the laboratory at temperatures corresponding to the lowest winter water temperatures (2°-3°C) in the Woods Hole area remain viable and hatch on being warmed to 19°C. Thus, resting eggs are a mechanism which permits this species to occur in temperate latitudes and serves to repopulate the area.
Diagnosis for L. aestiva in the 'darwinii' species group after Prusova & Al-Yamani (2014, p.1165) :
Females:
- 1: Urosome of 2 somites.
- 2: Genital somite symmetrical.
- 3: Exopod of symmetrical P5 with 3 terminal points.
Males:
- 1: Urosome of 4 somites.
- 2: Left P5 endopod extends to or slightly beyond distal border of exopodal segment 1.
- 3: Right A1 segment's XXI-XXIII toothed process does not reach proximal margin of segment XXIV.
Supplementary data of Gemmell & al., 2012: High speed recording (500 fps) video of a copepod (Labidocera aestiva) making an aerial escape in the laboratory  | | | Last update : 17/09/2020 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed February 27, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |