|
|
 |
|
Calanoida ( Order ) |
|
|
|
Diaptomoidea ( Superfamily ) |
|
|
|
Acartiidae ( Family ) |
|
|
|
Acartia ( Genus ) |
|
|
|
Odontacartia ( Sub-Genus ) |
|
|
| |
Acartia (Odontacartia) erythraea Giesbrecht, 1889 (F,M) | |
| | | | | | | Syn.: | Acartia erythräa Giesbrecht, 1889; 1892 (p.508, 523, figs.F,M);
? Acartia laxa Dana,1849; Brady, 1883 (p.73, figs.F, Rem.). | | | | Ref.: | | | Giesbrecht & Schmeil, 1898 (p.155); Wolfenden, 1905 (1906) (p.1023); Carl, 1907 (p.12, 17, Rem. F); A. Scott, 1909 (p.187, Rem.); Sewell, 1912 (p.354, 377); Pesta, 1912 a (p.53, figs.F,M); 1913 (p.32); Sewell, 1914 a (p.241); Steuer, 1923 (p.30, figs.F,M, Rem.); Früchtl, 1924 b (p.58, 60, Rem.); Sewell, 1932 (p.396); 1933 (p.29); Mori, 1937 (1964) (p.102, figs.F); Sewell, 1947 (p.252); Chiba, 1956 (p.38, 50, figs. Juv.F, figs.F,M, Descr.M); Chiba & al., 1957 (p.310); 1957 a (p.12); Chen & Zhang, 1965 (p.113, figs.F,M); Saraswathy, 1966 (1967) (p.85); Kasahara & al., 1974 (p.170, fig. egg); Marques, 1982 (p.768, figs.F,M); Yoo & Hue, 1983 (p.11, figs.F,M); Nishida, 1985 (p.137); Ueda, 1986 a (p.16, 18, figs.F,M, Rem.); Chihara & Murano, 1997 (p.672, Pl.21,22: F,M); Conway & al., 2003 (p.101, figs.F,M, Rem.); Mulyadi, 2004 (p.143, figs.F,M, Rem.); Ferrari & Ueda, 2005 (p.341, figs. juv, F,M); Ferrari & Dahms, 2007 (p.70, fig.25.); Phukham, 2008 (p.55, figs.F,M); Srinui & al., 2019 ( p.90, Rem. F, M); Lee S. & al., 2019 (p.86, Table 3). | 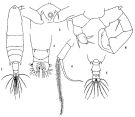 issued from: Q.-c Chen & S.-z. Zhang in Studia Marina Sinica, 1965, 7. [Pl.50, 1-6]. Female (from E China Sea): 1, habitus (dorsal); 2, forehead (ventral); 3, urosome (dorsal); 4, right P5 (anterior). Male: 5, urosome (dorsal); 6, P5 (posterior).
|
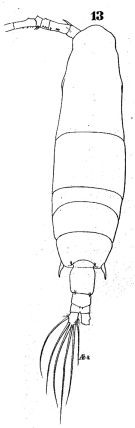 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.43, Fig.13]. Female: 13, habitus (dorsal).
|
 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.43, Fig.12]. Male: 12, thoracic segment 5 and urosome (dorsal).
|
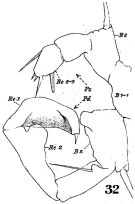
 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.30, Fig.5]. Male: 5, right A1 (segments 18 and 19-21).
|
 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.30, Fig.32]. Male: 32, P5.
|
 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.30, Fig.19]. Female: 19, P5.
|
 issued from : T. Mori in The pelagic Copepoda from the neighbouring waters of Japan, 1937 (2nd edit., 1964). [Pl.50, Figs.1-4]. Female: 1, habitus (dorsal); 2, P5; 3, A1; 4, forehead (frontal view).
|
 issued from : A. Steuer in Arb. zool. Inst. Innsbruck, 1923, 1 (5). [Taf. III, Figs.23, 24]. Female: 23, genital segment (lateral); 24, idem (ventral).
|
 Issued from : G.S. Brady in Rep. Scient. Results Voy. Challenger, Zool., 1883, 8 (23). [Pl.XXXII, Figs.1-11]. With doubt as Acartia laxaFemale: 1,habitus (dorsal); 2, A1; 3, A2; 4, Md; 5, Mx1; 6, Mx2; 7, Mxp; 8, P1; 9, exopodite of one of the swimming leg; 10, P5; 11, last thoracic segment and urosome.
|
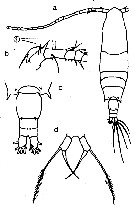
 issued from : N. Phukham in Species diversity of calanoid copepods in Thai waters, Andaman Sea (Master of Science, Univ. Bangkok). 2008. [p.140, Fig.14]. Female (from W Malay Peninsula): a, habitus (dorsal); b, A1; c, proximal segments of A1; d, distal part of thorax and urosome (dorsal); e, P5. Male: f, habitus (dorsal); g, posterior part of thorax and urosome (dorsal); h, P5. Body length after the drawings: F = 1.267 mm; M = 1.183 mm.
|
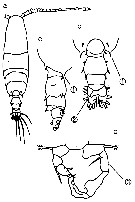
 issued from : Mulyadi in Published by Res. Center Biol., Indonesia Inst. Sci. Bogor, 2004. [p.143, Fig.81]. Female (from Indonesian Seas): a, habitus (dorsal); b, posterior part of last thoracic segment and urosome (dorsal); c, proximal segments of A1; d-h, P1 to P5. Male: i, habitus (dorsal); j, P5.
|
 Issued from : R. Hirota & S. Uno in Bull. Plankton Soc. Japan, 1977, 24 (2). [p.80, Fig.3, E]. Pelagic eggs of Acartia erythraea from vicinity of Amakusa-Matsushima (western Kyushu, Japan).
|
 Issued from : M. Chihara & M. Murano in An Illustrated Guide to Marine Plankton in Japan, 1997. [p.679, Pl. 21, fig.13 a-d]. Female: a, habitus (dorsal); b, A1 (proximal segments); c, last thoracic segment and urosome (dorsal); d, P5. Nota: numbers show caracteristics of this species to compare with A. bispinosa, A. japonica, A. pacifica.
|
 Issued from : M. Chihara & M. Murano in An Illustrated Guide to Marine Plankton in Japan, 1997. [p.680, Pl. 22, fig.13 a-d]. Male: a, habitus (dorsal); b, last thoracic segment and urosome (dorsal); c, same (lateral, right side); d, P5. Nota: numbers show caracteristics of this species to compare with A. bispinosa, A. japonica, A. pacifica.
|
 Erratum: read Srinui and not Siruani. rFemale: 1 - Genital double-somite having paired posyerodorsal processes. 2 - 2nd segment of A1 without strong curved processes. 3 - Exopod of P5 not thickened proximally. 4 - Caudal ramus longer than wide by at most ca 2 times; 2nd free urosomite with small spinules diorsally and posteriorly. 5 - 5th to 7th segments of A1, each lacking hook posteriorly; genital double-somite with pair of small processes dorsally. 6 - 1st segment of A1 with 2 or more strong processes distally. 7 - 2nd segment of A1 with single spinule posteriorly. Male: 1 - Urosomite 3 without spine-like processes dorsally. 2 - Urosomite 4 with prominences dorally; number of dorsal prominenves on urosomite 4 fewer than 5. 3 - Terminal exopodal segment of left P5 with 3 elements.
|
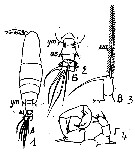 Issued from : M.C. Kos in Field guide for plankton. Zool Institute USSR Acad., Vol. I, 1972. As Acartia (Odontacartia) erythraea. After Steuer, 1923. Female: 1, habitus (dorsal); 2, last thoracic segment and abdomen (dorsal); 3, P5. Male: 4, P5.
|
 Issued from : R. Nakajima, T. Yoshida, B.H.R. Othman & T. Toda in Galaxea, J. Coral Reef Stu., 2013, 15; [p.27, Fig.1, C] C: Blue-pigmented copepod Acartia erythraea female, collected at Tioman Island, Malaysia. Picture taken using halogen white light. Scale bar = 500 µm.
|
 Issued from : S. Lee, H.Y. Soh & W. Lee in ZooKeys, 2019, 893. [p.84, Table 3]. Acartia (Odontacartia) erythraea: Morphological characters. Compare with other Odontacartia species. Nota: 1 - Presence of spine on 1st to 2nd segments of female A1 .............. 2. 2 - Strong spines present on dorsal surface of female genital double-somite .... 3. 3 - 2 strong spines present on dorsal surface of 2nd urosomite.
| | | | | Compl. Ref.: | | | Thompson & Scott, 1903 (p.236, 254); Sewell, 1948 (p.357); Krishnaswamy, 1953 (p.139); Yamazi, 1958 (p.152, Rem.); Ganapati & Shanthakumari, 1962 (p.9, 15); Subbaraju, 1967 (p.18, Table I; developmental time vs latitude); Delalo, 1968 (p.138, as aerythraea); Wellershaus, 1969 (p.276); Patel, 1975 (p.660); Subbaraju & Krishnamurphy, 1972 (p.25, 26, Table 1, 2); Kasahara & al., 1975 (p.25, eggs, cycles); Madhu Pratap & al., 1977 (p.138, Table 3: abundance vs. stations); Tranter, 1977 (p.596); Carter, 1977 (1978) (p.36); Hanaoka, 1977 (p.267, 301, abundance); Hirota & Uno, 1977 (p.77, fig. egg, seasonal abundance); Sreekumaran Nair & al., 1981 (p.493), Grice & Marcus, 1981 (p.125, Dormant eggs, Rem.: p.132, 135, 136); Hirota 1981 (p.19, Table 1, length-weight-CHN); Ueda & al., 1983 (p.165, Table 1, 2, 4 swarms); Guangshan & Honglin, 1984 (p.118, tab.); Uye & al., 1984 (p.390, fig.5, resting eggs-survival); Stephen, 1984 (p.161, Distribution vs thermocline & geographic); Madhupratap & Haridas, 1986 (p.105, tab.2); Mitra & al., 1990 (fig.3); Yoo, 1991 (tab.1); Yoo & al., 1991 (p.258); Dai & al., 1991 (tab.1); Godhantaraman, 1994 (tab.5, 6, 7); Shih & Young, 1995 (p.66); Kotani & al., 1996 (tab.2); Madhupratap & al., 1996 (p.77, Table 2: resting eggs); Marcus, 1996 (p.143); Ohno & al., 1996 (p.231, fig.2); Sharaf & Al-Ghais, 1997 (tab.1); Go & al., 1997 (tab.1, as Acarita: lapsus calami); Ramaiah & Nair, 1997 (tab.1); Achuthankutty & al., 1998 (p.1, Table 2, seasonal abundance vs monsoon)); Hsieh & Chiu, 1998 (tab.2); Wong & al, 1998 (tab.2); Mauchline, 1998 (tab.40); El-Serehy, 1999 (p.172, Table 1, occurrence); Dolganova & al., 1999 (p.13, tab.1); Ueda & al., 2000 (tab.1); Dalal Goswami, 2001 (p.22, fig.2); Hwang & al., 2003 (p.193, tab.2); Shimode & Shirayama, 2004 (p.607, tab.1, 2); Rezai & al., 2004 (p.486, tab.2, 3, abundance); Lo & al., 2004 (p.468, tab.2); Yin & al., 2004 (p.3); Kurihara & al., 2004 (p.721, Rem.: CO2 effects); Rezai & al., 2005 (p.157, Table 2: spatial & temporal variations); Zuo & al., 2006 (p.163: tab.1); Rakhesh & al., 2006 (p.93, Table 2, spatial distribution); Hwang & al., 2006 (p.943, tab.I); Dur & al., 2007 (p.197, Table IV); McKinnon & al., 2008 (p.843: Tab.I, p.848: Tab. IV); Ohtsuka & al., 2008 (p.115, Table 4, 5); Rakhesh & al., 2008 (p.154, abundance vs stations); Ayon & al., 2008 (p.238, Table 4: Peruvian samples); Tseng L.-C. & al., 2008 (p.46, table 2, abundance vs moonsons, fig.8, table 3: indicator species); Hwang & al., 2009 (p.49, fig. 4, 5); Cornils & al., 2010 (p.2076, Table 3, Fig.5); Fazeli & al., 2010 (p.153, Table 1); W.-B. Chang & al., 2010 (p.735, Table 2, abundance); Zhang G.-T. & Wong, 2011 (p.277, fig.6, 7, 8, abundance, indicator); Maiphae & Sa-ardrit, 2011 (p.641, Table 2); Saitoh & al., 2011 (p.85, Table 5); Chen & Liu, 2011 (p.65, figs.5, 6, 7, 8, feeding); Chew & Chong, 2011 (p.127, Table 3, abundance vs location); Beltrao & al., 2011 (p.47, Table 1, density vs time); Kang J.-H., 2011 (p.219, occurrences, inter-annual variability vs t° & Sal., Chl.a, Rem.: p.226); Hsiao & al., 2011 (p.317, Table 2, indicator of seasonal change); Tseng L.-C. & al., 2011 (p.47, Table 2, occurrences vs mesh sizes); Johan & al., 2012 (2013) (p.1, Table 1); Mulyadi & Rumengan, 2012 (p.202, Rem.: p.204); Tseng & al., 2012 (p.621, Table 3: abundance); Jose & al., 2012 (p.20, fig.3 a,b,c: % vs monsoon); Yoshida & al., 2012 (p.644, fig.1, 3, Table 1, egg development time and hatching vs temperature); Chen M. & al., 2012 (p.95, food vs egg production); Jagadeesan & al., 2013 (p.27, Table 3, 4, 5, 6, fig.11, seasonal abundance); Anjusha & al., 2013 (p.40, Table 3, abundance & feeding behavior); Rakhesh & al., 2013 (p.7, Table 1, abundance vs stations); Suzuki, K.W; & al., 2013 (p.15, Table 2, 3, 4, estuaries, annual occurrence); Seo & al., 2013 (p.448, Table 1, occurrence); Varadharajan & Soundarapandian, 2013 (p.2: occurrence vs stations); Oh H-J. & al., 2013 (p.192, Table 1, occurrence); Chen M. & al., 2013 (p.47, clearance & ingestion rate); Nakajima & al., 2013 (p.27, pigmentation); Hwang & al., 2014 (p.43, Appendix A: seasonal abundance); Nakajima & al., 2015 (p.19, Table 3: abundance); Rojas-Herrera & al., 2016 (p.40, Table 2: temporal abundance); Ohtsuka & Nishida, 2017 (p.565, 578, Table 22.1, Fig.22.3: central station-innermost station in Japanese waters) | | | | NZ: | 8 | | |
|
Distribution map of Acartia (Odontacartia) erythraea by geographical zones
|
| | | | | | | | |  Chart of 1996 Chart of 1996 | |
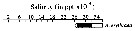 issued from : C.T. Achuthankutty, N. Ramaiah & G. Padmavati in Pelagic biogeography ICoPB II. Proc. 2nd Intern. Conf. Final report of SCOR/IOC working group 93, 9-14 July 1995. Workshop Report No. 142, Unesco, 1998. [p.8, Fig.6]. issued from : C.T. Achuthankutty, N. Ramaiah & G. Padmavati in Pelagic biogeography ICoPB II. Proc. 2nd Intern. Conf. Final report of SCOR/IOC working group 93, 9-14 July 1995. Workshop Report No. 142, Unesco, 1998. [p.8, Fig.6].
Salinity ranges for A. erythraea in coastal and estuarine waters of Goa (India).
Shaded area indicates the range of higher abundance. |
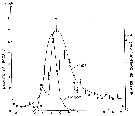 issued from : S. Kasahara, S. Uye & T. Onbé in Mar. Biol.,; 1975, 31. [p.26, Fig.4]. issued from : S. Kasahara, S. Uye & T. Onbé in Mar. Biol.,; 1975, 31. [p.26, Fig.4].
Seasonal cycles of abundance of Acartia erythraea populations and eggs in sea-bottom mud from Sensui-jima (Fukuyama, Japan).
Plankton collected in vertical hauls from 1.5 m above the bottom to the surface (copepods adults and late copepodids. Bottom sediment consisting of soft mud, collected by means of an Eckman-Birge grab-sampler. |
 Issued from : T. Yoshida, C.-F. Liong, A.M. Majid, T. Toda & B.H.R. Othman in Zoological Studies, 2012, 51 (5). [p.651, Fig.3]. Issued from : T. Yoshida, C.-F. Liong, A.M. Majid, T. Toda & B.H.R. Othman in Zoological Studies, 2012, 51 (5). [p.651, Fig.3].
Geographical records of A. pacifica, A. spinicauda, A. erythraea occurrences throughout the East Asian region. |
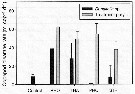 Issued from : M. Chen, H. Liu & B. Chen in Mar. Ecol. Prog. Ser., 2012, 455. [p.100, Fig.1]. Issued from : M. Chen, H. Liu & B. Chen in Mar. Ecol. Prog. Ser., 2012, 455. [p.100, Fig.1].
Acartia erythraea. Clearance rates on different prey species among different treatments and control.
Error bar indicates SD among triplicates.
Treatments combined Dunaliella sp. with either Rhodomonas sp. (RHO), Thalassiosira weissflogii (THA), Prorocentrum dentatum (PRO) or Strombidium sulcatum (STR).
Nota: The clearance rates on PRO and STR at respective treatments were significantly higher than those on Dunaliella indicating an apparent feeding preference on these 2 large-sized prey.
Clearance rates on Dunaliella sp. in the RHO and THA treatments were significantly higher than that in controls, suggesting that the feeding activity was increased in the presence of RHO and THA compared to the controls. |
 Issued from : M. Chen, H. Liu & B. Chen in Mar. Ecol. Prog. Ser., 2012, 455. [p.102, Fig.4]. Issued from : M. Chen, H. Liu & B. Chen in Mar. Ecol. Prog. Ser., 2012, 455. [p.102, Fig.4].
Acartia erythraea. Egg production rates of females fed on different diets.
Error bar indicates SD among triplicates.
Treatments combined Dunaliella sp. with either Rhodomonas sp. (RHO), Thalassiosira weissflogii (THA), Prorocentrum dentatum (PRO) or Strombidium sulcatum (STR).
Nota: Egg production rates of the copepods fed the different diets were significantly different among treatments. The highest egg production rates were observed in the PRO treatment with an average of 38 eggs per female per day, indicating the high food quality of the mixture of PRO and Dunaliella sp. The lowest egg production rate was found in the STR treatment , about 6 eggs per female per day, which was not significantly different from the control, suggesting nutritional inadequacy of the ciliate. |
 Issued from : M. Chen, H. Liu & B. Chen in Mar. Ecol. Prog. Ser., 2012, 455. [p.103, Table 6]. Issued from : M. Chen, H. Liu & B. Chen in Mar. Ecol. Prog. Ser., 2012, 455. [p.103, Table 6].
Acartia erythraea. Coefficients of partial correlation between ingested specific polyunsaturated fatty acids (PUFAs) and egg production rates and egg hatching success.
Ingestion rate of total PUFAs was controlled as the first order factor to avoid co-linearity.
*p <0.05, **p < 0.01, ns: not significant.
Nota: The table illustrates the effect of individual fatty acids among PUFAs on egg production rates, partial correlation coefficients of specific PUFAs are compared.
Significance levels indicated that omega3 unsaturated fatty acids (DHA, EPA, 18:5omega3 and ALA) were highly positively correlated with egg production rates, while stearidonic acid (SDA) and linoleic acid (LIN) were negatively correlated. Overall, the 2 long-chain fatty acids (DHA and EPA) had higher coefficients than the 2 short-chain fatty acids (18:5omega3 and ALA).
EPA = eicosapentaenoic acid. DHA = docosahexaenoic acid. ALA = alpha-linolenic acid. |
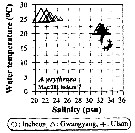 Issued from : J.-H. Kang in Ocean Sci. J., 2011, 46 (4). [p.228, Fig.5] Issued from : J.-H. Kang in Ocean Sci. J., 2011, 46 (4). [p.228, Fig.5]
Abundance-temperature-salinity diagram of A. erythraea observed in the seaports from Korea during 3 years (2007-2009). |
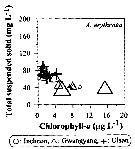 Issued from : J.-H. Kang in Ocean Sci. J., 2011, 46 (4). [p.229, Fig.6] Issued from : J.-H. Kang in Ocean Sci. J., 2011, 46 (4). [p.229, Fig.6]
Abundance-total suspended solid-chlorophyll-a diagram of A. erythraea observed in the seaports from Korea during 3 years (2007-2009). |
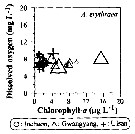 Issued from : J.-H. Kang in Ocean Sci. J., 2011, 46 (4). [p.230, Fig.7] Issued from : J.-H. Kang in Ocean Sci. J., 2011, 46 (4). [p.230, Fig.7]
Abundance-dissolved oxygen-chlorophyll-a diagram of A. erythraea observed in the seaports from Korea during 3 years (2007-2009). |
| | | | Loc: | | | Suez Canal, Red Sea, Gulf of Oman, Arabian Sea, Arabian Gulf, UAE coast, , SE South Africa (Durban, Natal), Indian, Laccadive Is., Karavatti Is., Maldives Is., Seychelles , India (Cochin Backwater, G. of Mannar, Palk Bay, Saurashtra coast, Mangalore coast, S, Bombay, Goa, Pointcalimere-Manamelkudi, Visakhapatnam, Mandapam, Trivandrum coast, Madras, Lawson's Bay, Mandarmani), Sri Lanka, E India (coast, Godavari region, Porto Novo, Madras, Kakinada Bay, Calicut), Bay of Bengal, Nicobar Is., S Burma, Straits of Malacca, Sangga estuary (nearshore-offshore), E Thailand, Nankauri Harbour, Kurau Riv., W Malay Peninsula (Andaman Sea), Cilacap Bay, off Labuan, Indonesian Seas (Jakarta-Seribu Islands, off Tegal, off Surabaya, Ambon Bay, Tioman Is., SW Celebes), Australia (W, Shark Bay, North West Cape, G. of Carpentaria), Indonesia-Malaysia, Tioman Is.), Bintulu coast, Viet-Nam (Cauda Bay), Hainan (Sanya Bay), China Seas (South China Sea, East China Sea, Yellow Sea, Xiamen Harbour), Hong Kong, Taiwan Strait, Taiwan (S, N, SW, Tapong Bay, off Danshuei River, NE), Okinawa, S Korea (S, Gwangyang & Ulsan Harbors, Mokpo), Geumo Is., Japan Sea, Japan (Kyushu: Ariake Sea, Ariake Bay, Tanabe Bay, Fukuyama harbor, Seto Inland Sea, south estuaries, Honshu: Maizuru Bay), W Pacif. (equatorial), Palau Is., off Peru, Acapulco Bay (rare) | | | | N: | 131 | | | | Lg.: | | | (46) F: 1,25; M: 1,1; (164) F: 1,378-1,274; M: 1,196-1,183; (172) F: 1,33; M: 1,18; (290) F: 1,45-1,5; M: 1,35-1,4; (530) F: 1,2; M: 1,1; (795) F: 1,2; M: 1; (866) F: 1,1-1,5; M: 1,1-1,4; (991) F: 1,4; M: 1,2; (1122) F: 1,15; M: 1,08; (1230) F: 1,25-1,4; M: 1,1-1,25; {F: 1,10-1,50; M: 1,00-1,40} | | | | Rem.: | estuary-neritic.
Thompson (1900 c, p.284) suggests that Acartia laxa could be a synonym of A. erythraea taken into account its locality record of origin (Sulu Sea).
Acartia erythraea brehmi Früchtl,1923 (F,M)
Ref.: Früchtl, 1923 (p.449,451); 1924 b (p.58,60, figs.)
Loc.: Indonesia-Malyasia
Acartia erythraea valdiviae Steuer,1923 (F,M)
Ref.: Steuer, 1923 (p.31, figs.F,M); Früchtl, 1923 (p.449); 1924 b (p.58)
Loc.: Molucca Is.
See Acartia australis Table 1 from Ueda (1986, p.18).
See in DVP Conway & al., 2003 (version 1)
After Ueda & al., 1983 (p.167: Table 2 & p.169: Table 4) at Maizuru Bay (Japan): (1)-Shape and diameter of swarm, (2)-depth (m), (3)- swarming position, (4)-number of samples examined, (5)-Mean % of adults (range), (6)- Mean % of females among adults (range) :
(1)- irregular balls (10-30 cm); (2)- o.5-1.5 m; (3)- over white objects; (4)- 3; (5)- 93.5 (85.7-98.3); (6)- 92.9 (89.2-95.8).
Mulyadi (2004, p.151) the species is principally neritic (euryhaline marine).
After Wellershaus (1969), this spcsies were found in samples with more than 29/1000 salinity.
Ohtsuka & Nishida point to in the Inlet and brackish waters on the main islands of Japan, this species is dominant.
After observed and calculated developmental time in weeks: 1.0, 0.95 at 11°39', respectively. | | | Last update : 03/12/2020 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed February 10, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |