|
|
 |
|
Calanoida ( Order ) |
|
|
|
Pseudocyclopoidea ( Superfamily ) |
|
|
|
Pseudocyclopidae ( Family ) |
|
|
|
Pseudocyclops ( Genus ) |
|
|
| |
Pseudocyclops umbraticus Giesbrecht, 1893 (F,M) | |
| | | | | | | Syn.: | Pseudocyclops umbricatus : Vervoort,1964 a (p.14: lapsus calami) | | | | Ref.: | | | Giesbrecht, 1893 a (p.64, figs.F,M); Giesbrecht & Schmeil, 1898 (p.125); Andronov, 1986 a (p.461, figs.F, Rem.); Othman & Greenwood,1989 (p.74); Vives & Shmeleva, 2007 (p.979, figs.F,M, Rem.) | 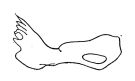 issued from : Andronov V.N. in Zool. Zh., 1986, 65 (3). [Fig.2 (19), p.459]. Mandible (gnathobase and coxa).
|
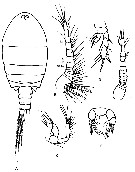 Issued from : F. Vives & A.A. Shmeleva in Fauna Iberica, 2007, 29. [p.980, Fig.566]. After Andronov, 1986 b. Female: A, habitus (dorsal); B, A1; C, A2; D, P5. Male: E, A1; F, P5.
| | | | | Compl. Ref.: | | | Gurney, 1927 d (p.45); Sewell, 1948 (p.441, 469); Costanzo & al., 2004 (p.49, N & juv.); Giacomo & al., 2005 (p.98); Zagami & al., 2008 (p.612, Rem.: egg production); Pansera & al., 2014 (p.221, Table 2, abundance); Brugnano & al., 2014 (p.215, fecundity, development vs temperature, egg-laying behaviour); Belmonte, 2018 (p.273, Table I: Italian zones) | | | | NZ: | 3 | | |
|
Distribution map of Pseudocyclops umbraticus by geographical zones
|
| | | | | |  Chart of 1996 Chart of 1996 | |
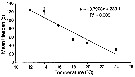 Issued from : C. Brugnano, A. Granata, L. Guglielmo, R. Minutomi & G. Zagami in Aquat. Biol., 2014, 20. [p.250, Fig.5]. Issued from : C. Brugnano, A. Granata, L. Guglielmo, R. Minutomi & G. Zagami in Aquat. Biol., 2014, 20. [p.250, Fig.5].
Relationship between temperatrure and mean lifespan for female P. umbraticus.
Circle symbols withour error bars represent interpolated values from the regression line. (d) in days.
Nota: At 12*C and 32*C, females were able to survive, but they stopped egg production. Temperature also affected female copepode lifespan, shorter at highter temperature. |
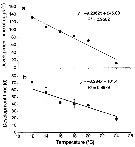 Issued from : C. Brugnano, A. Granata, L. Guglielmo, R. Minutomi & G. Zagami in Aquat. Biol., 2014, 20. [p.251, Fig.6]. Issued from : C. Brugnano, A. Granata, L. Guglielmo, R. Minutomi & G. Zagami in Aquat. Biol., 2014, 20. [p.251, Fig.6].
Relationship between temperature (a) mean development time of P. umbraticus eggs, and (b) development time from naupliar stage to adulthood.
Circle symbols without error bars in (b) represent interpolated values from the regression line. (d) in days); (h) in hours.
Nota: Development time for the eggs deereased with increasing temperature, as did development time from egg to adulthood |
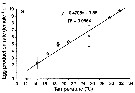 Issued from : C. Brugnano, A. Granata, L. Guglielmo, R. Minutomi & G. Zagami in Aquat. Biol., 2014, 20. [p.251, Fig.8 a]. Issued from : C. Brugnano, A. Granata, L. Guglielmo, R. Minutomi & G. Zagami in Aquat. Biol., 2014, 20. [p.251, Fig.8 a].
Relationships between temperature and mean egg production rates in (a) P. umbraticus.
Symbols without error bars represent interpolated values from the regression line.
Nota: The egg production rates in P. umbraticus is relatively low compared to other pelagic copepods, but within the ranges reported for egg-carrying species.
Within 2 to 3 days after achieving maturity, the females release eggs more or less continously throughout the day, almost until the end of their life.
The females do not release yhese eggs directly into the water; instead, after a pair of eggs is extruded from the paired gonopores, the female swims with the pair of eggs attached on either wide of the genital double-somite until the eggs are released by a rapid urosome stroke.
After releasing the egg pair, the female swims over them with a rotatory motion, apparently secreting a substance that facilitates the adhesion of the eggs to the bottom of the Petri dish or any hard surface. This adhesive substance is secreted from the female mouthparts, which become so sticky that any encountered fragments can become stuck.
By carrying the eggs, the female would be more susceptible to visually hunting predators (Webb & Weaver, 1988). The attachment of the eggs upon a substrat would be an protection with respect to predators.
For explained the competition between P. umbraticus and P. xiphophorus in the same locality (Lake Faro, NE Sicily), the authors note that P. umbraticus is more fecund at all temperatures, but less reactive than P. xiphophorus to a temperature increment of as little as 6¨C. This was demonstrated by the similar (not significantly different) egg production at 18*C and 24°C by P. umbraticus. It appears well adaptated to the high variability within shallow shore waters, which are more likely to undergo rapid changes in environmental parameters, even though it was not possible to demonstrate any significant negative effect of salinity on P. umbraticus abundance. |
| | | | Loc: | | | W Medit. (Napoli, Sicily: Lake Faro), Port Said, Ismaïlia, Suez Canal, Mauritania (Cap Blanc) | | | | N: | 9 | | | | Lg.: | | | (47) F: 0,65-0,6; M: 0,54; (487) F: 0,7-0,64; M: 0,58; 0,57; {F: 0,60-0,70; M: 0,54-0,58} | | | | Rem.: | hyperbenthic.
According to Andronov (1986 a, p.462) the specimens in Levrier Bay are practically undistinguishable from those from the naples area with the exception of the structures of the Md and the 2nd segment of the male left leg P5 exopodite which looks like a semitransparent formation with blurred contour.
After Brugnano & al. (2014), this egg-laying behaviour has never been observed before for copepods. | | | Last update : 06/02/2020 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed February 10, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |








