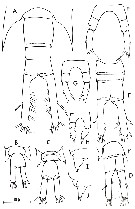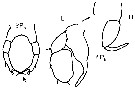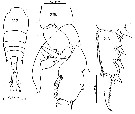|
|
 |
|
Calanoida ( Order ) |
|
|
|
Diaptomoidea ( Superfamily ) |
|
|
|
Temoridae ( Family ) |
|
|
|
Temora ( Genus ) |
|
|
| |
Temora stylifera Dana, 1849 (F,M) | |
| | | | | | | Syn.: | Temora armata Claus, 1863 (p.195); Brady, 1883 (p.80);
? no T. stylifera : Chiba, 1953 c (p.695, figs.M); 1953 e (p.722, figs.M); Chiba & al., 1957 (p.308); 1957 a (p.11); Yamazi, 1958 (p.150);
no T. stylifera : Mori, 1937 (1964) (p.66, figs. juv.F); Tanaka, 1963 (p.14, Rem.F,M); ? Anraku & Azeta, 1965 (p.13, Table 2, fish predator);
Temora discaudata : Grice & Hart, 1962 (p.287, table 3 as discaudatus) | | | | Ref.: | | | Giesbrecht, 1892 (p.328, 337, 775, figs.F,M); Giesbrecht & Schmeil, 1898 (p.101, Rem. F,M); Thompson & Scott, 1903 (p.234, 249); Wolfenden, 1911 (p.357); Sewell, 1912 (p.353, 366); 1914 a (p.227); Pesta, 1920 (p.526); Sars, 1925 (p.193); Farran, 1929 (p.209, 257); Rose, 1929 (p.28); Sewell, 1932 (p.246); Wilson, 1932 a (p.104, figs.F,M); Sewell, 1933 (p.27); Rose, 1933 a (p.170, figs.F,M); Lysholm & al., 1945 (p.29); Carvalho, 1952 a (p.147, figs.F,M); Marques, 1953 (p.106, figs.F,M); Marques, 1959 (p.213); Gaudy, 1961 (p.115, figs. Nauplius, juv.); 1962 (p.93, 99, Rem.: p.108, 128, Pl.V-VIII: Nauplius-juv., Tableau 6: development), P.145: biological annual cycle; Fish, 1962 (p.15); Kasturirangan, 1963 (p.40, 41, figs.F,M); Paiva, 1963 (p.52); ? Chen & Zhang, 1965 (p.66, figs. juv.F,M); Vervoort, 1965 (p.100, Rem.); Gonzalez & Bowman, 1965 (p.248, figs.F,M, Rem.); Ramirez, 1966 (p.13, figs.F,M); Marques, 1966 (p.5); Owre & Foyo, 1967 (p.68, figs.F,M); Vinogradov, 1968 (1970) (p.158); Vilela, 1968 (p.22, figs.M); Corral Estrada, 1970 (p.168); Ramirez, 1971 (p.84, fig.F); Björnberg, 1972 (p.28, figs., Rem.:N); Razouls, 1972 (p.94, Annexe: p.66, figs.F,juv.); Fleminger & Hulsemann, 1973 (p.344, figs.F,M, Rem., carte); Marques, 1973 (p.242); 1974 (p.15); Fleminger, 1975 (p.395, 397); Greenwood, 1978 (Rem.: p.3, 4); Schnack, 1982 (p.145, fig.Mx, Md); Sazhina, 1982 (p.1157, 1158, fig.N); Riera, 1972 (p.67, morphometry); Razouls S., 1975 (p.297: eggs.); Marques, 1982 (p.760); Riera, 1983 (p.363, Rem.: morphometric variations); Nival & Nival, 1978 (p.78, figs.F,M, Rem.: md); Goswami & Goswami, 1978 (p.11, figs.); Séret, 1979 (p.128, fig.M, Rem.); Arnaud & al., 1980 (p.213, gut structure); Björnberg & al., 1981 (p.640, figs.F,M); Koga, 1984 (p.43, figs.); Sazhina, 1985 (p.52, figs.N); Razouls S. & al., 1986 (p.875, genital system, eggs); 1987 (p.653: genital system); Schnack, 1989 (p.137, tab.1, fig.6: Md); Arcos & Fleminger, 1991 (p.1177, figs.F,M, juv.5, Rem.); Kim & al., 1993 (p.270); Bundy & Paffenhöfer, 1993 (p.3, fig.F); Barthélémy & al., 1998 (p.721, genital area); Bradford-Grieve & al., 1999 (p.884, 954, figs.F,M); Barthélémy, 1999 a (p.9, Fig.11, A); Paffenhöfer & Loyd, 1999 (p.101, Rem., figs.F); Carotenuro, 1999 (p.1613, larval stages); Paffenhöfer & Loyd, 2000 (p.171, fig.F); Corni & al., 2001 (p.79, reproductive morphology); G. Harding, 2004 (p.14, figs.F,M); Conway, 2006 (p.17, copepodides 1-6, Rem.); Ferrari & Dahms, 2007 (p.62, 63, Rem.); Avancini & al., 2006 (p.91, Pl. 60, figs.F,M, Rem.); Vives & Shmeleva, 2007 (p.524, figs.F,M, Rem.); Martinelli-Filho & al., 2009 (p.455, figs F,M: anomalies); Lacuna & al., 2013 (p.99, 105, figs.F,M, Rem.); Kos, 2016 (p.48, figs. F, M, Rem.) | 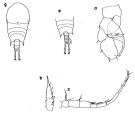 issued from : A. Fleminger & K. Hulsemann in B. Zeitschel, ed., The Biology of the Indian Ocean. 1973. [Fig. 6, p.345; Fig.7, p.346]. Female: g, habitus (dorsal view). Male: a, P5 (anterior); b, exopodite of P2 (dorsal); c, right A1; e, habitus (dorsal).
|
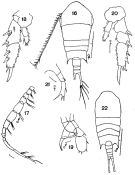
 issued from : M. Rose in Faune de France. 26. Paul Lechevalier ed., 1933. [Fig. 193, p.170]. Female and Male.
|
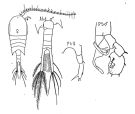
 issued from : F.C. Ramirez in Bol. Inst. Biol. Mar., Mar del Plata, 1966, 11. [Lam.V, Figs.16-22]. Female (from off Mar del Plata): 16, habitus (dorsal); 18, P2; 20, P2 (abnormal); 21, P5. Male: 17, right A1; 19, P5; 22, habitus (dorsal).
|
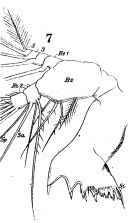 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.17, fig.7]. Female: Md (anterior view).
|
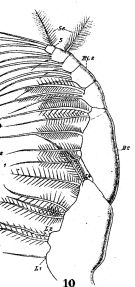 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.17, fig.10]. Female: 10, Mxp (anteror view).
|
 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.17, fig.13]. Female: 13, P4 (posterior view).
|
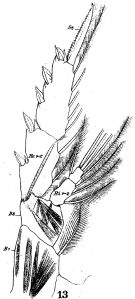
 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.17, fig.22]. Female: 22, P5.
|
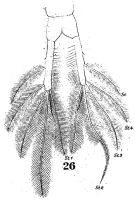 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.38, fig.26]. Female: 26, anal segment and caudal rami (ventral).
|
 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.17, fig.2]. Male: 2, terminal seta of exopodite 3 of P1.
|
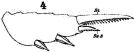 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.17, fig.4]. Male: 4, exopodite 3 of P2 (anterior view).
|
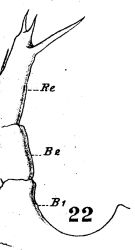
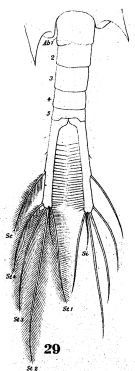 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.38, fig.29]. Male: 29, last thoracic segment and urosome (dorsal).
|
 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.17, fig.6]. Male: right A1 (posterior view).
|
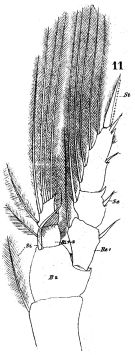 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.17, fig.11]. Male: 11, P1 (posterior view).
|
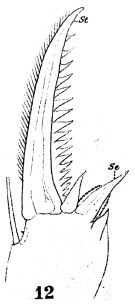 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.17, fig.12]. Male: 12, terminal portion of exopodite 3 of right P2.
|
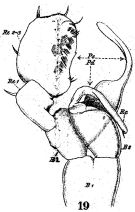 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.17, fig.19]. Male: 19, P5 (anterior view).
|
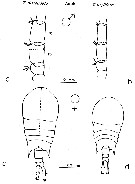 issued from : F. Arcos and A. Fleminger in J. Plankton Res., 1991, 13 (6). p.1183, Fig.5]. Comparison between T. discaudata (from W coast of Mexico) and T. stylifera (from Caribbean Sea off Honduras). a, b, right A1 male (segments 14-16); c, d, habitus (dorsal).
|
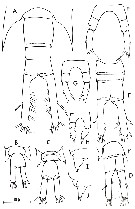 issued from : C. Razouls in Th. Doc. Etat Fac. Sc. Paris VI, 1972, Annexe. [Fig.50]. Female (from Banyuls): F, P5. Copepodite stage 1: B, corners of the distal metasomal segment and urosome (dorsal); I, I, left corner. Cpepodite stage 2: C, same; J, corner. Copepodite stage 3: D, same. Copepodite stage 4: E, same; H, P5. Copepodite stage 5: A, same; G, P5
|
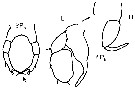 issued from : G. Harding in Key to the adullt pelagic calanoid copepods found over the continental shelf of the Canadian Atlantic coast. Bedford Inst. Oceanogr., Dartmouth, Nova Scotia, 2004. [p.14]. Female: P5. Nota: inner terminal spine longer than other three. Male: P5 (L = left leg; R = right leg).
|
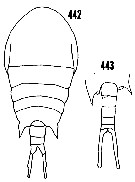 issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1, Crustacea, 1: Copepoda. Copepods of the Florida Current. [p.69, Figs.442, 443]. Female: 442, habitus (dorsal). Male: corners of the last thoracic segment and urosome (dorsal).
|
 issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1, Crustacea, 1: Copepoda. Copepods of the Florida Current. [p.70, Figs.449-451]. Female: 449, P5. Male: 450, right P5; 451, left P5.
|
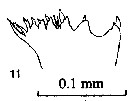 issued from : P.E. Lapernat & C. Razouls in Vie Milieu, 2002, 52 (1). [p.28, Pl. VI, fig.11]. Masticatory edge of Md gnathobase female (from off Malta, Mediterranean Sea). Nota: Itoh's index value of the mandibular gnathobase = 746.8 (number of teeth : 8), and 813.3 (number of teeth : 9); and after Schnack (1989) the Itoh's index value = 798.
|
 issued from : S.B. Schnack in Crustacean Issue, 1989, 6. [p.143, Fig.6: 10]. 10, Temara stylifera (from off NW Africa, upwelling region): Cutting edge of Md.
|
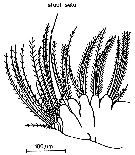 issued from : S.B. Schnack in Crustacean Issue, 1989, 6. [p.147, Fig.9]. Mx2. Nota: Temora species are apparently able to switch from one feeding mode (herbivore and omnivore).: the Maxillae bear a single setule-free, long, and powerful seta suitable for grasping prey items (see Schnack, 1982 a). besides this seta, Mx2 has small intersetule distances (between 4 and 11 micometers).
|
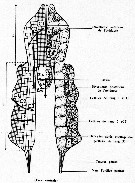 issued from : S. Razouls, S. Nival & P. Nival in J. Plankton Res., 8 (5) [p.879, Fig.2) Schematic representation of the female gonad and evolution of ovocytes. 3-9: index of measures (length-width). Zm = multiplication zone; Zc = growth zone; C1, C2, C3 = different stages of vitellogenesis maturation.
|
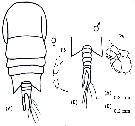 issued from : C. de O. Dias & A.V. Araujo in Atlas Zoopl. reg. central da Zona Econ. exclus. brasileira, S.L. Costa Bonecker (Edit), 2006, Série Livros 21. [p.67]. Female and Male.
|
 issued from : A. Ianora, B. Scotto di Carlo & P. Mascellaro in Mar. Biol., 1989, 101. [p.189, Fig.2]; Temora stylifera females from Gulf of Naples (Italy): Unripe female with transparent gonads (a) and dark (b) ripe female wit gonads containing mature oocytes.
|
 issued from : A. Ianora, B. Scotto di Carlo & P. Mascellaro in Mar. Biol., 1989, 101. [p.190, Fig.3]; Temora stylifera females from Gulf of Naples (Italy). Histological sections: a, previtellogenic phase of oogenic cycle, corresponding to light or unripe females; b, vitellogenic phase with dense yolk granules filling oocytes running parallel to inner margins of oviducal diverticulae, corresponding to semi-dark females; c, final vitellogenic phase with diverticulae containing mature oocytes, corresponding to dark or ripe females; d, moment of egg-laying and ensuing light or unripe gonadal condition. i.o. = immature oocytes; m.o. = mature oocytes; m.g. = mid-gut; h.g. = hind-gut; m.b. = muscle bands.
|
 issued from : J.E. Martinelli-Filho, M. Melo-Junior, D.R. Cunha & R.M. Lopes in Braz. J. Biol., 2009, 69 (2). [p.455, Fig.1]. Anomalous male from Temora stylifera (from Southern Brazilian Bight), bearing geniculation on both A1. Nota: Geniculation in both A1 is common in cyclopoid families, but not in calanoids.
|
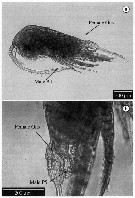 issued from : J.E. Martinelli-Filho, M. Melo-Junior, D.R. Cunha & R.M. Lopes in Braz. J. Biol., 2009, 69 (2). [p.456, Fig.2]. Aberrant individual displaying male characters and a female double-genital somite complex (Gns) from Temora stylifera (from Southern Brazilian Bight): a, whole specimen; b, detail of female Gns and male P5. Nota: The animal shows a female genital somite and reproductive structures including ovary and oviducts. The remaining sexual characters such as A1 and P5 are typically male. The gynandromorphic individual anomaly was not induced by parasites (no evidence of infections); Intersexuality is often found in P5 (see Ianora & al., 1987), or A1 (see Fleminger, 1985) rather than on the genital somite. Intersex individuals were described in sevaral calanoid families (Acartiidae, Calanidae, Diaptomidae, Euchaetidae, Metridinidae and Paracalanidae. Similar data has not been found for the Temoridae and the authors cannot confirm that the gynandromorphic specimen is an intersex. The fact that A1 and P5 are identical to male appendages is not an ordinary pattern in intersex as well.
|
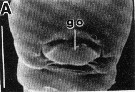 issued from : R.-M. Barthélémy in Thèse Doct. Univ. Provence (Aix-Marseille I), 1999. [Fig.11, A]. Female (from Gulf of Marseille, NW Mediterranean Sea): A, external ventral view genital double-somite. go = genital operculum. Scale bar: 0.050 mm.
|
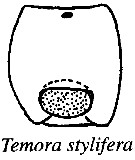 issued from : R.-M. Barthélémy, C. Cuoc, D. Defaye, M. Brunet & J. Mazza in Phil. Trans. R. Soc. Lond., B, 1998, 353. [p.733, Fig.63]. Schematic representation of external genital area in the species studied; Dashed line, limit of the anterior pad and lateral thickenings; shading, posterior pad; dots, genital operculum.
|
 issued from : M.L.D.G. Lacuna, D.C. Sagrado, N.B. España, D.D. Simyunn & R.O. Mejorada in ABAH Bioflux, 2013, 5 (1). [p.108, Fig.11, e; p.109, Fig.12, e]. Female (from Mindanao bays); F: P5. Male: M, P5.
|
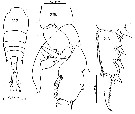 Issued from : C. Séret in Thesis 3ème Cycle, UPMC, Paris 6. [Pl. XXXIV, Figs.217-219]. Male (from off Kerguelen Is.): 217, habitus (dorsal); 218, exopodal segment 3 of P2; 219, P5.
|
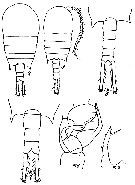 Issued from : M.S. Kos in Zoological Institut RAS, St. Petersburg, 2016, 179. [p.49, Fig. 22]. Temora stylifera female and male from 39°34' N, 147°47' E.
| | | | | Compl. Ref.: | | | Cleve, 1904 a (p.198); Carl, 1907 (p.16); Sewell, 1912 (p.366); Carazzi & Grandori, 1912 (p.12, 38); Chatton, 1920 (p.17); Rose, 1924 d (p.479); Gurney, 1927 (p.151, Rem.); Wilson, 1942 a (p.209); Massuti Alzamora, 1942 (p.91); Oliveira, 1945 (p.191); Sewell, 1948 (p.391, 406); C.B. Wilson, 1950 (? part., p.343); Krishnaswamy, 1953 (p.125); Deevey, 1960 (p.5, Table II, annual abundance); Cervigon, 1962 (p.181, tables: abundance distribution); Grice & Hart, 1962 (p.287, table 3); V.N. Greze, 1963 a (tabl.2); Shmeleva, 1963 (p.141); Gaudy, 1963 (p.24, Rem.); Björnberg, 1963 (p.46, Rem.); Mazza, 1964 (p.293, weight); De Decker & Mombeck, 1964 (p.14); Roehr & Moore, 1965 (p.565, vertical distribution); Shmeleva, 1965 b (p.1350, lengths-volume-weight relation); Neto & Paiva, 1966 (p.26, Table III, annual cycle); Vives, 1966 (p.81); Pavlova, 1966 (p.43); Mazza, 1966 (p.71); 1967 (p.356, 377, 399, fig.76); Ehrhardt, 1967 (p.738, geographic distribution, Rem.); Séguin, 1968 (p.488); Evans, 1968 (p.15); Delalo, 1968 (p.138); Berdugo & Kimor, 1968 (p.447); Champalbert, 1969 a (p.636); Moraitou-Apostolopoulou, 1969 (p.2, fig.1); El-Maghraby & Dowidar, 1970 (p.81); Dowidar & El-Maghraby, 1970 (p.269); M. Bernard, 1970 (p.75); Park, 1970 (p.476); Shih & al., 1971 (p.49); Salah, 1971 (p.319); Deevey, 1971 (p.225); Gamulin, 1971 (p.381, tab.2); Carli, 1971 (p.372, tab.1); Gaudy, 1971 (p.65, Tabl. 1, fig.1, egg production); 1972 (p.175, 200, figs.13-18, annual cycle); Bainbridge, 1972 (p.61, Appendix Table I: vertical distribution vs. day/night, Table II: %), Table IV; Brodsky, 1972 (p.256); Binet & al., 1972 (p.71); Della Croce & al., 1972 (p.1, Rem.); Riera, 1972 (p.67, morphometry); Razouls S., 1972 (p.95, metabolism); 1972 a (p.145, respiration); 1972 b (p.2, respiration); Champalbert & Gaudy, 1972 (p.159, respiration vs temperature); Valentin, 1972 (p.377: egg diameter = 80 µm); Ibanez & Seguin, 1972 (p.81, annual cycle, multivarite analysis); Nival & al., 1972 (p.63, respiration); Apostolopoulou, 1972 (p.328, 352, fig.4); Boucher & Thiriot, 1972 (p.47, Tableau 4); Roe, 1972 (p.277, tabl.1, tabl.2); C. Razouls & Guinness, 1973 (p.413, size variations & temperature); Champalbert & al., 1973 (p.529, CHN composition); Gaudy, 1973 (p.267, Table I, II, fig.2, 5, respiration); P. Nival & S. Nival, 1973 (p.135, mouth parts, grazing); Desgouille, 1973 (p.1, 131, Rem.: p.138); Guglielmo, 1973 (p.399); C. Razouls, 1973 a (p.361, annual abundance); 1974 (p.51, life history); S. Razouls, 1974 (147, oxygen rate); Corral Estrada & Pereiro Muñoz, 1974 (tab.I); Nival & al., 1974 (p.231, respiration & excretion); Gaudy, 1975 (p.109, Table I, respiration); Le Ruyet-Person & al., 1975 (p.283, comparative biology, metabolism activity); S. Razouls, 1975 (p.297, egg production); Vives & al., 1975 (p.43, tab.II, III, IV); Boucher & al., 1975 (p.85, nutrition/enzyme); Fernandez, 1975 (p.1, fig.1, 2, 3, 4, metabolism/lux), Boucher & al., 1976 (p.61, elementary composition); Lakkis & Abboud, 1976 (p.81); Razouls & Razouls, 1976 (p.281, annual variations); Boucher & al., 1976 (p.61, 62); C. Razouls & S. Razouls, 1976 (p.281, caloricity, growth); S. Razouls, 1977 (p.265, caloricity); Weikert, 1977 (p.351, Rem.: p.353, exuviae); Deevey & Brooks, 1977 (p.256, tab.2, Station "S"); Madhu Pratap & al., 1977 (p.138, Table 3: abundance vs. stations); Scaramuzza, 1978 (p.17); Paffenhöfer & Knowles, 1978 a (p.143, feeding); Fernandez, 1978 (p.97, metabolism/food, Rem.: Table 19); S. Razouls & Apostolopoulou, 1978 (p.13, metabolism); Binet, 1979 (p.400); Dessier, 1979 (p.100, 201, 206); Paffenhöfer & Knowles, 1979 (p.35, fecal pellets); Stephen & Iyer, 1979 (p.228, tab.1, 3, 4, figs.3, 4); Vaissière & Séguin, 1980 (p.23, tab.2); Andronov & Maigret, 1980 (p.71, Table 1, 2, 3); Paffenhöfer & Knowles, 1980 (p.355, feeding); Yassen, 1981 (p.125, rearing, mortality rate); Sreekmaran Nair & al., 1981 (p.493, Fig.2); Madhupratap & al., 1981 (p.266, fig.2h: abundance vs. geographic transect); Kovalev & Shmeleva, 1982 (p.84); Vives, 1982 (p.293); Castel & Courties, 1982 (p.417, Table II, spatial distribution); Boucher, 1982 a (p.199, fig.2); Yassen, 1982 (p.125, culture, mortality rate); Riera, 1983 (p.363, biometry); Abou Debs & Nival, 1983 (p.125, egg production vs. diet, temperature); Rivière, 1983 (p.19, enzymes); Greze & al., 1983 (p.17, Rem.: p.24); Turner & Dagg, 1983 (p.16, 22); Moore E.A. & Sander, 1983 (p.113, fig.1: variation % sex ratio); De Decker, 1984 (p.316, 364: chart); Tremblay & Anderson, 1984 (p.7); Turner, 1984 a (p.73, Table 1, fig.4-7: pellet contents); Scotto di Carlo & al., 1984 (1041); Cummings, 1984 (p.163, Table 2); ? Guangshan & Honglin, 1984 (p.118, tab.); Miller & al., 1984 (p.1274, Rem.: p.1278, molting rates); Abou Debs, 1984 (p.213, egg & fecal pellets porduction, respiration rate, C & N budget vs. diet); Gruzov, 1985 (p.633, age dependant feeding); Regner, 1985 (p.11, Rem.: p.34); Brenning, 1985 (p.5, spatial distribution/TS); 1985 a (p.23, Table 2); Jansa, 1985 (p.108, Tabl.I, II, III, IV, V); Petipa & Borichenko, 1985 (tab.2); Gaudy, 1985 (p.279, Tab.3); Garcia-Rodriguez, 1985 a (p.41, 42); Moraitou-Apostolopoulou, 1985 (p.303, occurrence/abundance in E Mediterranean Sea); Valentin & al., 1986 (p.117, Table V); Riera & Alcaraz, 1986 (p.127, biometry); Ambler, 1986 a (p.957, fig.7, selectivity); Comaschi Scaramuzza, 1987 (tab.1); Boucher & al., 1987 (p.133, spatial distribution/physical structure); Lozano Soldevilla & al., 1988 (p.59); Daan & al., 1988 (p.45, carnivorous behaviour); Ianora & al., 1989 (p.187, reproduction); Kouwenberg & Razouls, 1990 (p.23, climatic change); Suarez & al., 1990 (tab.2); Yoo, 1991 (tab.1); Lindo, 1991 (tab.3); Suarez & Gasca, 1991 (tab.2); Samba Diouf, 1991 (p.104); Carlotti & Nival, 1991 (p.801, variability of development); Suarez, 1992 (App.1); Huntley & Lopez, 1992 (p.201, Table 1, eggs, temperature-dependent production); C. Razouls & S. Razouls, 1992 (p.259, life history); Ayukai & Hattori, 1992 (p.163, Table 5, fecal pellet production rate); Kouwenberg, 1993 (p.215, fig.3, seasonal abundance); 1993 a (p.281, fig.3, 4, sex ratio); Ianora & Poulet, 1993 (p.1615, egg production & viability/food); Seguin & al., 1993 (p.23); Lopes, 1994 (tab.1); Kouwenberg, 1994 (tab.1); Hajderi, 1995 (p.542); Webber & Roff, 1995 (tab.1); Shih & Young, 1995 (p.74); Roff & al., 1995 (p.165, Table 5: naupllii vs. bacteriophagy/picoplankton); Kotani & al., 1996 (tab.2); Webber & al., 1996 (tab.1); Jamet & Ferec-Corbel, 1996 (p.17, tab.1); Paffenhöfer & al., 1996 (p.1699, motion behavior); Ban S, Burns C. & al., 1997 (p.287, fig.1, Table 1, 2, feeding, reproduction); Suarez-Morales & Gasca, 1997 (p.1525); Park & Choi, 1997 (Appendix); Hure Krsinic, 1998 (p.59, 101); Gilabert & Moreno, 1998 (tab.1, 2); Lopes & al., 1998 (p.195); Alvarez-Cadena & al., 1998 (tab.1,3,4); Kouwenberg, 1998 (p.203, seasonal variation, fig.3: global change); Vigoni & al., 1998 (tab.2); Hopcroft & al., 1998 (tab.2); Noda & al., 1998 (p.55, Table 3, occurrence); Hsieh & Chiu, 1998 (tab.2); Suarez-Morales, 1998 (p.345, Table 1); Suarez-Morales & Gasca, 1998 a (p.111); Mauchline, 1998 (tab.8, 16, 21, 25, 26, 27, 30, 38, 45, 47, 48, 58, 61, 62, 63); Lopes & al., 1999 (p.215, tab.1); Neumann-Leitao & al., 1999 (p.153, tab.2); Miralto & al., 1999 (p.173, Rem.: p.175, egg production vs diatoms effect of inhibition); Lapernat, 1999 (p.29, 55); Siokou-Frangou, 1999 (p.476); Lapernat, 2000 (tabl.3, 4); El-Sherif & Aboul Ezz, 2000 (p.61, Table 3: occurrence); Suarez-Morales & Gasca, 2000 (1247, tab.1); Seridji & Hafferssas, 2000 (tab.1); Plounevez & Champalbert, 2000 (p.175, Table III, IV, V, abundance vs fish, Rem.: p.185); Pakhomov & al., 2000 (p.1663, Table 2, transect Cape Town-SANAE antarctic base); Lopez-Salgado & al., 2000 (tab.1); d'Elbée, 2001 (tabl. 1); Lapernat & Razouls, 2001 (p.123, tab.1); Pardal & Azeiteiro, 2001 (p.25); El-Serehy & al., 2001 (p.116, Table 1: abundance vs transect in Suez Canal); Calbet & al., 2001 (p.319, Fig.7); Halsband-Lenk & al., 2001 (p.597, seasonal cycle, egg production); Zerouali & Melhaoui, 2002 (p.91, Tableau I); Beaugrand & al., 2002 (p.179, figs.5, 6); Bressan & Moro, 2002 (tab.2); Dunbar & Webber, 2003 (tab.1); Vukanic, 2003 (139, tab.1); Bode & al., 2003 (p.85, Table 1, abundance); Fernandez de Puelles & al., 2003 (p.123, fig.5); Ceballos & Ianora, 2003 (p.189, egg production); Hsiao & al., 2004 (p.326, tab.1); Hsieh & al., 2004 (p.397, tab.1, p.398: Rem., p.399, tab.2); Rezai & al., 2004 (p.486, p.490: tab.2, p.495: tab.8, abundance); Chang & Fang, 2004 (p.456, tab.1); Daly Yahia & al., 2004 (p.366, fig.4, tab.1); Lan & al., 2004 (p.332, tab.1); Di Capua & Mazzocchi, 2004 (p.632); Halsband-Lenk & al., 2004 (p.709, figs.6,7); CPR, 2004 (p.63, fig.189); Vukanic & Vukanic, 2004 (p.9, tab. 3); Wang & Zuo, 2004 (p.1, Table 2, dominance, origin); Dias & Bonecker, 2005 (p.100 + poster); Di Capua & al., 2005 (p.72 + poster); Lakkis & al., 2005 (p.152); Uriarte & Villate, 2005 (p.863, tab.I); Frangoulis & al., 2005 (p.254, Table I: C/N/P fecal pellet composition); Molinero & al., 2005 (p.640, climate forcing); Lindley & Daykin, 2005 (p.869, occurrence between 1959 to 2000); Zuo & al., 2006 (p.159, tab.1, abundance, fig.8: stations group); Isari & al., 2006 (p.241, tab.II); Sterza & Fernades, 2006 (p.95, Table 1, occurrence); Dias & Araujo, 2006 (p.67, Rem., chart); Zervoudaki & al., 2006 (p.149, Table I); De Olazabal & al., 2006 (p.966); Koppelmann & Weikert, 2007 (p.266: tab.3); Fernandez de Puelles & al., 2007 (p.338, fig.7); Albaina & Irigoien, 2007 (p.435: Tab.1); Valdés & al., 2007 (p.104: tab.1, figs.7, 8); Khelifi-Touhami & al., 2007 (p.327, Table 1); Busatto, 2007 (p.26, Tab.2); Dur & al., 2007 (p.197, Table IV); Aldamman, 2008 (p.1-154); Kiørboe, 2008 (p.155, Table 2, swimming speed); Cabal & al., 2008 (289, Table 1); Rossi, 2008 (p.90: Tableau XII); Gugliandolo & al., 2008 (p.580, bacteria assiociated); Jerling, 2008 (p.55, Tabl.1); Neumann-Leitao & al., 2008 (p.799: Tab.II, fig.6); Goetze & Kiørboe, 2008 (p.185, mating recognition); Fernandes, 2008 (p.465, Tabl.2); Muelbert & al., 2008 (p.1662, Table 3); Ayon & al., 2008 (p.238, Table 4: Peruvian samples); C.-Y. Lee & al., 2009 (p.151, Tab.2); Miyashita & al., 2009 (p.815, Tabl.II); Zhang W & al., 2009 (p.261); Brugnano & al., 2010 (p.312, Table 2, 3); Hafferssas & Seridji, 2010 (p.353, Table 2); Eloire & al., 2010 (p.657, Table II, temporal variability); Lidvanov & al., 2010 (p.356, Table 3); Drira & al., 2010 (p.145, Tanl.2); Cornils & al., 2010 (p.2076, Table 3); Schnack-Schiel & al., 2010 (p.2064, Table 2: E Atlantic subtropical/tropical); Hidalgo & al., 2010 (p.2089, Table 2); Dias & al., 2010 (p.230, Table 1); Xu & Gao, 2011 (p.514, figs.3, 4, Table 2: optimal salinity); Mazzocchi & Di Capua, 2010 (p.428); Medellin-Mora & Navas S., 2010 (p.265, Tab. 2); Yen & Lasley, 2010 (p.177, Rem.: p.183, mating): Hafferssas & al., 2010 (p.1281, Table III, abundance vs spatial distribution); Fazeli & al., 2010 (p.153, Table 1); W.-B. Chang & al., 2010 (p.735, Table 2, 4, abundance); Ianora & Miralto, 2010 (p.493, Table I: reproductive responses vs diatom diets); Nowaczyk & al., 2011 (p.2159, Table 2); Shanthi & Ramanibai, 2011 (p.132, Table 1); Hsiao S.H. & al., 2011 (p.475, Appendix I); Maiphae & Sa-ardrit, 2011 (p.641, Table 2, 3, Rem.); Magris & al., 2011 (p.260, abundance, interannual variability); Costa R.G. da & al., 2011 (p.364, Table 1, seasonal occurrence); Moscatello & al., 2011 (p.80, Table 4); Selifonova, 2011 a (p.77, Table 1, alien species in Black Sea); Barreiro & al., 2011 (p.13, egg production, hatching success, ingestion rate vs food selection); Mazzocchi & al., 2011 (p.1163, Table I, II, fig.6, long-term time-series 1984-2006); Andersen N.G. & al., 2011 (p.71, Fig.3: abundance); Isari & al., 2011 (p.51, Table 2, abundance vs distribution); Ianora & al., 2011 (p.265, diet vs egg production, egg viability, fecal pellets production, survival); ); Tutasi & al., 2011 (p.791, Table 2, abundance distribution vs La Niña event); Delpy & al., 2012 (p.1921, Table 2); Shiganova & al., 2012 (p.61, Table 4); Uysal & Shmeleva, 2012 (p.909, Table I); Almeida LR. & al., 2012 (p.13, Table 1, abundance); Salah S. & al., 2012 (p.155, Tableau 1); Zizah & al., 2012 (p.79, Tableau I, Rem.: p.89); Miloslavic & al., 2012 (p.165, Table 2, transect distribution); Vidjak & al., 2012 (p.243, Rem.: p.254); Aubry & al., 2012 (p.125, table 1, 3, fig. 6, 8 a, b, interannual variation); Johan & al., 2012 (2013) (p.1, Table 1); Turner & al., 2012 (p.63, toxic dinoflagellate effects); Drillet & al., 2012 (p.155, Table 1, culture); Bode & al., 2012 (p.108, spatial distribution vs time-series, % biomass); Mazzocchi & al., 2012 (p.135, annual abundance 1984-2006); Miyashita & al., 2012 (p.1557, Table 2: occurrence); Neffati & al., 2012 (p.80, egg production & nauplii survival vs environmental factors); Buttino & al., 2012 (p.247, culture); Gusmao & al., 2013 (p.279, Table 4, sex ratio); Nikolioudakis & al., 2012 (p.173, Table 2: Ivelv's selectivity index); Gubanova & al., 2013 (in press, p.4, Table 2); Belmonte & al., 2013 (p.222, Table 2, abundance vs stations); Teuber & al., 2013 (p.1, Table 1, 2, abundance vs oxygen minimum zone, respiration rates); Jagadeesan & al., 2013 (p.27, Table 3, fig.11, seasonal abundance); Anjusha & al., 2013 (p.40, Table 3, abundance & feeding behavior); Sobrinho-Gonçalves & al., 2013 (p.713, Table 2, fig.8, seasonal abundance vs environmental conditions); Terbiyik Kurt & Polat, 2013 (p.1163, Table 2, fig.9, seasonal distribution); Lidvanov & al., 2013 (p.290, Table 2, % composition); El-Serehy & al., 2013 (p.2099, Rem.: p.2101, as Tempora stylifera); Varadharajan & Soundarapandian, 2013 (p.2: occurrence vs stations); Fernandez de Puelles & al., 2014 (p.82, Table 3, seasonal abundance); Bonecker & al., 2014 (p.445, Table II: frequency, horizontal & vertical distributions); Pansera & al., 2014 (p.221, Table 2, abundance); Zakaria, 2014 (p.3, Table 1, abundance vs. 1960-2000); Zaafa & al., 2014 (p.67, Table I, occurrence); Mazzocchi & al., 2014 (p.64, Table 3, 4, 5, spatial & seasonal composition %); Dias & al., 2015 (p.483, Table 2, abundance, biomass, production, Table 4: % vs. season, fig.8); Araujo & al., 2016 (p.1, Table 3, abundance, %) ; Zakaria & al., 2016 (p.1, Table 1, 3); Benedetti & al., 2016 (p.159, Table I, fig.1, functional characters); Marques-Rojas & Zoppi de Roa, 2017 (p.495, Table 1); Atique & al., 2017 (p.1, Table 1); El Arraj & al., 2017 (p.272, table 1, 2, spatial distribution); Benedetti & al., 2018 (p.1, Fig.2: ecological functional group); Chaouadi & Hafferssas, 2018 (p.913, Table III, fig.4, 5: occurrence, abundance, seasonal variation); Belmonte, 2018 (p.273, Table I: Italian zones); Dias & al., 2018 (p.1, Tables 2, 4, 5: vertical distribution, abundance vs. season); Dias & al., 2018 a (p.189, Rem.: p.196); Hure M. & al., 2018 (p.1, Rem: p.11); Yebra & al., 2019 (p.1, Table 4: length, width, juv., F, M); Richirt & al., 2019 (p.3, Table 1, figs. 2, 3, 4, 5, abundance changes vs. years 1998-2014, table 2: diversity index); Acha & al., 2020 (p.1, Table 3: occurrence % vs. ecoregions, Table 5: indicator ecoregions). | | | | NZ: | 14 + 1 doubtful | | |
|
Distribution map of Temora stylifera by geographical zones
|
| | | | | | | | | | | |  Chart of 1996 Chart of 1996 | |
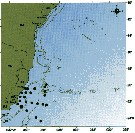
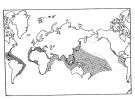 Distribution of Temora discaudata (hatching) and T. stylifera (cross-hatching); modified from Fleminger and Hulsemann; issued from : F. Arcos and A. Fleminger in J. Plankton Res., 1991, 13 (6). [Fig.7, p.1184] Distribution of Temora discaudata (hatching) and T. stylifera (cross-hatching); modified from Fleminger and Hulsemann; issued from : F. Arcos and A. Fleminger in J. Plankton Res., 1991, 13 (6). [Fig.7, p.1184] |
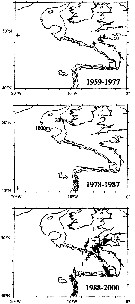 issued from : J.A. Lindley & S. Daykin in ICES Journal. Mar. Sc., 2005, 62. [p.871, Fig.1]. issued from : J.A. Lindley & S. Daykin in ICES Journal. Mar. Sc., 2005, 62. [p.871, Fig.1].
The geographical distribution of occurrences of Temora stylifera in CPR samples east of 20°W in the years 1959-1977, 1978-1987, and 1988-2000. Note that the Iberian coastal waters south of 42°N were only sampled from 1978 to 1986 and from 1997 onwards and the Bay of Biscay east of the line between Ushant and Cape Finisterre was sampled only on a few occasions in 1958 and 1959 and regularly from mid-1997 onwards.
See chart in Centropages chierchiae |
 issued from : A.A. Shmeleva in Bull. Inst. Oceanogr., Monaco, 1965, 65 (n°1351). [Table 6: 24 ]. Temora stylifera (from South Adriatic). issued from : A.A. Shmeleva in Bull. Inst. Oceanogr., Monaco, 1965, 65 (n°1351). [Table 6: 24 ]. Temora stylifera (from South Adriatic).
Dimensions, volume and Weight wet. Means for 50-60 specimens. Volume and weight calculated by geometrical method. Assumed that the specific gravity of the Copepod body is equal to 1, then the volume will correspond to the weight. |
 issued from : C. de O. Dias & A.V. Araujo in Atlas Zoopl. reg. central da Zona Econ. exclus. brasileira, S.L. Costa Bonecker (Edit), 2006, Série Livros 21. [p.67]. issued from : C. de O. Dias & A.V. Araujo in Atlas Zoopl. reg. central da Zona Econ. exclus. brasileira, S.L. Costa Bonecker (Edit), 2006, Série Livros 21. [p.67].
Chart of occurrence in Brazilian waters (sampling between 22°-23° S).
Nota: sampling 131 specimens. |
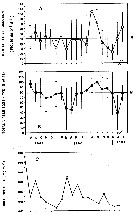
 issued from : R. Gaudy in Rec. Trav. St. Mar. End., 1962, 27 (42). [p.146, Fig.5]. issued from : R. Gaudy in Rec. Trav. St. Mar. End., 1962, 27 (42). [p.146, Fig.5].
Population dynamics of Temora stylifera in the Gulf of Marseille (43°15'30''N, 5°17'02''E) during 1960-1961.
A: Frequenciy distributions of size classes (cephalothoracic lengths females from 1 to 10); B: Number of adults (males and females) and nauplii by haul; C: Percentage of the different stages (N: naulii, 1-5: copepodites, 6: adults); D: Interpretation of successive generations.
Class of sizes (in mm): 1 = 0.800-0.849; 2 = 0.850-0.899; 3: 0.900-0.949; 4 = 0.950-0.999; 5: 0.1.000-1.049; 6 =1.050-1.099; 7 = 1.100-1.149; 8 = 1.150-1.199; 9 = 1.200-1.249; 10 = 1.250-1.299.
Gaudy (p.138) points to 5 generations by year in the Gulf of Marseille (NW Mediterranean Sea). |
 issued from : J.T. Turner in Mar. Biol., 1984 a, 82. [p.79, Fig.5]. issued from : J.T. Turner in Mar. Biol., 1984 a, 82. [p.79, Fig.5].
Contents of fecal pellets of Temora stylifera females from the Station in slope water (off W Mississipi River mouth) on 9 December 1945.
a: intact Thalassiosira sp. cell (T); b: fragmented Thalassiosira sp.cells (T) and Chaetoceros spp. spines (Ch); c and d: fragmented Thalassiosira sp. cells (T) showing inner and outer surfaces; e: intact Ciscinodiscus sp. cell (C); f: fragments of Coscinodiscus sp. (C) and Skeletonema costatum (S) cells. |
 issued from : U. Brenning in Wiss. Z. Wilhelm-Pieck-Univ. Rostock -34. Jahrgang 1985. Mat.-nat. wiss. Reihe, 6. [p.10, Figs.6, 7]. issued from : U. Brenning in Wiss. Z. Wilhelm-Pieck-Univ. Rostock -34. Jahrgang 1985. Mat.-nat. wiss. Reihe, 6. [p.10, Figs.6, 7].
Spatial distribution and T-S Diagram for Temora stylifera from from 8° S - 26° N; 16°- 20° W for different expeditions (V1: Dec. 1972-Jan. 1973; V2: Feb./mar. 1973; VI: May 1974; IV: Jun./Jul. 1972).
Nota: SO: Southern Surface Water (S °/oo: 34,50; T°C: 29,0); ND: Northern Water of the Surface Layer (S °/oo: 37,5; T°C: 21,0); SD: Southern Deep Water of the surface layer (S °/oo: 35,33; T°C: 13,4).
Water types basing on the works of Sverdrup & al. (1942), Wolf (1978) and Wolf & Kaiser (1978) distinguishing various quasi-permanent water types in the top 200 m of the water column in the NW Africa upwelling region. The water type SD, which usually forms the upper limit of the SACW (South Atlantic Central water: S °/oo: 34,35-35,33 & T°C: 5,15-13,4), can enter the surface layer as a result of upwelling, so that water types ND, SD and SO can be expected in this layer in upwelling regions. The boundary separating the NACW (North Atlantic Central Water: S °/oo: 35,10-37,35; T°C: 8,0-21,0) and SACW water masses is situated in the region between Bahia de Gorrei and Cap Blanc (Mauritania) throughout the whole year. The distribution of the water types SACW in the surface layer off North West Africa varies with the season and the meridional migration of the wind field. See in Brenning (1985 a, p.6).
Nota: Brenning (1985 a, p.28), taking into account the seasonal temperature variations, the number of generations to be expected in a given region when the duration of upwelling in the region concerned is known, obtained: ± 15 generations at Nouachott (Mauritania), ± 12 at Cap Vert (Dakar) and ± 10 at Cap Roxo (Casamance). Binet (1977), for instance, counting 25 generations in 14 months, i.e. 1 generation every 17 days, off the Ivory Coast. |
 issued from : U. Brenning in Wiss. Z. Wilhelm-Pieck-Univ. Rostock -34. Jahrgang 1985. Mat.-nat. wiss. Reihe, 6. [p.7, Fig.4]. issued from : U. Brenning in Wiss. Z. Wilhelm-Pieck-Univ. Rostock -34. Jahrgang 1985. Mat.-nat. wiss. Reihe, 6. [p.7, Fig.4].
Vertical distribution for Temora stylifera from from NW Africa.
T: daylight; N: night. |
 issued from : A. Ianora & S.A. Poulet in Limnol. Oceanogr., 1993, 38 (8). [p.1617, Fig.1]. issued from : A. Ianora & S.A. Poulet in Limnol. Oceanogr., 1993, 38 (8). [p.1617, Fig.1].
Field estimates of seasonal variations in mean monthly egg production rate (A) and egg viability (B) of Temora stylifera and chlorophyll a (C) sampled in the Bay of Naples (Italy).
M: mean of monthly values; bars: standard deviation of the monthly mean.
Nota: In the study, the authors show that egg production and egg viability are related to each other but not to phytoplankton stocks as determinated by in itu Chl a concentrations. It follows that the fecundity and egg viability-phytoplankton relationship is not reliable, because Chl a gives no indication of the composition and properties of the food (chemical composition: C:N ratio, proteins, free amino acids, vitamins, lipids). Also this species is omnivorous. |
 issued from : C. Razouls in Cah. Biol. Mar., 1974, 15. [p74, Fig.7] issued from : C. Razouls in Cah. Biol. Mar., 1974, 15. [p74, Fig.7]
Life history pattern of Centropages typicus at Banyuls-sur-Mer (western Gulf of Lion, Mediterranean Sea) from 1965 to 1969 in relation to the mean temperature (depth 50 m). Arrowhead denote the maximum of adults. J in days
Nota: The number of generations is etimated betwenn 6 or 7 according to the years. A partir from the variations of copepodites (Ci to C5) and adults, the class sizes and the development times. |
 issued from : C. Razouls & C. Guiness in Cah. Biol. Mar., 1973, 14. [p.422, Fig.6]. issued from : C. Razouls & C. Guiness in Cah. Biol. Mar., 1973, 14. [p.422, Fig.6].
Cephalothoracic length variations (in micrometers) during 20 months in relation with the temperature (mean 0-30 m; depth 50 m) and quantitative variations of phytoplancton at Banyuls-sur-Mer (western Gulf of Lion, Mediterranean Sea).
A: number of cells per liter of peridinians and diatomacae; B: number of cells of nannoplankton forms.
Nota: Using total and partial correlation, the relation between length cephalothoracic and temperature is very high (probability 95-99 %) whereas the correlations with the phytoplankton is not significant. The temperaure preponderance masks the effects of trophic factor, and probaly the omnivorous mode of food of Temora stylifera. |
 issued from : R. Gaudy in Tethys, 1972, 4 (1). [p.237, Fig.36]. issued from : R. Gaudy in Tethys, 1972, 4 (1). [p.237, Fig.36].
Schematic quantitative abundance of Temora stylifera in the Gulf of Marseille (Mediterranean Sea) established from samples during the period between ending 1960 to ending 1967.
Gaudy (p.208) points to 5 generations per year. The sex ratio is about equal, but sometimes with a slight dominance of males. |
 Issued from : T. Kiørboe in Oecologia, 2008, 155. [p.185, Table 2]. Issued from : T. Kiørboe in Oecologia, 2008, 155. [p.185, Table 2].
Grand average swimming speeds (±SD) and cephalothorax lengths of males and females of Temora stylifera)
Nota: Experimental animals were reared in the laboratory at 22°C.
Compare with Temora longicornis |
 Issued from : T. Kiørboe in Oecologia, 2008, 155. [p.186, Table 3]. Issued from : T. Kiørboe in Oecologia, 2008, 155. [p.186, Table 3].
Horizontal (Vx) and vertical (Vy) average swimming speeds (±SD) in Temora stylifera.
Compare with Temora longicornis. |
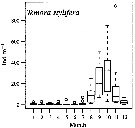 Issued from : M.G. Mazzocchi, L. Dubroca, C. Garcia-Comas, I. Di Capua & M. Ribera d'Alcalà in Prog. Oceanogr., 2012, 97-100. [p.141, Fig.7]. Issued from : M.G. Mazzocchi, L. Dubroca, C. Garcia-Comas, I. Di Capua & M. Ribera d'Alcalà in Prog. Oceanogr., 2012, 97-100. [p.141, Fig.7].
Average annual cycle of abundance (ind. m3) of Temora stylifera at station MC in the period 1984-2006.
iNota: Individual samples collected by vertical tows in the upper 50 m at station MC in the Gulf of Napoli, depth < 100m. |
 Issued from : M.G. Mazzocchi, L. Dubroca, C. Garcia-Comas, I. Di Capua & M. Ribera d'Alcalà in Prog. Oceanogr., 2012, 97-100. [p.139, Fig.3]. Issued from : M.G. Mazzocchi, L. Dubroca, C. Garcia-Comas, I. Di Capua & M. Ribera d'Alcalà in Prog. Oceanogr., 2012, 97-100. [p.139, Fig.3].
Average annual cycle of (a) temperature (*C), (b) salinity (psu), and (c) chlorophyll a concentration (mg m3) integrated in the 0-50 m layer at Station MC (Gulf of Naples) in the period 1984-2006. Box-and-whisker plots have whisker range fixed at 1.5 times the interquartile interval. |
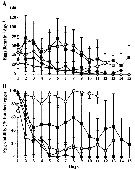 Issued from : A. Barreiro, Y. Carotenuto, N. Lamari, F. Esposito, G. D'Ippolito, A. Fontana, G. Romano, A. Ianora, A. Miralto & C. Guisande in J. Exp. Mar. Biol. Ecol., 2011, 401. [p.15, Fig.1]. Issued from : A. Barreiro, Y. Carotenuto, N. Lamari, F. Esposito, G. D'Ippolito, A. Fontana, G. Romano, A. Ianora, A. Miralto & C. Guisande in J. Exp. Mar. Biol. Ecol., 2011, 401. [p.15, Fig.1].
Egg production rates (A) and hatching success (B) of Temora stylifera during the reproduction experiments (mean ±SD).
Symbols: open circle (PRO), dark square (TR1), grey square (TR2), dark diamond (SM), and grey diamond (SPC).
SPC = Skeletonema pseudocostatum; SM = Skeletonema marinoi; TR1 = Thalassiosira rotula; TR2 = another Thalassiosira rotula ; PRO = Prorocentrum minimum (Dinoflagellate).
Nota: Strains TR1 and TR2 induced highest egg production rates, even if there was a decrease from the first half of the experiment (meandays 2-7 : 61.9 ± 12 SD and 59.5 ± 11.7 SD eggs /female/day respectively) with respect to the second half (mean since day 8 to the end : 31.8 ±9.8 SD and 35.4 ± 15.5 SD eggs/female/day respectively), representing a decrease of 48.7 % and 40.4 % respectively. PRO supported moderate-low but constant egg production rates over the whole experimental period (mean days 2-7 : 13.6 ± 5.3 SD eggs/female/day, mean as of day 8 to the end : 14.4 ± 5.7 SD eggs /female/day.
In contrast, SM and SPC resulted in higher initial egg production rates that decreased sharply with time (mean days 2-7 : 21.7 ±9.6 SD and 26.4 ± 17.5 SD eggs/female/day, respectively mean as of day 8 to the end : 4.5 ± 2.2 SD and 1.5 ±2.7 SD eggs /female/day, respectively), representing a decrease of 94.1 % and 79.2 % respectively, and the lowest egg production rates recorded in this set of experiment.
Optimal hatching rates were obseved on day 1 with all diets (mean 87.3 % ± 12.6 SD, but decreased thereafter with all tested diatoms. Only PRO supported optimal egg hatching rates throughout the experiment (mean 89.9 % ± 8.9 SD. Using an ANCOVA analysis, eggs viability with PRO was significantly different compared to all other treatments.
With TR1 and TR2 diets egg hatching success decreased slowly (mean day 2-day 7 : 50.7 % ± 7.3 SD and 19.4 % ± 8.7 SD respectively.
SPC and SM induced strong egg hatching inhibition after the first few days, with a drastic negative trend (mean day 2-day 7: 20.5% ±20.18 SD and 22 % ± 29.4 SD respectively. There were no differences in egg viability between TR2, SM and SPC. |
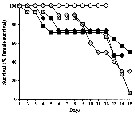 Issued from : A. Barreiro, Y. Carotenuto, N. Lamari, F. Esposito, G. D'Ippolito, A. Fontana, G. Romano, A. Ianora, A. Miralto & C. Guisande in J. Exp. Mar. Biol. Ecol., 2011, 401. [p.16, Fig.3]. Issued from : A. Barreiro, Y. Carotenuto, N. Lamari, F. Esposito, G. D'Ippolito, A. Fontana, G. Romano, A. Ianora, A. Miralto & C. Guisande in J. Exp. Mar. Biol. Ecol., 2011, 401. [p.16, Fig.3].
Female survival of Temora stylifera during reproduction experiments.
Symbols as drawing above.
Nota: PRO supported 100 % survival throughout the experimental period, and diatoms, in contrast, showed lower values at the end of the experiment : 50 %, 6.6 %, 40.7 %, and 30 % respectively in TR1, TR2, SM and SPC. |
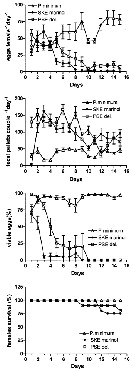 Issued from : A. Ianora, G. Romano, Y. Carotenuto, F. Espositi, V. Roncalli, I. Buttino & A. Miralto in Hydrobiologia, 2011, 666. [p.269, Fig.1] Issued from : A. Ianora, G. Romano, Y. Carotenuto, F. Espositi, V. Roncalli, I. Buttino & A. Miralto in Hydrobiologia, 2011, 666. [p.269, Fig.1]
Egg production, fecal pellet production, egg viability (%) and female survival (%) of Temora stylifera fed with monoalgal diets of the control dinoflagellate Prorocentrum minimum and the test diatoms Skeletonema marinoi and Pseudo-nitzschia delicatissima for 15 days.
Nota: Temora stylifera from the Gulf of Naples (Italy) was fed with the pennate diatom Pseudo-nitzschia delicatissima for 15 days. This diatom does not produce polyunsaturated aldehydes (PUAs) but only synthesizes other oxylipins. Effects of this monoalgal diet were compared to copepods fed with the PUA-producing diatom Skeletonema marinoi and the control dinoflagellate Prorocentrum minimum which does not produce any oxylipins.
Both egg production and hatching success were negatively affected by the two diatoms compared to the control diet. Both diatoms induced malformations in the offspring, with the number of malformed nauplii increasing dramatically with time
The results indicate that P. delicatissima induced negative effects comparable to those of S. marinoi even though it did not produce PUAs. |
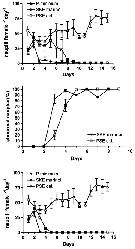 Issued from : A. Ianora, G. Romano, Y. Carotenuto, F. Espositi, V. Roncalli, I. Buttino & A. Miralto in Hydrobiologia, 2011, 666. [p.270, Fig.2] Issued from : A. Ianora, G. Romano, Y. Carotenuto, F. Espositi, V. Roncalli, I. Buttino & A. Miralto in Hydrobiologia, 2011, 666. [p.270, Fig.2]
Expected recruitment (nauplii /female/day), percentage of abnormal nauplii (%) and effective recruitment (nauplli/*female/day) of Temora stylifera fed with monoalgal diets of the control dinoflagellate Prorocentrum minimum and the test diatoms Skeletonema marinoi and Pseudo-nitzschia delicatissima for 15 days. |
 Issued from S. Razouls in XXIII rd Congress of Athens, 3-11 November 1972. [p.2]. Oxygen consumed by individual (adult) in the Banyuls Bay and equivalent carbon asked. Issued from S. Razouls in XXIII rd Congress of Athens, 3-11 November 1972. [p.2]. Oxygen consumed by individual (adult) in the Banyuls Bay and equivalent carbon asked.
(1) Hydrological season in the stability period: Eté = Summer: 18-20 °C; Hiver = Winter: 13-10°C.
Espèces = species; Saison = Season; Lg céph.= cephalothoracic length; an = individual. |
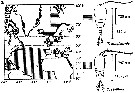 Issued from : C. Halsband-Lenk, H.-J. Hirche & F. Carlotti in J. Exp. Mar. Biol. Ecol., 2002, 271. [p.124, Fig.1, b]. Issued from : C. Halsband-Lenk, H.-J. Hirche & F. Carlotti in J. Exp. Mar. Biol. Ecol., 2002, 271. [p.124, Fig.1, b].
Habit (after Rose, 1957; modified), range of prosome length in situ (Halsband-Lenk, partly unpublished) and geographical distribution in the Atlantic and adjacent seas of Temora longicornis and Temora stylifera (b).
The target is the temperature impact on reproduction and development of two congener copepod pairs inhabiting different biogeographic regions to their geographic distribution patterns (from two stations in the North Sea, Helgoland Island and the Mediterranean Sea, Villefrance -sur-Mer).
See Centropages typicus in commentaries by the same authors (p.124, Fig. 1) and Temora longicornis for conclusion. |
 Issued from : C. Halsband-Lenk, H.-J. Hirche & F. Carlotti in J. Exp. Mar. Biol. Ecol., 2002, 271. [p.127, Fig.3, c, e]. Issued from : C. Halsband-Lenk, H.-J. Hirche & F. Carlotti in J. Exp. Mar. Biol. Ecol., 2002, 271. [p.127, Fig.3, c, e].
Total mortality of adult females after 5 days of incubation at different temperatures., individuals from two sites the North Sea (Helgoland Island) and Medioterranean Sea (Villefranche-sur-Mer)
Nota: well introduced by the superficial current from the Atlantic through Gibraltar Strait, inversely to T. longicornis rare in the W Mediterranean Sea, |
 Issued from : C. Halsband-Lenk, H.-J. Hirche & F. Carlotti in J. Exp. Mar. Biol. Ecol., 2002, 271. [p.128, Fig.4, c, e]. Issued from : C. Halsband-Lenk, H.-J. Hirche & F. Carlotti in J. Exp. Mar. Biol. Ecol., 2002, 271. [p.128, Fig.4, c, e].
Cumulative proportions of dead females incubated 5 days at different temperatures. |
 Issued from : C. Halsband-Lenk, H.-J. Hirche & F. Carlotti in J. Exp. Mar. Biol. Ecol., 2002, 271. [p.129, Fig.5, c, e]. Issued from : C. Halsband-Lenk, H.-J. Hirche & F. Carlotti in J. Exp. Mar. Biol. Ecol., 2002, 271. [p.129, Fig.5, c, e].
Temperature impact on egg production rates. |
 Issued from : C. Halsband-Lenk, H.-J. Hirche & F. Carlotti in J. Exp. Mar. Biol. Ecol., 2002, 271. [p.130, Fig.6, c, e]. Issued from : C. Halsband-Lenk, H.-J. Hirche & F. Carlotti in J. Exp. Mar. Biol. Ecol., 2002, 271. [p.130, Fig.6, c, e].
Cumulative egg production during 5 days at different temperatures. |
 Issued from : C. Halsband-Lenk, H.-J. Hirche & F. Carlotti in J. Exp. Mar. Biol. Ecol., 2002, 271. [p.139, Table 3]. Issued from : C. Halsband-Lenk, H.-J. Hirche & F. Carlotti in J. Exp. Mar. Biol. Ecol., 2002, 271. [p.139, Table 3].
Stage durations (day) and mortality rates (day) of Temora stylifera at different temperatures in the Mediterranean Sea (Villefranche-sur-Mer).
Compare with Temora longicornis in Table 3, Tl, for the same authors. |
 Issued from : C. Halsband-Lenk, H.-J. Hirche & F. Carlotti in J. Exp. Mar. Biol. Ecol., 2002, 271. [p.140, Table 4]. Issued from : C. Halsband-Lenk, H.-J. Hirche & F. Carlotti in J. Exp. Mar. Biol. Ecol., 2002, 271. [p.140, Table 4].
Egg production rates (EPR) and development times of Temora stylifera.
NS = North Sea; M = Mediterranean; A = Atlantic.
I.g. = Isochrysis galbana; H.e.= Hymenomonas elongata; T.s. = Tetraselmis suecica; D.t. = Dunalliella tertiolecta; T.w. = Thalassiosira weissflogii; T.r. = Thalassiosira rotula; R.b. = Rhodomonas baltica; R.sp. = Rhodomonas sp.; O.m. = Oxyrrhis marina. |
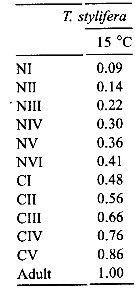 Issued from : C. Halsband-Lenk, H.-J. Hirche & F. Carlotti in J. Exp. Mar. Biol. Ecol., 2002, 271. [p.144, Table 6]. Issued from : C. Halsband-Lenk, H.-J. Hirche & F. Carlotti in J. Exp. Mar. Biol. Ecol., 2002, 271. [p.144, Table 6].
Proportion of total development time (egg-laying to adult) spent in each stage (cumulative median development time/generation time in Temora stylifera. |
 Issued from : C. Halsband-Lenk, H.-J. Hirche & F. Carlotti in J. Exp. Mar. Biol. Ecol., 2002, 271. [p.143, Table 5]. Issued from : C. Halsband-Lenk, H.-J. Hirche & F. Carlotti in J. Exp. Mar. Biol. Ecol., 2002, 271. [p.143, Table 5].
Temora stylifera (from the Mediterranean Sea, Villefrance-sur-Mer): Synthesis of results from incubation in a temperature gradient from 0 to 35°C.
FTT = female thermal tolerance. RTR = Reproductive thermal response = . ETR = Embryonic thermal response.
See in the same authors Temora longicornis (Table 5). |
 Issued from : C. Halsband-Lenk, H.-J. Hirche & F. Carlotti in J. Exp. Mar. Biol. Ecol., 2002, 271. [p.143, Table 5]. Issued from : C. Halsband-Lenk, H.-J. Hirche & F. Carlotti in J. Exp. Mar. Biol. Ecol., 2002, 271. [p.143, Table 5].
Temora stylifera and Temora longicornis: Synthesis of results from incubation in a temperature gradient from 0 to 35°C.
FTT = female thermal tolerance. RTR = reproductive thermal response. ETR = embryonic thermal response. |
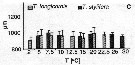 Issued from : C. Halsband-Lenk, H.-J. Hirche & F. Carlotti in J. Exp. Mar. Biol. Ecol., 2002, 271. [p.132, Fig.8]. Issued from : C. Halsband-Lenk, H.-J. Hirche & F. Carlotti in J. Exp. Mar. Biol. Ecol., 2002, 271. [p.132, Fig.8].
Prosome length of females incubated in experiments. vertical bars indicate standard deviation.
Nota: Prosome length of females used in experiments showed little variability within species in comparison to the range of prosome length compiled from different seasons and locations see in fig. 1 for the same authors).
Temora longicornis and Temora stylifera females had a similar mean prosome length of 951.1 ±71.3 and 965.7 ±47.9 µm, respectively.
The size range of T. longicornis from 774.3 to 1212.6 µm (mean: 951.1 ±71.3 µm) was broader than that of T. stylifera with 876.8-1073.7 µm (mean: 9565.7 ±47.9 µm).
Size differences between experiments were not significant in a given population. |
 Issued from : M. Madhupratap, S.R. Sreekumaran Nair, C.T. Achuthankutty & V.R. Nair in Indian J. Mar. Sci., 1981, 10. [p.267, Fig.2 g, h, i]. Issued from : M. Madhupratap, S.R. Sreekumaran Nair, C.T. Achuthankutty & V.R. Nair in Indian J. Mar. Sci., 1981, 10. [p.267, Fig.2 g, h, i].
Distribution of copepod species in the Andaman Sea (around Andaman-Nicobar Islands). g: Lucicutia sp.; h: Temora stylifera; i: Temora discaudata.
Samples collected in vertical hauls (200-0 m) using a net (mesh aperture 500 µm). Cf. Madhupratap & al., 1981: Indian J. Mar. Sci., 10, p.258.
No. = number of individuals. |
 Issued from : C. de O. Dias, A. V. de Araujo & S.L.C. Bonecker in Zoologia, 2018, 35. [p.5, Table 2]. Issued from : C. de O. Dias, A. V. de Araujo & S.L.C. Bonecker in Zoologia, 2018, 35. [p.5, Table 2].
Vertical distribution of the mean (S.D.) density (ind. m3) of the two congeners Temora stylifera and Temora turbinata in The Campos Basin (23°55'- 22°N, 41°44'-38°41'W) during the rainy (RS) and dry (DS) seasons, from the continental shelf (0-130 m) to the depth line 3000 m.
Mesozooplankton samples collected by horizontal hauls with MultiNet type (200 µm mesh aperture).
Rainy season: February-April and dry season: August- September 2009.
The sampling stations distributed along 6 transects perpendicular to the coast in a north-south ditrection. |
 Issued from : J.C. Roff, J.T. Turner, M.K. Webber & R.R. Hopcroft in Aquat. microb. Ecol., 1995, 9. [p.170, Table 5]. Issued from : J.C. Roff, J.T. Turner, M.K. Webber & R.R. Hopcroft in Aquat. microb. Ecol., 1995, 9. [p.170, Table 5].
Summary observations on naupliar bacterivory on FLB (fluorescently labelled bacteria) a partir Escherichia coli (±0.7 µm3 cell volume) in Lime Cay (Jamaica).
Fluorescence levels: 0 = no materal for observation; - = no fluorescence observed; + = low fluorescence due to few FLB observe; * = highly fluorescence due to many FLB. |
 Issued from : S. Ban, C. Burns, J. Castel, Y. Chaudron & al. in Mar. Ecol. Prog. Ser., 1997, 157. [p.289, Table 1]. Issued from : S. Ban, C. Burns, J. Castel, Y. Chaudron & al. in Mar. Ecol. Prog. Ser., 1997, 157. [p.289, Table 1].
Synopsis of feeding/reproduction experiments. A total of 17 diatom and 16 copepod species, representative of a variety of worldwide temperate and subarctic environments (E: estuarine, C: coastal ocean), were screened.
Data for fecundity and hatching success are average values measured at the start and end of the inciubations in a minimum of 3 replicate batches, showing the variation in time of the effects of diatoms on copepod reproduction.
Level of significance of the diatom effect between treatments and controls (e.g. non-diatom diets) in categories I to III was p < 0.01. No.. rank (for comparison with Table 2, p.290).
For the signification of the categories I to IV (fecundity vs. Hatching success vs. Duration of experiment days-1): See Eurytemora affinis, Calanus pacificus, Calanus finmarchicus, Temora stylifera.
List of the 12 marine or estuarine species studied (fresh water excluded. CR): Acartia clausi, A. grani, A. steueri, A. tonsa, Calanus finmarchicus, C. helgolandicus, C. pacificus, Centropages hamatus, C. typicus, Eurytemora affinis, Temora longicornis, T. stylifera. |
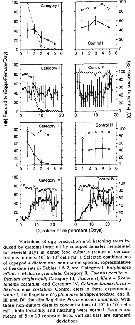 Issued from : S. Ban, C. Burns, J. Castel, Y. Chaudron & al. in Mar. Ecol. Prog. Ser., 1997, 157. [p.288, Fig. 1]. Issued from : S. Ban, C. Burns, J. Castel, Y. Chaudron & al. in Mar. Ecol. Prog. Ser., 1997, 157. [p.288, Fig. 1].
Variations of egg production and hatching rates induced by diatoms ingested by copepod females maintained for several days in dense food cultures. Selected combinations of the data sets in Table 1 & 2. For categories in function of the duration of experiments (days).
See explanations in Calanus finmarchicus (same Fig. 1). |
 Issued from : S. Ban, C. Burns, J. Castel, Y. Chaudron & al. in Mar. Ecol. Prog. Ser., 1997, 157. [p.290, Table 2]. Issued from : S. Ban, C. Burns, J. Castel, Y. Chaudron & al. in Mar. Ecol. Prog. Ser., 1997, 157. [p.290, Table 2].
Combinations of copepod and non-diatom diets in controls in concentrations ranging from 104 to 105 cells ml-1. Data for fecundity and hatching success are average values measured at the start and end of incubation in a minimum of 3 replicate batches.
Nos 17, 18: In Category I; No29: In Category II; No36: In Category III |
| | | | Loc: | | | Sub-Antarct. (50°S, 70°E in C. Séret, 1979, exceptional), South Africa (E), South Africa (W, Richard's Bay Harbour and Mhlathuze Estuary)], Angola, Baia Farta, Congo, G. of Guinea, off Lagos, Ivorian shelf, Argentina, Uruguay (continental shelf), Brazil (S, Paranagua Bay, Sao Joao, Bracui, off Rio de Janeiro, Campos Basin, Cabo Frio, Vitoria Bay, Vitoria-Cabo de Sao Tomé, off Macaé, Guarairas Lagoon, Mucuri estuary, Camamu, off Natal, Guarau estuary, Ajuruteua Bay, Caeté Estuary), off Amazon, G. of Guinea, Abidjan, Dakar, Cape Verde Is., Mauritania, Banc d'Arguin, Morocco-Mauritania, Cap Ghir (Morocco), Canary Is., off Madeira, Ibero-moroccan Bay, Portugal, Vigo, off Coruña, Bilbao & Urdaibai estuaries, S Bay of Biscay, Arcachon Bay, Gironde estuary, Azores, Barbados Is., Caribbean Sea, Caribbean Columbia, Cariaco Basin, Bahia de Mochima (Venezuela), Yucatan, Caribbean, Jamaica (Lime Cay), G. of Mexico, off W Mississipi River mouth, Cuba, Straits of Florida, Floride, Sargasso Sea, off Bermuda: Station ‘’ S’’ (32°10’N, 64°30’W), Delaware Bay (outside), Long Island, Bay of Fundy, Nova Scotia, Labrador, off W Tangier, Gibraltar, Medit. (M'Diq, Alboran Sea, Habibas Is., Sidi Fredj coast, Gulf of Annaba, El Kala shelf, laguna Mar Menor, Castellon, Baleares, off Barcelona, Banyuls, Berre Lagoon, Marseille, Toulon Harbour, Villefranche-sur-Mer, Genova, Tyrrhenian Sea, Napoli, Milazzo, Lake Faro (Sicily), Messina, Gulf of Taranto, Taranto Harbour, NW Tunisia, Bizerte Channel, G. of Gabès, Malta, Adriatic Sea, Vlora Bay, Venice, Po delta, Trieste, Island of Pag, Ionian Sea, Aegean Sea, Thracian Sea, Iskenderun Bay, Lebanon Basin, W Egyptian coast, Alexandria, Marmara Sea, Black Sea), ? Suez Canal, Sharm El-Sheikh, Safaga, Red Sea, Gulf of Oman, Maldive Is., Laccadives Is., Kavaratti Is., Natal, off Cochin, Sri Lanka, Coom & Adyar, Pointcalimere, Madras, G. of Mannar, Palk Bay, E India, Bay of Bengal, Andaman Sea, Malaysia (Kurau River), Straits of Malacca, G. of Thailand, Malaysia (Sarawak: Bintulu coast), Ambon Bay, Celebes Sea, Philippines (Mindanao bays), Viet-Nam (Cauda Bay), G. of Tonkin, China Seas (Yellow Sea, East China Sea, South China Sea), Taiwan (S, SW, E, W, Kaohsiung Harbor, NW, N), S Korea, Japan Sea, Japan, Kuchinoerabu Is., Pacif. (tropical), Pacif. (W equatorial), Galapagos-Ecuador (inTutasi & al., 2011); Peru (inIMARPE unpublished), Chile (N-S).
Locality from Kos (2016, p.50): 39°34' N, 147°47' E. | | | | N: | 429 ? | | | | Lg.: | | | (16) F: 2,05-1,5; M: 1,8-1,5; ? (26) F: 1,34; (45) F: 1,75-1,45; M: 1,55-1,35; (46) F: 1,7-1,45; M: 1,55-1,4; (54) M: 1,88-1,79; (73) M: 1,74-1,68; (75) F: 2,03-1,65; M: 1,8-1,59; (180) F: 1,7-1,35; 2,05-1,8; M: 1,61-1,3; (199) F: 1,44; M: 1,44; (237) F: 1,05; M: 1,7-1,55; (322)* F: 3,0; M: 3,0; (327) F: 1,77-1,57; M: 1,8-1,46; (332) F: 1,21-1,19; M: 1,22-1,16; (334) F: 1,35; M: 1,3; (340) M: 1,55; (449) F: 1,7-1,45; M: 1,55-1,4; (578) M : 1,04-1,01; (580) F: 1,88-1,48; M: 1,88-1,43; (785) F: 1,67-1,28; (795) F: 1,5; M: 1,2; (920) F: 1,54; M: 1,58; (927) F: 1,67-1,71 [winter]; F: 1,43-1,45 [summer]; M: 1,71-1,75 [winter]; M: 1,47-1,49 [summer]; (1009) F: 1,82-1,90; M: 1,58-1,88; (1140)** F: 2,7; M: 3,51; (1220) F: 1,34-1,9; M: 1,4-1,5; (1225) F: 0,93; M: 0,88; {F: 0,93-2,05; M: 0,88-1,88}
Ramirez (1966)* indicates body lengths exceptionally high: Female and Male = 3.00 mm., as Lacuna and al. (2013)**: F = 2.7 and male = 3.51 mm. | | | | Rem.: | Epipelagic, 2000 m (off Malta). Sargasso Sea: 0-500 m (Deevey & Brooks, 1977, Station "S");
For Gurney (1927, p.151) , the distribution of T. stylifera and T. discaudata, two very closely allied species is rather obscure, same if Wolfenden found T. stylifera common in the Maldives, while Thompson & Scott record it from the southern parts of the Red Sea and from Sri Lanka, but not from the northern Red Sea. After Gurney, T. deiscaudata is a purely Indo-Pacific species, but was identified by Thompson & Scott between Messina and Port Said (Mediterranean Sea). In the expedition's collections T. discaudata was common in the neighbourhood of Port Taufik, and was taken at KM. 152 and 146 in the Suez Canal, but entirely absent from the Canal north; T. stylifera was common at Poert Said, and was taken as far as El Tineth (Km. 24 from Port Said).
This form seems essentially present in the tropical and sub-tropical Atlantic, although Wilson (1936 d) reports the species from Labrador, and in Pacific Ocean (Philippines, Fiji Islands, Gilbert Islands, Galapagos) with other species of Temora (discaudata, longicornis, turbinata in the "Albatross" expedition (1950). Since this expedition, it is rarely reported from the Indian and Pacific Oceans, sometimes in confusion with T. discaudata (Mori, 1937 (1964). Reported by C. Séret (1979, p.128) near the Kerguelen Islands from one male specimen (that could have been introduced at the time of the expedition and after ranalysed at Abidjan, but it could not correspond to Temora kerguelensis.
The fauna of Japan (1997) does not mention the presence of this species anymore in the Japanese waters, although Yamazi (1958, p.150) registered T. stylifera outside of the Tanabe Bay in warm season. A re-examination of this species in the Indo-Pacific seems necessary. According to Arcos & Fleminger (1991, p. 1185) this species is restricted to the Atlantic Ocean (40°N-35°S along the American coasts and 45°N-5°S in the eastern Atlantic and the Mediterranean).
After El-Serehy & al. (2001, p.119) the species is very rare in the Suez Canal but present and not abundant in the Gulf of Suez.
Sewell points to the occurrence of tis species in the Bay of Bengal, but only a few specimens. Madhupratap & al. (1981) frequent in the Andaman Sea. Rezai & al. (2004) in the Straits of Malacca (with T. discaudata and T. turbinata; W. Zhang (pers. comm. 2006, 2010) confirms its presence in his samples from the Chinese Seas, and Johan (2013) in Malaysian waters, like Lacuna & al. (2013) in the bays of Mindanao (Southern Philippines, also W.-B. Chang & al. (2010) in the embayment of south Taiwan and Ayukai & Hattori (1992) off south Shikoku Island (Japan).
The habitus female and male, P5 female in figure by Dias & Araujo (2006, p.67) seem more closer than T. discaudata.
For Lacuna & al. (2013, p.109) the morphology of T. stylifera is similar with T. discaudata except in the anal segment and caudal rami where the former species exhibited symmetry in structures for both sexes but the latter species showed strong assymmetry in female only.
See remarks in T. discaudata.
Cf. in Conway & al., 2003 (p.138: Rem.: Temora disscaudata.
First occurrence in Chilean waters by Hidalgo & al. (2010).
Gaudy (1962, p.145) points to 5 generations by year in the Gulf of Marseille (NW Mediterranean Sea).
After Benedetti & al. (2018, p.1, Fig.2) this species belonging to the functional group 4 corresponding to small filter feeding herbivorous and mixed feeding omnivorous (mostly broadcasters).
Bonecker A.C.T. & al. (2017), point out on 117 taxa, with 81 species identified, this species is one of the most abundant in the Campos Basin with 206, 32 ind.m-3. | | | Last update : 19/06/2023 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2025. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed December 01, 2025] © copyright 2005-2025 Sorbonne University, CNRS
|
|
 |
 |