|
|
 |
|
Calanoida ( Order ) |
|
|
|
Diaptomoidea ( Superfamily ) |
|
|
|
Acartiidae ( Family ) |
|
|
|
Acartia ( Genus ) |
|
|
|
Acartiura ( Sub-Genus ) |
|
|
| |
Acartia (Acartiura) tranteri Bradford, 1976 (F,M) | |
| | | | | | | Syn.: | Acartia clausi : Dakin & Colefax, 1940 (p.106, figs.); Greenwood, 1974 (p.224, fig.35, a-g). | | | | Ref.: | | | Bradford, 1976 (p.192, figs.F,M); Greenwood, 1978 (p.18, figs.F,M); McKinnon & al., 1992 (p.239, 248, figs.F,M, Rem.); Bradford-Grieve, 1999 b (p.225, figs.F,M, figs.187, 195). | 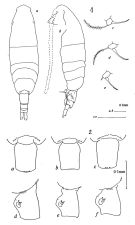 issued from : J.M. Bradford in N.Z. J. Mar. Freshw. Res., 1976, 10 (1). [p.193, 194, Figs.29, 30]. Female (From Port Hacking, New South Wales, Australia).: 1a, habitus (dorsal view); 1b, idem (lateral view); 1c-e, P5; 2a-c, genital segment (dorsal view); 2d-f, idem (lateral view).
|
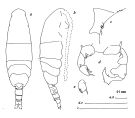 issued from : J.M. Bradford in N.Z. J. Mar. Freshw. Res., 1976, 10 (1). [p.195, Fig.31]. Male ( From Port Hacking, New South Wales, Australia): a, habitus (dorsal view); b, idem (lateral view); c, posterior metasome (right lateral view); d, posterior surface of P5; e, terminal segment of left P5.
|
 issued from : J.G. Greenwood in Proc. R. Soc. Qd, 1978, 89. [p.17, Fig.8]. Female (from Moreton Bay, E Australia): a, P5; b, posterior metasome and urosome (lateral right side); g, P5. Male: c, P5; d, right end metasome and urosome (dorso-lateral); e, right P5; f, left P5.
|
 issued from : A.D. McKinnon, W.J. Kimmerer & J.A.H. Benzie in J. Crustacean Biol., 1992, 12 (2). [p.249, Fig.5]. Female (from Port Hacking, S. Australia): b, P5. Male: a, P5. Scale bar: 0.100 mm.
|
 issued from : A.D. McKinnon, W.J. Kimmerer & J.A.H. Benzie in J. Crustacean Biol., 1992, 12 (2). [p.256, Table 7]. Morphological comparison of species closely related to Acartia tranteri. Data for A. ensifera, A. jilletti, A. simplex, and A. tranteri are taken from Bradford (1976). TL: total length (from the tip of the caudal rami to the front of the head); Th6S: mean number of spines on the thoracic segment 6; GC: Presence/absence of spinules on the posterior dorsal margin of the genital complex; GCP: presence/absence of protuberances posterior to the genital apertures on the female genital complex; LP5 a.s.: relationship of length of the anterior spine of the left male P5 to the posterior spine (see Bradford, 1976); LGC: length of the female genital complex along the dorsal midline; WGC: maximum width of the female genital complex in dorsal view; LCR: length of right caudal ramus along outer margin (measured to insertion of outer terminal seta in dorsal view); WCR: width of right caudal ramus at base in dorsal view; Th6: presence/absence of hairs on the posterior ventral margin of thoracic segment 6; C: presence/absence of spinules on the posterior lateral face of genital complex; U2: both sexes, as GC, but urosome segment 2; U3: males, as GC, but for urosome segment 3; U4: males, as GC, but for urosome segment 4; p: presence; a: absence; for the characters C, GC, Th6, U2, U3 and U4 percentage of occurrence is given.
|
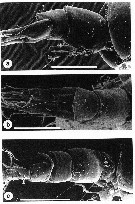 issued from : A.D. McKinnon, W.J. Kimmerer & J.A.H. Benzie in J. Crustacean Biol., 1992, 12 (2). [p.251, Fig.7]. Female: a, urosome (lateral); b, idem (dorsal). Nota: Last thoracic segment (Th6) with 4-7 spines (average 5), and with fine hairs on ventroposterior margin. Mean length:width ratio of genital complex, in dorsal view, 1.10. Lateral faces of genital complex with rows of denticles always extending on to posterior half of segment. Mean length : width ratio of caudal ramus 2.72. Dorsoposterior margin of 2nd, 3rd and 4th urosome segments with row of denticles. Male: c, urosome (dorsolateral). Nota: Last thoracic segment (Th6) with 3-6 dorsolateral spines. Length : width ratio of caudal ramus 1.66 Scale bars: 0.100 mm.
| | | | | Compl. Ref.: | | | Nyan Taw & Ritz, 1979 (p.189); Greenwood, 1981 (p.591, fig.1: co-occurrence); Kimmerer & McKinnon, 1985 (p.149); 1987 (p.14); Kimmerer & McKinnon, 1986 (p.1003, preservation); Jenkins, 1988 (p.233, Table 1, 2, 3, figs.3,4, 5); Mauchline, 1998 (tab.33, 45, 54). | | | | NZ: | 1 | | |
|
Distribution map of Acartia (Acartiura) tranteri by geographical zones
|
| | |  Issued from : J.G. Greenwood in Estuar. Coast. Shelf Sci., 1981, 13. [p.593, Fig. 1, a, b). Issued from : J.G. Greenwood in Estuar. Coast. Shelf Sci., 1981, 13. [p.593, Fig. 1, a, b).
Salinity-temperature-dominance relationships in occurrences of two congeneric species pairs: Acartia tranteri (a) and Acartia pacifica (b).
DS scale refers to grades of dominance (see methods).
This diagram is used to demonstrate differences in ecological requirments of the congenors.
Samples taken by sampling monthly over three years at several stations within the Moreton Bay estuarine system.
The existence of such closely related sympatric species is of great interest concerning interspecific competition and the notion of 'niche theory'. The principles of these topics are summarized by Whittaker (1975), Odum (1971). Possible ways in which zooplanctonic populations may avoid or reduce direct competition have been summatrized by Pejler (1962) as 1- spatial (e.g. by differing horizontal/or vertical distributions; 2- functional differences, e.g. by selective feeding preferences; 3- temporal differences by species populations reaching maxima at different times of the year; and other factors as predators or toxicity.
The two sympatric species coexist within the total range of salinity and temperature conditions sampled in Moreton Bay, they have differing optimal ranges, with one species (A. tranteri) being more eurytopic and in this sense a 'generalist', the other (A. pacifica) finding optimal conditions toward one end of the range of conditions and being more 'specialist', at least in terms of these environmental features. The two species are apparently in close competition during spring and autumn when temperatures are suboptimal for both, the result of this competition depending on wether temperatures are rising or falling. Superimposed on this influence is a difference in salinity optima, with the less euryhaline and more warm thermophilie. A. pacifica competing successfully within its optimal temperature range in the absence of reduced salinities. Populations of A. pacifica may be more variable on a year-to-year basis than those of A. tranteri, depending on the degree of summer (wet season) dilution and that during rising temperatures, replacement of A. tranteri by A. pacifica may occur progressively from outer Bay areas toward inner and estuarine-influenced areas with a dominant status in the community being attained in estuary-mouth regions during periods of low flow. |
| | | | Loc: | | | Australia (Western Port Bay, Swan River estuary, Moreton Bay, Melbourne, Port Hacking, Sydney, Tasmania). | | | | N: | 8 | | | | Lg.: | | | (174) F: 1,11-0,97; M: 1-0,9; {F: 0,97-1,11; M: 0,90-1,00} | | | | Rem.: | epipelagic.
For Greenwood (1978, p.18) an important Bay species. | | | Last update : 02/12/2017 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed March 05, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |









