|
|
 |
|
Calanoida ( Order ) |
|
|
|
Clausocalanoidea ( Superfamily ) |
|
|
|
Scolecitrichidae ( Family ) |
|
|
|
Neoscolecithrix ( Genus ) |
|
|
| |
Neoscolecithrix caetanoi Alvarez, 1985 (F,M) | |
| | | | | | | Syn.: | Cenognatha caetanoi : Bradford-Grieve, 2004 (p.285) | | | | Ref.: | | | Alvarez, 1985 a (p.198, Descr.F,M, figs.F,M); Bradford-Grieve & al., 1999 (p.880, 928, 929, figs.F,M, as caetanovi); Bradford-Grieve, 2001 a (p.790: Rem.) |  issued from : M.P.J. Alvarez in Rev. bras. Zool., S. Paulo, 1985, 3 (4). [p.201, Figs.1-7]. Female (from 21°37'S, 40°03'W): 1-2, habitus (dorsal and lateral, respectively); 3, forehead (ventral); 4, 5th metasomic segment (lateral, right side); 5, urosome (ventral); 6, posterior margin of the 2nd urosome segment (dorsal); 7, A2.
|
 issued from : M.P.J. Alvarez in Rev. bras. Zool., S. Paulo, 1985, 3 (4). [p.202, Figs.8-12]. Female: 8, Md (mandibular palp); 9, Md (masticatory edge); 10, distal tooth of the mandible gnathobase); 11, Mx1; 12, Mx2.
|
 issued from : M.P.J. Alvarez in Rev. bras. Zool., S. Paulo, 1985, 3 (4). [p.203, Figs.13-22]. Female: 13, Mxp; 14, distal region of 1st basipod of Mxp; 15, P1; 16, spines of 2nd basipod of P1; 17, P2 (incomplete); 18, spines of 2nd basipod of P2 (enlarged); 19, P3 (incomplete); 20, spines of 2nd basipod of P3 (enlarged); 21, P5; 22, P5 (paratype). Nota: The paratype female is sexually mature, and is not so long as the holotype (3.2 mm and 3.9 mm respectively), but both are equal in the other features, excepting in the aspect of P5. This is a little different as to the position of the lateral spines of the 3rd segment. It looks as if the fifth pair of legs is not fully developed. Something similar to that was observed by Sewell (1929) in Eucalanus subcrassus. But Vervoort (1941) when studying the morphology and biology of this same species did not confirm the possibility of sexual maturity occurring in copepodite V as Sewell (1929) had suggested. Vervoort observed a very incomplete development of the genital segment of the copepodite V of the species and concluded that the precocious sexual maturity would occur only in some exceptional cases. He added that the different degrees of dimorphism observed in the mature specimens resulted from different rhythms of growth. Sewell (1929) found that the rhythm of development diminishes considerably when maturation begins and is probably influenced by different conditions of the environment. Thus, the differences observed in the 5th these appendages may vary intraspecifically as a function of the growth rhythm, and that in this species the sexual maturity can precede the complete development of the secondary sexual characteristics.
|
 issued from : M.P.J. Alvarez in Rev. bras. Zool., S. Paulo, 1985, 3 (4). [p.204, Figs.23-29]. Male: 23-24, habitus (dorsal and lateral, respectively); 25, forehead (ventral); 26, idem (lateral); 27, A1; 28, urosome (dorsal); 29, A2.
|
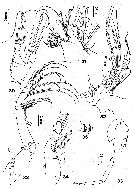 issued from : M.P.J. Alvarez in Rev. bras. Zool., S. Paulo, 1985, 3 (4). [p.205, Figs.30-36]. Male: 30, Md (mandibular palp); 31, Mx1; 32, Mx2; 33, Mxp; 34, P1; 35, spines of 2nd basipod of P1; 36, P5.
|
 issued from : M.P.J. Alvarez in Rev. bras. Zool., S. Paulo, 1985, 3 (4). [p.206]. Groups of Neoscolecithrix species according to P5. Group \"koekleri\": N. kohleri after Canu (1896) (figs.37, 38); N. caetoni after Alvarez (1985) (figs.39, 40); N. magna after Grice (1972) (fig.41). Group \"farrani\": N. farrani after Fosshagen (1972) (figs.42, 43); N. watersae after Grice (1972) (figs.44, 45). Nota: The species of the genus Neoscolecithrix can be distributed into 2 groups considering the rostrum, the structure of the gnathobase of the mandible, the type of setae of the 5th lobe of Mx2 and the morphology of the legs.
| | | | | NZ: | 1 | | |
|
Distribution map of Neoscolecithrix caetanoi by geographical zones
|
| | | | Loc: | | | S Brazil | | | | N: | 1 | | | | Lg.: | | | (585) F: 3,9; 3,2; M: 3,4; {F: 3,20-3,90; M: 3,40} | | | | Rem.: | hyperbenthic.
? Cf. Cenognatha.
According to Alvarez (1985, p.200) the majority ofthe authors (Rose, 1933; Brodsky, 1967; Fosshagen, 1972 among others) included the genus in the Phaennidae. Bradford (1973) redefining the family, did not accept the above opinion, because the Neoscolecithrix species lack an important feature of the Phaennidae: the presence of spines on the endopods of P2 to P4. But she did not suggest to which family the genus should belong. Canu (1896) had already called attention to the similarity of the genera Scolecithrix, Xanthocalanus, and Neoscolecithrix, proposing that the last one be intermediate between the two. Fosshagen (1972) remarked that N. farrani is an association of characteristics of several families, and concluded that a revision of the systematic position of this genus and of the families related to it was necessary. The different modes of life have influence on the morphology of the animals, and this may mask the phylogenetic relationships existing between the several copepod genera. | | | Last update : 03/02/2015 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed February 10, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |









