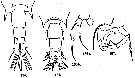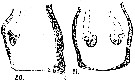|
|
 |
|
Calanoida ( Order ) |
|
|
|
Diaptomoidea ( Superfamily ) |
|
|
|
Acartiidae ( Family ) |
|
|
|
Acartia ( Genus ) |
|
|
|
Odontacartia ( Sub-Genus ) |
|
|
| |
Acartia (Odontacartia) pacifica Steuer, 1915 (F,M) | |
| | | | | | | Syn.: | Acartia spinicauda : Mori, 1937 (1964) (p.103, figs.F);
no Acartia pacifica : Brodsky, 1948 (p.73, figs.); 1950 (1967) (p.422, figs.F,M); Shen & Lee, 1963 (p.582); Chen & Zhang, 1965 (p.112, figs.F,M); Wellershaus, 1969 (p.275, figs.F);
Acartia pacifica forma typica : Steuer, 1923 (p.29, Rem.); Greenwood, 1978 (p.15, figs.F,M);
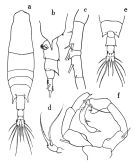
No Acartia pacifica: Al-Yamani & I. Prusova, 2003, (p.91, Figs.F,M). | | | | Ref.: | | | Steuer, 1915 a (p.397, figs.F,M); 1923 (p.28, figs.F,M); Sewell, 1932 (p.397); ? Farran, 1936 a (p.120, figs.F,M); Tanaka, 1965 (p.391, figs.F,M); Greenwood, 1978 (p.15, figs.F,M, Rem.); Yoo & Hue, 1983? (p.11, figs.F,M); Chihara & Murano, 1997 (p.672, Pl.21, 22: F,M); Bradford-Grieve, 1999 b (p.226, figs.F,M, Rem., figs.187, 195); Al-Yamani & Prusova, 2003 (p.92, figs.F,M); Mulyadi, 2004 (p.146, figs.F,M, Rem.); Ueda & Bucklin, 2006 (p.84, figs.F,M, Rem.); Phukham, 2008 (p.58, figs.F,M); Srinui & al., 2019 (p.71, Rem.: p.82, Table 2, fig. 6, 7, table 3: phylogeny); Lee.S & al., 2019 (p.86, Table 3). | 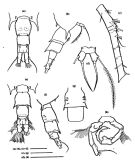 issued from : Ueda H. & Bucklin A.C. in Hydrobiologia, 2006, 500. [Fig.5]. Female: a, b, fifth pediger and urosome (dorsal, lateral view); csecond to sixrth of right antennule (dorsal view); d, fifth legs (posterior view using a cover slip). Male: e, f, fifth pediger and urosome (dorsal, lateral view); g, second and third urosomites (ventral view); h, fifth leg (posterior view using a cover slip). Scales = 0.1 mm.
|
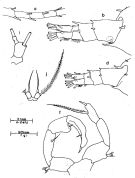
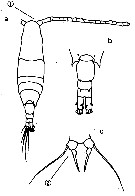
 issued from : O. Tanaka in Publs Seto Mar. Biol. Lab., 1965, XII (5). [p.392, Fig.247]. Female: a, habitus (dorsal); b, last thoracic segment and urosome (left lateral side); c, proximal portion of A1; d, P5. Nota: The urosome segments and furca are in proportional lengths as 43:21:15:21 = 100 Male: e, last thoracic segment and urosome (dorsal); f, P5.
|
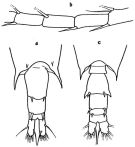
 issued from : J.G. Greenwood in Proc. R. Soc. Qd, 1978, 89. [p.16, Fig.7, a, b, d, f, i, j]. As Acartia pacifica forma typica. Female (from Moreton Bay, E Australia): a, A1 (segments15-19); b, posterior metasome and urosome (dorso-lateral); i, j, P5. Male: d, posterior metasome and urosome (dorso-lateral); f, P5. Remarks: males A. pacifica and A. mertoni with ventrolateral setae on 2nd urosomal segment; 3rd urosomal segment ofmale mertoni with 2 larger spines dorsolaterally on posterior margin, row of 6-8 spinules between. Male P5 slightly different proportions in the two species: in A. pacifica inner projection of right exopodal segment 2 is larger (length from outer border similar to that of segment) with ridge on posterior surface giving slightly bifid appearance; length of left exopodal segment 1 similar to exopodal segment 2 and less than basal segment, inner spine on exopodal segment 2 less than twice length of segment, in A. mertoni length of inner projection on right exopodal segment 2 less than that of segment, inner end rounded, distal end flattened; left exopodal segment 1 longer than exopodal segment 2 or basal segment, inner spine on exopodal segment 2 more than twice length of segment. Females in both species with base of terminal spine gibbose and margins of spine finely denticulate, but seta and spine of similar length in A. mertoni, seta twice length of spine in A. pacifica.
|
 issued from : G.P. Farran in Great Barrier Reef Expedition 1928-29. Scientific Reports, V, No.3. Copepoda. 1936 [p.120, Fig.22]. With doubt. Female: a, last thoracic segment and urosome (dorsal); b, A1 (15th-18th segments). Nota: Acute lateral prolongations of the 5th thoracic segment. Long furcal rami. A pair of large spines on the posterior margin of the genital segment. Slender spines of the 15th, 16th and 8th segments of A1. The terminal spine of P5 has a small lobe at its base. Male: c, last thoracic segment and urosome (dorsal).
|
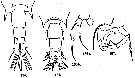 issued from : A. Steuer in Arb. zool. Inst. Innsbruck, 1923, 1 (5). [p.29, Figs.134-137]. Female (from 32°N, 157°W): 134, last thoracic segment and urosome (dorsal); 136a, P5 (ventral); 136b, distal segment of P5 (lateral). Male: 135, last thoracic segment and urosome (dorsal); 137, P5.
|
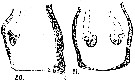 issued from : A. Steuer in Arb. zool. Inst. Innsbruck, 1923, 1 (5). [Taf. III, Figs.20, 21]. Female: genital segment (lateral); 21, idem (ventral).
|
 issued from : A. Steuer in Zool. Anz., 1915, 45 (9). [p.397, Figs.5, 6]. Female: 5, P5. male: 6, P5.
|
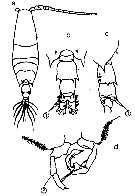
 issued from : N. Phukham in Species diversity of calanoid copepods in Thai waters, Andaman Sea (Master of Science, Univ. Bangkok). 2008. [p.142, Fig.16]. Female (from W Malay Peninsula): a, habitus (dorsal); b, A1; c, posterior part of thorax and urosome (left side); d, urosome (dorsal); e, P5. Male: f, habitus (dorsal); g, last thoracic segment and urosome (dorsal); h, P5. Body length after the drawings: F: = 1.159 mm; M = 1.10 mm.
|
 issued from : Mulyadi in Published by Res. Center Biol., Indonesia Inst. Sci. Bogor, 2004. [p.146, Fig.83]. Female (from Indonesian Seas): a, habitus (dorsal); b, posterior part of last thoracic segment with P5 and genital segment (lateral); c, P1; d, P4; e, P5; f, A1. Male: g, habitus (dorsal); h, P5. Nota Female: Posterolateral ebds of last thoracic segment produced into strong and long spiniform processes, reaching to middle of urosomal segment 1, with a pair of small spines pn dorsal surface (Steuer’s specimen has a pair of single spine instead 2). Dorsal surface of urosomal segments 1 and 2 each with 2 spinules on their dorsal margins distally ; spinules on urosomal segment 2 larger than those of urosomal segment 1. Urosomal segment 1 with a tuft of hairs on ventral surface near periphery of genital opening. Caudal ramus long. A1 reaches to distal end of urosomal segment, 1st and 2nd segments without spines. Nota Male: Urosomal segment 1 with rows of short hairs on lateral and posterior margins ; urosomal segment 2 long and large with 2 ventral small spines ; urosomal segment 3 and 4 each with a pair of spines dorsolaterally ; urosomal 4 shortest. Caudal rami shorter than that of female. Right A1 with segments 13 and 14 finely serrated on anterior margin. Distal segment of left P5 with a long and straight spine.
|
 Issued from : M. Chihara & M. Murano in An Illustrated Guide to Marine Plankton in Japan, 1997. [p.679, Pl. 21, fig.15 a-c]. Female: 15 a, habitus (dorsal); b, last thoracic segment and urosome (dorsal); c, P5. Nota: 1, proximal segment of A1 without spine; 2, P5 with swollen base of spine. Compare with A. japonica, A. bispinosa, A. erythraea.
|
 Issued from : M. Chihara & M. Murano in An Illustrated Guide to Marine Plankton in Japan, 1997. [p.680, Pl. 22, fig.15 a-d]. Male: 15a, habitus (dorsal); 15b, last thoracic segment and urosome (dorsal); 15c, same (lateral); d, P5. Nota: numbers show characteristics of this species to compare with A. erythrea, A. bispinosa, A. japonica.
|
 Issued from : K. Srinui, S. Ohtsuka, E.B. Metillo & M. Nishibori in ZooKeys, 2019, 814. [p.83, Table 2]. Morphological characteristics in Acartia (Odontacartia) pacifica from Japanese and Korean waters. Nota: Compare with Acartia (o) edentata and A. (O. ohtsukai, closely related species.
|
 Issued from : K. Srinui, S. Ohtsuka, E.B. Metillo & M. Nishibori in ZooKeys, 2019, 814. [p.73, Fig.1]. Distribution of Acartia (Odontacartia) pacifica and its sibling species.
|
 Erratum: read Srinui and not Siruani. Female: 1 - Genital double-somite having paired posterodorsal processes. 2 - 2nd segment of A1 without strong curved processes. 3 - Exopod of P5 thickened proximally. 4 - Exopod of P5 with thickened proximal part confined to base of exopod. 5 - Length ratio of outer basal setae to exopod of P5: ca 2.
Male: 1 - Urosomite 3 with large spine-like processes dorsally. 2 - Dorsal processes of urosomite 3 long, reaching half-length of anal somite. 3 - Urosomite 4 lacking spine-like processes between pair of dorsal processes. Genital somite lacking spinular rows along posterodorsal border.
|
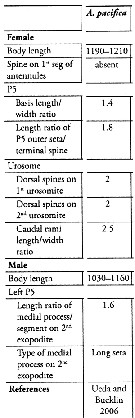 Issued from : S. Lee, H.Y. Soh & W. Lee in ZooKeys, 2019, 893. [p.84, Table 3]. Acartia (Odontacartia) pacifica: Morphological characters. Compare with other Odontacartia species. Nota: 1 - Absence of spine on 1st to 2nd segments of female A1 .....2. 2 - Spine present on dorsal surface of female genital double-somite ..... 3. 3 - Dorsal surface of female genital bouble-somite and 2nd urosomite with 2 strong spines .....4. 4 - Female P5 outer seta longer than terminal spine ..... 5. 5 - Length-width ratio of female caudal rami as 2.5; medial process on 2nd exopodite male left P5 as long seta.
| | | | | Compl. Ref.: | | | Sewell, 1948 (p.322); Yamazi, 1958 (p.153, Rem.); Hanaoka, 1977 (p.267, 301, abundance); Chen Q-c, 1980 (p.794); Greenwood, 1981 (p.K591, fig.1: co-occurrence); Hirota 1981 (p.19, Table 1, length-weight-CHN); Uye & al., 1984 (p.390, resting eggs-survival); Madhupratap & Haridas, 1986 (p.105, tab.2); Othman & al., 1990 (p.561, 564, Table 1); Yoo, 1991 (tab.1); Dai & al., 1991 (tab.1); Hattori, 1991 (tab.1, Appendix); Yoo & al., 1991 (p.259); Myung & al., 1994 (tab.1); Shih & Young, 1995 (p.66); Ohno & al., 1996 (p.231, fig.1); Madhupratap & al., 1996 (p.77, Table 2: resting eggs); Park & Choi, 1997 (Appendix); Ramaiah & Nair, 1997 (tab.1); McKinnon & Klumpp, 1998 (tab.1); Mauchline, 1998 (tab.40); Hsieh & Chiu, 1998 (tab.2); Noda & al., 1998 (p.55, Table 3, occurrence); Nair & Ramaiah, 1998 (p.274); Achuthankutty & al., 1998 (p.1, Table 2, fig.5, seasonal abundance vs monsoon); Dolganova & al., 1999 (p.13, tab.1); Hsiao & al., 2004 (p.325, tab.1); Rezai & al., 2004 (p.486, tab.2, 3, abundance); Chang & Fang, 2004 (p.456, tab.1); Wang & Zuo, 2004 (p.1, Table 2, dominance, origin); Lan & al., 2004 (p.332, tab.1); Lo & al., 2004 (p.89, tab.1); Jiang X. & al., 2004 (p.89, resting eggs vs sediment depth); Choi & al., 2005 (p.710: Tab.III); Rezai & al., 2005 (p.157, Table 2: spatial & temporal variations); Zuo & al., 2006 (p.159, tab.1, abundance, fig.8: stations group); Hwang & al., 2006 (p.943, tab.I); Dur & al., 2007 (p.197, Table IV); Madhu & al., 2007 (p.54, Table 4, abundance vs monsoon); Jitlang & al., 2008 (p.65, Table 1); McKinnon & al., 2008 (p.843: Table. I, p.848: Tab. IV); Ohtsuka & al., 2008 (p.115, Table 5); Moon & al., 2008 (p.61); Lan Y.C. & al., 2008 (p.61, Table 1, % vs stations); Lan & al., 2009 (p.1, Table 2, % vs hydrographiv conditions); Jiang Z.-B. & al., 2009 (p.196, Table 1, 2); Zhang G.-T. & al., 2010 (p.492, Table 1, 2); Cornils & al., 2010 (p.2076, Table 3); Zengling & al., 2010 (p.233, UV-radiation response); Sun & al., 2010 (p.1006, Table 2); Fazeli & al., 2010 (p.153, Table 1); Hsiao S.H. & al., 2011 (p.475, Appendix I); Guo & al., 2011 (p.597, Table 2, fig.9, indicator, abundance); Maiphae & Sa-ardrit, 2011 (p.641, Table 2); Sun X.-H. & al., 2011 (p.741, figs. 5, 3, 4, tab.I, II, production); Zhang D. & al., 2011 (p.86, pH effects); Hsiao & al., 2011 (p.232, Table 1: abundance, %, Table 2: trace metal concentration); Beltrao & al., 2011 (p.47, Table 1, density vs time); Kang J.-H., 2011 (p.219, occurrences, inter-annual variability vs t° & Sal., Chl.a, Rem.: p.224); Hsiao & al., 2011 (p.317, Table 2, indicator of seasonal change); Johan & al., 2012 (2013) (p.1, Table 1); Mulyadi & Rumengan, 2012 (p.202, Rem.: p.204); Yoshida & al., 2012 (p.644, fig.1, 3, Table 1, egg development time and hatching vs temperature); Lee D.B. & al., 2012 (p.88, co-occurrence, abundance, feeding); Huo & al., 2012 (p.1, Table 1: dominance); Yoshida & al., 2012 (p.251, feeding, egg production, Table 2: other data); Lee J. & al., 2012 (p.95, Table 2: abundance, annual & seasonal distribution); Zhang H. & al., 2013 (p.57, Rem. p.58); Suzuki, K.W. & al., 2013 (p.15, Table 2, 3, 4, estuaries, annual occurrence); Seo & al., 2013 (p.448, Table 1, occurrence); Oh H-J. & al., 2013 (p.192, Table 1, occurrence); Nakajima & al., 2013 (p.27, cooccurence); Nakajima & al., 2015 (p.19, Table 3: abundance); Trottet & al., 2017 (p. 7, Table 3: resting stage); Ohtsuka & Nishida, 2017 (p.565, Table 22.1) | | | | NZ: | 7 | | |
|
Distribution map of Acartia (Odontacartia) pacifica by geographical zones
|
| | | | | | | | |  Chart of 1996 Chart of 1996 | |
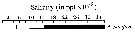 issued from : C.T. Achuthankutty, N. Ramaiah & G. Padmavati in Pelagic biogeography ICoPB II. Proc. 2nd Intern. Conf. Final report of SCOR/IOC working group 93, 9-14 July 1995. Workshop Report No. 142, Unesco, 1998. [p.8, Fig.6]. issued from : C.T. Achuthankutty, N. Ramaiah & G. Padmavati in Pelagic biogeography ICoPB II. Proc. 2nd Intern. Conf. Final report of SCOR/IOC working group 93, 9-14 July 1995. Workshop Report No. 142, Unesco, 1998. [p.8, Fig.6].
Salinity ranges for A. pacifica in coastal and estuarine waters of Goa (India).
Shaded area indicates the range of higher abundance. |
 Issued from : T. Yoshida, C.-F. Liong, A.M. Majid, T. Toda & B.H.R. Othman in Zoological Studies, 2012, 51 (5). [p.651, Fig.3]. Issued from : T. Yoshida, C.-F. Liong, A.M. Majid, T. Toda & B.H.R. Othman in Zoological Studies, 2012, 51 (5). [p.651, Fig.3].
Geographical records of A. pacifica, A. spinicauda, A. erythraea occurrences throughout the East Asian region. |
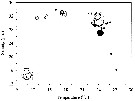 Issued from : S.E. Moon, S. Ohtsuka, H. Ueda & H.Y. Soh in Zootaxa, 2008, 1841. [p.63, Fig.2]. Issued from : S.E. Moon, S. Ohtsuka, H. Ueda & H.Y. Soh in Zootaxa, 2008, 1841. [p.63, Fig.2].
Temperature-salinity-abundance diagram for A. ohtsukai and A. pacifica.
Aundance (ind./m3) of each species is estimated by multiplying numbers on scale by 100 for A. ohtsukai (black circle) and A. pacifica (white circle).
See nota in A. ohtsukai for explanation of the segregation of these two sibling species. |
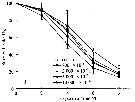 Issued from : D. Zhang, S. Li, G. Wang & D. Guo in Acta Oceanol. Sin., 2011, 30 (6). [p.89, Fig. 1 d]. Issued from : D. Zhang, S. Li, G. Wang & D. Guo in Acta Oceanol. Sin., 2011, 30 (6). [p.89, Fig. 1 d].
Influence of seawater acidification on survival rate in Acartia pacifica.
Average pH of each pCO2 seawater culture. Control (380) 8.16-8.17; (800) : 7.84-7.85; (2000): 7.39-7.37; (5000): 7.19-7.24; (10 000): 6.92-6.94. |
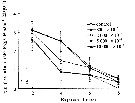 Issued from : D. Zhang, S. Li, G. Wang & D. Guo in Acta Oceanol. Sin., 2011, 30 (6). [p.90, Fig. 2 d]. Issued from : D. Zhang, S. Li, G. Wang & D. Guo in Acta Oceanol. Sin., 2011, 30 (6). [p.90, Fig. 2 d].
Influence of seawater acidification on egg producton rate in Acartia pacifica.
Average pH of each pCO2 seawater culture. Control (380) 8.16-8.17; (800) : 7.84-7.85; (2000): 7.39-7.37; (5000): 7.19-7.24; (10 000): 6.92-6.94. |
 Issued from : J.G. Greenwood in Estuar. Coast. Shelf Sci., 1981, 13. [p.593, Fig. 1, a, b). Issued from : J.G. Greenwood in Estuar. Coast. Shelf Sci., 1981, 13. [p.593, Fig. 1, a, b).
Salinity-temperature-dominance relationships in occurrences of two congeneric species pairs: Acartia tranteri (a) and Acartia pacifica (b).
DS scale refers to grades of dominance (see methods).
This diagram is used to demonstrate differences in ecological requirments of the congenors.
Samples taken by sampling monthly over three years at several stations within the Moreton Bay estuarine system.
The existence of such closely related sympatric species is of great interest concerning interspecific competition and the notion of 'niche theory'. The principles of these topics are summarized by Whittaker (1975), Odum (1971). Possible ways in which zooplanctonic populations may avoid or reduce direct competition have been summatrized by Pejler (1962) as 1- spatial (e.g. by differing horizontal/or vertical distributions; 2- functional differences, e.g. by selective feeding preferences; 3- temporal differences by species populations reaching maxima at different times of the year; and other factors as predators or toxicity.
The two sympatric species coexist within the total range of salinity and temperature conditions sampled in Moreton Bay, they have differing optimal ranges, with one species (A. tranteri) being more eurytopic and in this sense a 'generalist', the other (A. pacifica) finding optimal conditions toward one end of the range of conditions and being more 'specialist', at least in terms of these environmental features. The two species are apparently in close competition during spring and autumn when temperatures are suboptimal for both, the result of this competition depending on wether temperatures are rising or falling. Superimposed on this influence is a difference in salinity optima, with the less euryhaline and more warm thermophilie. A. pacifica competing successfully within its optimal temperature range in the absence of reduced salinities. Populations of A. pacifica may be more variable on a year-to-year basis than those of A. tranteri, depending on the degree of summer (wet season) dilution and that during rising temperatures, replacement of A. tranteri by A. pacifica may occur progressively from outer Bay areas toward inner and estuarine-influenced areas with a dominant status in the community being attained in estuary-mouth regions during periods of low flow. |
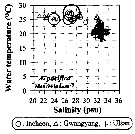 Issued from : J.-H. Kang in Ocean Sci. J., 2011, 46 (4). [p.228, Fig.5] Issued from : J.-H. Kang in Ocean Sci. J., 2011, 46 (4). [p.228, Fig.5]
Abundance-temperature-salinity diagram of A. pacifica observed in the seaports from Korea during 3 years (2007-2009). |
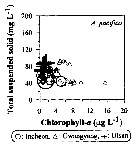 Issued from : J.-H. Kang in Ocean Sci. J., 2011, 46 (4). [p.229, Fig.6] Issued from : J.-H. Kang in Ocean Sci. J., 2011, 46 (4). [p.229, Fig.6]
Abundance-total suspended solid-chlorophyll-a diagram of A. pacifica observed in the seaports from Korea during 3 years (2007-2009). |
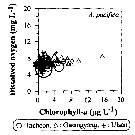 Issued from : J.-H. Kang in Ocean Sci. J., 2011, 46 (4). [p.230, Fig.7] Issued from : J.-H. Kang in Ocean Sci. J., 2011, 46 (4). [p.230, Fig.7]
Abundance-dissolved oxygen-chlorophyll-a diagram of A. pacifica observed in the seaports from Korea during 3 years (2007-2009). |
| | | | Loc: | | | Korea (Gwangyang & Icheon Harbors, Geumo Is.), Japan Sea, Japan, Ariake Bay, Fukuyama Harbor, Seto Inland Sea, Tanabe Bay, south estuaries, off Sanriku, Kuchinoerabus Is., Nakajima Is., W, S & E Korea , Chunsu Bay, Asan Bay, Gwangyang Bay, S Kuril Is., China Seas (Bohai Sea, Yellow Sea, East China Sea, South China Sea, Pearl River, Changjian River estuary, Xiamen Harbour, Jiaozhou Bay), Taiwan Strait, Taiwan (N, E, SW, Tapong Bay, Kaohsiung Harbor, N: Mienhua Canyon, NW), Hong-Kong, off N Hawaii, Australia (Great Barrier, Moreton Bay, Haughton Riv., North West Cape, G. of Carpentaria), India (Gulf of Oman, Bombay, Goa, Penang Is., Cochin, Kerala, Porto Novo), Bay of Bengal, W Malay Peninsula (Andaman Sea), Straits of Malacca, E Thailand, Singapore, Tioman Island; Indonesia-Malaysia, Cilacap Bay (S Java), off Labuan, Jakarta Bay-Seribu Is., off Tegal, off Surabaya, Lombok Sea, Manado Bay (S Celebes Sea), Tioman Is., Bintulu coast (Sarawak, NW Borneo), Viet-Nam (Cauda Bay). | | | | N: | 100 ? (any being confused) | | | | Lg.: | | | ?(34) F: 1,34-1,32; M: 1,3-1,26; (119) F: 1,23; M: 1,23; (164) F: 1,196-1,15; M: 1,124; (290) F: 1,2-1,35; M: 1-1,2; (866) F: 1-1,3; M: 1-1,2; (1122) F: 1,06; M: 1,0; {F: 1,00-1,35; M: 1,00-1,30} | | | | Rem.: | Littoral, ± brackish. Epipelagic.
Existence of resting eggs in sediments (Jiang & al., 2004, p.89).
Acartia pacifica mertoni Steuer,1923 (F,M)
: Cf. Acartia mertoni.
Confounded before 2006 with Acartia othsukai. Some complementary references are uncertain, lacking figures and precise description.
For Srinui & al. (2019, p.82) Acartia pacifica morphologically and genetically consists of several cryptic species (see in Ueda & Bucklin, 2006; Srinui & al., unpublished). In conclusion the japanese and Korean specimens of A (O) pacifica analysed clearly coincided with A (O) pacifica s.s. as morphologically/genetically redefined by Ueda & Bucklin (2006). After these authors, temperature and salinity appear to be important factors determining the distribution and abundance of this species in the Indo-West Pacific. In the East China Sea (subtropical zone), the species was abundant in August (salinity 15.0 per 1000) in the Changiiang (Yangtze River) Estuary, China, while in Korean waters, this species is strictly stenohaline, occuring waters more than 32 in salinity. Kang (2011) observed A (O.) pacifica and A (O.) erythraea in Korean waters with temperature ranges of 18.0-27.2 °C and 14.6-26.4 °C and salinity ranges of 21.0-32.9 and 21.0-33.7, respectively. In Japanese waters A (O.) ohtsukai was found in the estuary of the Rokkaku River, Ariake Bay in surface waters, where water temperature was 29.0 °C and 33.0 in the Seto Inland Sea (Japan) (Ueda & Bucklin, 2006). Furthermore A (O.) pacifica was dominant in Moreton Bay (Queensland) waters with temperature ranges above 22.0 °C and salinities ranging from 34.0 to 36.5 (Greenwood, 1981). In Bintulu, Sarawak (Malaysia), Johan & al. (2013) compared to A (O.) pacifica in costal waters with temperatures of 28.8-29.0 °C and high salinities (24.0-32.0). In the Indian waters, Wellershaus (1969) recorded female speimens of this species in waters with salinity ranging from 10.0 to 230.0, including in the Andaman Sea, Thailand (Surin Islands) with temperatures of 29.7-31.0 and salinities ranging from 29.9 to 35.8. °C.
The authors conclude that three species of Acartia (i.e. A. (O) pacifica, A. (O.) edentata and A. (O. ohtsukai appear to occupy water bodies differing in temperature and salinityof the tropical and subtropical zone of the Pacific and Indian oceans. | | | Last update : 09/12/2021 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed January 31, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |