|
|
 |
|
Calanoida ( Order ) |
|
|
|
Calanoidea ( Superfamily ) |
|
|
|
Calanidae ( Family ) |
|
|
|
Calanoides ( Genus ) |
|
|
| |
Calanoides carinatus (Krøyer, 1848) (F,M) | |
| | | | | | | Syn.: | In litt. anterior to 2017 :
Calanus carinatus Krøyer, 1848; With, 1915 (p.11, Rem.F,M); Farran, 1926 (p.229); 1929 (p.207, 215, Rem.); Lysholm & al., 1945 (p.6); Sewell, 1948 (513); Brodsky, 1972 (1975) (p.9, 71, 86, 120, figs.F,M); Björnberg, 1973 (p.283: Rem.); Vyshkvartzeva, 1976 (p.14); 1977 a (p.97, figs.); Svetlichnyi, 1980 (p.28, Table 1, passive submersion); Schnack & Elbrächter, 1981 (p.433, fig.3, gut contents); Kovalev & Shmeleva, 1982 (p.82); Peterson & Painting, 1990 (p.283, developement rate); Gilabert & Moreno, 1998 (tab.1, 2);
no Calanoides carinatus : Vervoort, 1946 (p.29, figs. juv.5F, Rem.); Bradford, 1970 a (p.352, figs.M, Rem.); 1972 (p.32, figs.F);
Calanus frontatus mihi Dahl, 1894 (p.76);
(?) Calanus brevicornis Lubbock, 1856; Giesbrecht, 1892 (p.90, 127, figs.F,M); Giesbrecht & Schmeil, 1898 (p.16); Cleve, 1904 a (p.185); Wolfenden, 1911 (p.193); Sars, 1925 (p.7); Rose, 1929 (p.7); 1933 a (p.60, figs.F,M); Dakin & Colefax, 1933 (p.203); Oliveira, 1945 (p.191); Gaudy, 1963 (p.19, Rem.); Seguin & al., 1993 (p.23);
(?) Calanoides brevicornis : A. Scott, 1909 (p.10); Früchtl, 1924 b (p.33); Sars, 1925 (p.7); Sewell, 1929 (p.25: Rem.); Rose, 1929 (p.215); 1933 a (p.60, figs.F,M); Dakin & Colefax, 1940 (p.89, figs.F,M); Tanaka, 1937 (p.251, figs. juv.F); C.B. Wilson, 1950 (p.174); Marques, 1953 (p.91); 1958 (p.205); Fleminger, 1985 (p.275, 285, Table 1, 4, Rem.: A1); Aboul Ezz & al., 2014 (p.283, Rem.: p.284);
Non Calanoides nataliensis Brady, 1914 (see in: Höring & al., 2017 (p.618: genetic structure)
Syn. after Bradford-Grieve & al., 2017 (p.815) :
Calanus carinatus Krøyer, 1848/9 (p.554)
Calanus brevicornis Giesbrecht, 1892/3, pl.7, fig.10; pl.8, figs 5, 28.
Calanus frontatus Dahl, 1894 (p.74-76)
Calanoides carinatus: Sabatini & al., 2007 (p.345-354, figs 2-8)
non Calanus carinatus : Bode & al., 2013; 2014; 2015 [probably Calanoides natalis] | | | | Ref.: | | | In litt. before 2017 : Farran & Vervoort, 1951 (n°32, p.3, figs.F,M); Carvalho, 1952 a (p.137); Tanaka, 1958 (p.259); Vervoort, 1957 (p.29, Rem.); Tanaka, 1960 (p.16, figs.F, juv.F, Rem.); Vervoort, 1963 b (p.81, Rem.); Tanaka, 1964 (p.5); Chen & Zhang, 1965 (p.28, figs.F); Ramirez, 1966 (p.7, figs.F,M); Vilela, 1968 (p.8, Rem.); Kitou & Tanaka, 1969 (p.71, fig.F); Ramirez, 1969 (p.38, figs.F, Rem.); Corral Estrada, 1970 (p.68, figs.F,M, Rem.); Razouls, 1972 (p.94, Annexe: p.9); Bradford & Jillett, 1974 (p.6); Hirche, 1980 (p.115, figs.: O, N); Björnberg & al., 1981 (p.604, 619, fig.FM); Zheng & al., 1982 (p.9, figs.F); Alvarez-Marques, 1984 (p.117, figs.F,M); Roe, 1984 (p.356); Fleminger, 1985 (p.273, 285, Table 1, 4, Rem.: A1, Rem.); Sazhina, 1985 (p.27, figs.N); Bradford, 1988 (p.77, 78: Rem.); Nishida, 1989 (p.173, table 1, 2: dorsal hump); Schnack, 1989 (p.137, tab.1, fig.6, 7: Md); He & al., 1992 (p.250); Bradford-Grieve & al., 1999 (p.877, 907, figs.F); Ramirez & Sabatini, 2000 (p.26, figs.F,M); Sywula & al., 2002 (p.523, génétique); Conway, 2006 (p.9: copepodite stages 1-6, Rem.); Vives & Shmeleva, 2007 (p.890, figs.F,M, Rem.); Sabatini & al., 2007 (p.345, Redescr.F,M, figs.F,M, Rem); Blanco-Bercial & al., 2014 (p.5: Rem.: problematic taxa); Höring, 2014 (p.1, genetic diversity vs geographic distribution);
Viñas & al., 2015 (p.97, phylogeography, molecular index); Höring & al., 2017 (p.618, phylogeographic distribution, Rem.); Bradford-Grieve & al., 2017 (p.807, 815, Table 1, 5, fig.2, phylogeny) |  issued from: Q.-c Chen & S.-z. Zhang in Studia Marina Sinica, 1965, 7. [Pl.2, 9-11]. Female (from E China Sea): 9, habitus (dorsal); 10, idem (lateral left side); 11, forehead (ventral). = Calanoides not yet identified.
|
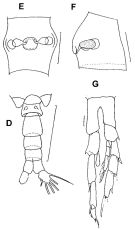 issued from : F.C. Ramirez & M.E. Sabatini in Hydrobiologia, 2000, 439. [p.29, Fig.5]. Female (from off Argentina coast): E, spermatheca (dorsal view); F, idem (lateral view). Male: D, urosome (dorsal); G, P5. Scale bars in mm: 0.03 (E, F); 0.2 (D); 0.025 (G).
|
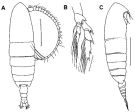 issued from : F.C. Ramirez & M.E. Sabatini in Hydrobiologia, 2000, 439. [p.29, Fig.5]. Female: A, habitus (dorsal); B, P5; C, habitus (lateral right side). Scale bars in mm: 1 (A, C); 0.2 (B).
|
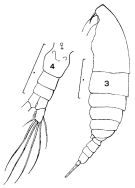 issued from : F.C. Ramirez in Bol. Inst. Biol. Mar., Mar del Plata, 1966, 11. [Lam.I, Figs.3, 4]. Female (from off Mar def Plata): 3, habitus (lateral left side); 4, urosome (dorsal). Scale bars in mm: 0.5 (3, 4).
|
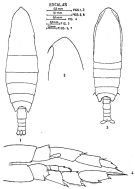 issued from : J. Corral Estrada in Tesis Doct., Univ. Madrid, A-129, Sec. Biologicas, 1970. [Lam.6, figs.1-4]. Female (from Canarias Is.): 1, habitus (dorsal); 2, forehead (lateral). Male: 3, habitus (dorsal); 4, P5. probably = C. natalis.
|
 issued from : Z. Zheng, S. Li, S.J. Li & B. Chen in Marine planktonic copepods in Chinese waters. Shanghai Sc. Techn. Press, 1982 [p.10, Fig.3]. Female: a, habitus (dorsal); b, forehead (lateral); c, urosome (lateral, left side); d, idem (ventral); e, forehead with rostrum (ventral); f, Md (mandibular palp); g, P1; h, P3; i, P5. Scale bar in mm. = Calanoides not yet identified .
|
 issued from : M. Kitou & O. Tanaka in Oceanogr. Mag., 1969, 21 (1). [p.71, Fg.3]. Female (from Antarctic): a-b, forehead (lateral and dorsal, respectively); c-d, last thoracic segment and urosome (lateral and dorsal, respectively); e, genital complex (ventral); f, A2; g, md; h, Mx12; i, Mx2; j, Mxp; k, P1; l, P2; m, P4; n, P5. = Calanoides not yet identified .
|
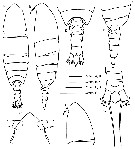 issued from : M. E. Sabatini, F. C. Ramirez & J. Bradford-Grieve in Invert. Syst., 2007, 21. [p.344, Fig.2]. Female (from Brazil, type locality): A-B, habitus (dorsal and lateral, respectively); C, forehead (dorsal); D, cephalosome (lateral); E, 5th thoracic somite and urosome (dorsal); F, 4th and 5th thoracic somites and urosome (lateral); G, 4th urosomal somite, anal somite and caudal rami (dorsal). Forehead triangular (dosal view), accentued anteriorly by low carina ; in lateral view, fronral margin projects anteriorly with 2 long rostral filaments. Urosome ± 25 % of prosome length. Caudal rami with 6 setae, 1 relatively short outer seta, 1 long seta located posterolaterally, 3 long, strong terminal setae and 1 small, curved inner seta.
|
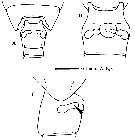 issued from : M. E. Sabatini, F. C. Ramirez & J. Bradford-Grieve in Invert. Syst., 2007, 21. [p.345, Fig.3]. Genital double-somite female: A, dorsal vies, B, ventral view; C, lateral view (right side). Nota: Genital double-somite swollen laterally, slightly anterior to mid-length; in lateral view, spermathecae extend parallel to posterior border of somite, extending just beyond central axis of segment; lateral margin of the genital operculum visible as a flap-like structure.
|
 issued from : M. E. Sabatini, F. C. Ramirez & J. Bradford-Grieve in Invert. Syst., 2007, 21. [p.347, Fig.4]. Female: A-B, Md, mandibular gnathobase (left and right respectively); C, Md, mandibular palp); D, A1; E, A2; F, Mx1; G, Mxp; H, Mx2. . A1 25-segmented, extending as far the distal border of caudal rami. A2 biramous with separate coxa and basis, bearing 1 and 2 distal setae on inner margin, respectively ; exopod 7-segmented, armatute formula on inner margin 2, 2, 1, 1, 1, 1, 4 setae ; endopod 2-segmented, segment 1 elongated with 2 distal setae on inner margin, segment 2, short, bilobed, with 9 and 7 setae on inner an douter lobes, respectively and 2 distinct rows of 2 and 5 small setules on outer margin. Md gnathobases symmetrical on right and left, each with 8 strong teeth that increase in size posteriorly and with 1 anterior small seta spinulose. Palp comprising basis with 4 inner setae, exopod 5-segmented with armature formula 1, 1, 1, 1, 2 setae, respectively ; endopod 2-segmented, 1st segment with inner prominent lobe bearing 4 setae, segment 2 with 11 setae. Mx1 praecoxa with well developed arthrite produced distally, bearing 15 setae (of which 6 are short, thick and curved located distally, 2 straight, thick, and 2 thin setae placed more proximally, another 4 placed on posterior surface and another thin, delicate setalocated on anterior surface) ; coxal epipodite bearing 9 setae, basal exite with 1 short seta ; exopod bears 11 setae ; endopod 3-segmented with setation formula 4, 4, 7 setae, 2 basal endites bearing 4 setae each ; coxal endite with 4 setae. Mx2 endites 1-4 with 6, 3, 3, 3 setae, respectively ; basal endite with 4 setae ; endopod with 3, 3, 2, 2 setae ; coxal epipodite with 1 plumose seta ; delicate relatively long hairs cover surfaces of internal lobes. Mxp 7-segmented ; syncoxa with 1, 2, 4, 4 setae on endites 1-4; basis and endopod segment 1 fused, with proximal row of spinules, 3 setae at midlength and 2 distal endopod setae ; free endopod segments with 4, 3, 3, 3 +1, 4 setae.
|
 issued from : M. E. Sabatini, F. C. Ramirez & J. Bradford-Grieve in Invert. Syst., 2007, 21. [p.348, Fig.5]. Female: A-E, P1 to P5, respectively (outer spineof the basis is shown for P2-P5). Nota: Swimming legs P1-P5 with all exopods and endopods 3-segmented. Armature formulae as in the Calanidae. P1 coxa has group of fine spinules near base of inner seta; basis inner distal seta S-shaped and directed externally; exopod segment 1 has small conical lobe on its posterior surface; endopod segments 1 and 2 have rounded outer borders (P2-P5 have these borders pointed).
|
 issued from : M. E. Sabatini, F. C. Ramirez & J. Bradford-Grieve in Invert. Syst., 2007, 21. [p.350, Fig.6]. Male (from Brazil): A, habitus (lateral); B, cephalic dorsal hump, (CDH sensu Nishida, 1989); C, posterior dorsal protrusion; D, P5; E, P5, detail of left basis; F, P5, detail of right basis (arrowhead indicates small tooth); G, P5, left endopod; H, P5, left exopod segment 3; I, P5, right endopod. Forehead viewed dorsally without carina. Anterior cephalosome broadly rounded in lateral view, with anterior cephalic dorsal hump (CDH sensu Nishida, 1989) opposite antenna and second posterior dorsal protrusion midway between Mxp and P1 (same appearance as CDH but slightly different in shape and size) . : P5 asymmetrical, extending slightly beyond caudal rami. Coxa with non serrate inner border, longer on left side than right. Left coxa noticeably wrinkled distally where it covers proximal part of basis in anterior view. Outer border of both left and right basis bears spine-like extension adjacent to blunt projection and fine hair. Distoanterior border of right basis, at base of exopod segment 1, bears small tooth. Right and leeft exopods 3-segmented, right wider than left. Right exopod shorter than the left esopod, with distal border of right segment 3 extending ± 0.80 of way along whole left exopod or 0.50 along left exopod segment 2. Right exopod segment 2 has short internal seta and long outer spine, slightly shorter than inner edge of exopod segment 3 ; Right exopod segment 3 with thick terminal spine that reaches distal border of left exopod segment 3. Left exopod segment 2 with long outer distal spine, 0.88-1.05 x length of exopod segment 3 measured along inner border. Left exopod segment 3 ratio of length (measured along outer berder) to widest width 2.26-2.59 ; ornamented on anterior surface with 7 transverse rows of long hair-like spinules ; with slender subterminal spine (widened at its base), which about equal to length of outer border of segment and sets at an angle of ± 20-40° to the inner border of segment (See fig.1 E) ; with 2 glandular pores (1 at base of outer, midlength spine on anterior surface and 1 on papilla at distal outer angle of segment adjacent to short spine ; Right endopod (fig. I) 3-segmented, segment 1 with either remnant of, or short seta, segment 2 smooth or with indication of insertion point of seta, segment 3 provided with 4 inner setae, proximal-most of which short and hardly extending beyond base of more distal seta. Right endopod segment 3 reaches distal border of right exopod segment 2 ; left endopod variable, reduced to 1-segmented stump ( although hints of limits to each segment may be discernible depending on plane of examination and focus) ; 2 subtle pumose setae (most often broken), and marginal group of spinules (evident at high magnification) (fig. G).
|
 issued from : M. E. Sabatini, F. C. Ramirez & J. Bradford-Grieve in Invert. Syst., 2007, 21. [p.351, Fig.7]. Male: A, Md (mandinular palp); B, Md (gnathobase); C, Mx1; D, A1; E, Mx2; F, A2; G, Mxp. . A1 23-segmented, extending to at least the anal somite. Ancestral segments I-IV and V-VI fused, although weal lines of separation occasionally evident. A2 with same structure as in female although reduced in size and setation. Mandibular palp similar tothat of female but setation differs. Only 3 slender setae on inner border of basis, proximal seta very small, prominent lobe on inner margin of endopod segment 1 carries only 2 plumose setae (4 in female), and segment 2 bears 9 setae (11 in female) ; mandibular gnathobase much reduced in size with degenerate teeth, cutting edge with only single tooth and 1 small plumose seta. Mx1 strongly reduced in size ; praecoxal arthrite with only 11 short plumose setae (one of them placed distally on anterior surface) ; coxal and basal endites 1 and 2 provided with 4 strong setae each ; endopod bearing 4, 4, 7 plumose setae on segments 1-2 ( and ; exopod bearing 11 plumose setae ; coxal epipodite with 9 strong setae and basal exite much reduced , with 1 thin seta. Mx2 extremely reduced and delicate,setation identical to female. Mxp reduced in length compared with female ; syncoxa with 2 short setae, thin, located near articulation with basis ; basis has row of short, delicate setules located proximally, 3 setae at midlength and 2 distal setae (1 very thin and other much thicker) on incorporated endopod segment 1 ; free endopod segments 2-6 bear 4, 3, 3, 3, 3 setae as well as 1 outer, strong plumose setae directed proximally on each two distal segments.
|
 issued from : M. E. Sabatini, F. C. Ramirez & J. Bradford-Grieve in Invert. Syst., 2007, 21. [p.352, Fig.8]. Male: A-D, P1 ti P4, respectively), outer spine of the basis is shown for P2-P4. Nota : P1-P4 similar, though smaller than those of female. P1 coxa lacks group of fine spinules near base of inner seta (present in the female).
|
 issued from : M. E. Sabatini, F. C. Ramirez & J. Bradford-Grieve in Invert. Syst., 2007, 21. [p.359, Fig.15]. Male: left P5 exopod segment 3from three locations: A-B, Calanoides carinatus s.s; from Brazil (cruise 2003); C-D, Calanoides cf. carinatus from Argentina (cruise 1989); E-G, Calanoides macrocarinatus from New Zealand).
|
 issued from : M. E. Sabatini, F. C. Ramirez & J. Bradford-Grieve in Invert. Syst., 2007, 21. [p.360, Fig.16]. Male P5 as figured in the literature: A, Calanoides cf. carinatus, Argentina (after Ramirez & Sabatini, 2000); B, Calanus carinatus, West coast of Africa, Tropic of Cancer (after Brodsky, 1972); C, Calanus carinatus, South Africa, Cape of Good Hope (after Brodsky, 1972); D, Calanoides carinatus, New Zealand (after Bradford, 1970) = C. macrocarinatus (Bradford, 1974); E, calanus brevicornis, Atlantic Ocean (re-drawn after Giesbrecht, 1892).
|
 issued from : M. E. Sabatini, F. C. Ramirez & J. Bradford-Grieve in Invert. Syst., 2007, 21. [p.362, Table 5]. Comparison characters to distinguish Males C. carinatus and C. macrocarinatus
|
 issued from : M. E. Sabatini, F. C. Ramirez & J. Bradford-Grieve in Invert. Syst., 2007, 21. [p.361, Table 4]. Comparison characters to distinguish Females C. carinatus and C. macrocarinatus.
|
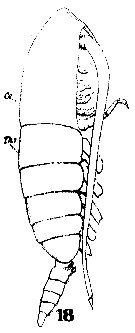 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf. 6, Fig.18]. As Calanus brevicornis. With doubt as Calanoides carinatus. Female: 18, habitus (lateral).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf. 7, Fig.11]. As Calanus brevicornis. With doubt as Calanoides carinatus. Female: 11, Md (masticatory edge; anterior view).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf. 8, Fig.5]. As Calanus brevicornis. With doubt as Calanoides carinatus. Male: 5, A1 (ventral view).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf. 7, Fig.10]. As Calanus brevicornis. With doubt as Calanoides carinatus. Male: 10, Md (mandibular blade).
|
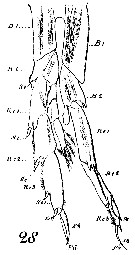 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf. 6, Figs. 7, 9]. As Calanus brevicornis. With doubt as Calanoides carinatus. Male: 28, P5 (anterior view).
|
 issued from : M. E. Sabatini, F. C. Ramirez & J. Bradford-Grieve in Invert. Syst., 2007, 21. [p.343, Fig.1]. Indication of the measurements made on Calanoides carinatus specimens from the South West Atlantic (DData in Table 2 & 3) in Table 2,. A, body size; B, shape of the head in female (X and Y are maximum width and 'height' in dorsal view); C, asymmetry of male P5; D-E, key characters of male P5 left exopods 2 and 3.
|
 issued from : M. E. Sabatini, F. C. Ramirez & J. Bradford-Grieve in Invert. Syst., 2007, 21. [p.346, Table 1]. Body dimensions (micrometers) of female Calanoides carinatus holotype and specimens from Brazil. s.d.: standard deviation; Cv: coefficient of variation.
|
 issued from : M. E. Sabatini, F. C. Ramirez & J. Bradford-Grieve in Invert. Syst., 2007, 21. [p.349, Table 2]. Body dimensions (micrometers) of male Calanoides carinatus holotype and specimens from Brazil. s.d.: standard deviation; Cv: coefficient of variation.
|
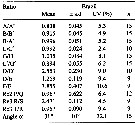 issued from : M. E. Sabatini, F. C. Ramirez & J. Bradford-Grieve in Invert. Syst., 2007, 21. [p.352, Tanle 3]. Assymmetry of male P5 and key features of left exopod segments 2 and 3 of male Calanoides carinatus from Brazil. s.d.: standard deviation; Cv: coefficient of variation.
|
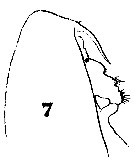 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.6, Fig.7]. As Calanus brevicornis. With doubt as Calanoides carinatus. Female: 7, forehead (lateral).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.6, Fig.9]. As Calanus brevicornis. With doubt as Calanoides carinatus. Female: 9, forehead (doesal).
|
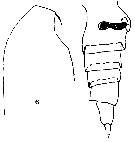 Issued from : F. Alvarez-Marques in Rev. Biol. Univ. Oviedo, 1984, 2. [p.116, Pl. II, Figs.6, 7]. Female (from off Gijon, NW Spain): 6, forehead (lateral); 7, urosome (lateral) with spermatheca.
|
 Issued from : F. Alvarez-Marques in Rev. Biol. Univ. Oviedo, 1984, 2. [p.116, Pl. II, Fig.8]. Male: 8, P5 (exopod of right leg incomplete, missing exopodal segments 2 and 3)
|
 Issued from : O. Tanaka in Spec. Publs. Seto mar. biol. Lab., 10, 1960 [Pl. IV]. Female (from off Cape of Good Hope): 1, habitus (dorsal); 2, forehead (lateral); 3, last thoracic segment and urosome (lateral); 4, genital segment (ventral); 5, P1; 6, P2; 7, P4; 8, P5. At the same scale for figs. 4-8. Immature female (5th copepodid stage): 9, last thoracic segment and urosome. Nota adult female: Cephalothorax and abdomen in the proportional lengths 82 to 18. Head in lateral aspect slightly crested on the apical portion. Rostral filaments long and slender. Posterior lateral margin of the thoracic segment narrowly rounded in lateral view, extends about to the 2/5 of the genital segment. Proportional lengths of the abdominal segments and caudal rami 41 : 16 : 12 : 12 : 19 = 100. Genital segment slightly wider than long.Genital boss produced slightly below. Receptacles large, extend horizontally, when viewed from the lateral, toward the dorsal. Genital flap semi-circular and small. Caudal rami about 1.6 times as long as wide. Basis of P4 with a small seta at the base of the outer edge spine; this spine is absent in P2 and P3. P1 has on the 1st exopodal segment a small conical process on the posterior surface about the middle of the segment which is clearly senn in lateral aspect. In P5 the outer marginal spine divides the outer margin of the 3rd exopodal segment in the proportions 5 : 3. The outer margin of the 3rd endopodal segment has no marginal seta; the basis has a small seta at the base of the outer edge spine. Calanoides species not yet identified
|
 Issued from : O. Tanaka in Spec. Publs. Seto mar. biol. Lab., 10, 1960 [p.17]. Female: Proportional length of segments of A1. Nota: A1 25-segmented. Calanoides species not yet identified
|
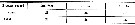 Issued from : O. Tanaka in Spec. Publs. Seto mar. biol. Lab., 10, 1960 [p.17]. Female: The outer marginal spine on the 3rd exopodal segment of the P2 to P4 divides the outer margin in the proportions as in the table herewith. These figures differ slightly from those given by Giesbrecht. Calanoides species not yet identified
|
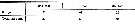 Issued from : O. Tanaka in Spec. Publs. Seto mar. biol. Lab., 10, 1960 [p.17]. Female: The 3rd exopodal segment of the P2 to P4 and the terminal spine of the same segment are in the proportions as in the table herewith. Calanoides species not yet identified
|
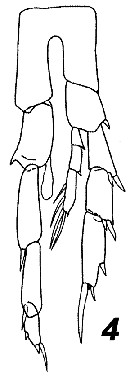 Issued from : V.N. Andronov in Russian Acad. Sci. P.P. Shirshov Inst. Oceanol. Atlantic Branch, Kaliningrad, 2014. [p.73, Fig.19, 4]. Calanoides carinatus after Ramirez, 1966. Male P5.
|
 Issued from M. Rose in Résult. Camp. scient. Prince Albert I, 1929, 78, p.7. Remarks concerning Calanoides brevicornis ( = Calanoudes carinatus) identifié aux cours des campagnes de 1908, 1910, 1912 et 1914. To translate as Calanoides natalis.
|
 Issued from : J.M. Bradford-Grieve, L. Blanco-Bercial & I. Prusova in J. Nat. Hist., 2017, 51 (13-14) [p.827, Fig. 12]. Charactristic to discriminate between females of C. natalis, C. carinatus and C. brevicornis.
|
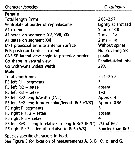 Issued from : J.M. Bradford-Grieve, L. Blanco-Bercial & I. Prusova in J. Nat. Hist., 2017, 51 (13-14) [p.828, Table 5]. Character states distinguished known of Calanoides. A1 = antennule, a = aesthetasc; approx. = approximately; CR = caudal rami; Gns = genital double-somite; Go = genital operculum; ms = modified seta; Pd5 = pedigerous somite 5; P5 = fifth leg; Re = exopod; Ri = endopod; SR = seminal receptacle; St = terminal spine.
|
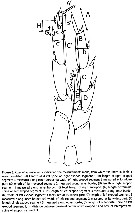 ssued from : J.M. Bradford-Grieve, L. Blanco-Bercial & I. Prusova in J. Nat. Hist., 2017, 51 (13-14) [p.812, Fig. 3]. Calanoides male P5 indicating the measurements made, from which the ratios in Table 5 were calculated.
| | | | | Compl. Ref.: | | | Confounded by numerous authors with Calanoides natalis mainly in the NE and SE Atlantic and NW and SW Indian Oceans.Sewell, 1948 (p.347, 513, 544, 555); Kott, 1957 (p.5, Rem.: p.13); Björnberg, 1963 (p.17, Rem.); Unterüberbacher, 1964 (p.13, Rem.); De Decker, 1964 (p.14, 17, 30); De Decker & Mombeck, 1964 (p.11); Neto & Paiva, 1966 (p.17, Table III, annual cycle); Mazza, 1966 (p.69); Ehrhardt, 1967 (p.736, 887, figs.10, 51-53, geographic distribution), Rem.); De Decker, 1968 (p.45); Vinogradov, 1968 (1970) (p.277); Bainbridge, 1972 (p.61, Appendix Table I: vertical distribution vs day/night, Table II: %, Table IV: seasonal abundance); Binet & al., 1972 (p.69); Ibanez & Seguin, 1972 (p.81, annual cycle, multivarite analysis); Roe, 1972 (p.277, tabl.1, tabl.2); 1972 a (p.321); Lefèvre-Lehoërff, 1972 (p.1681); Binet, 1973 (p.77); Corral Estrada & Pereiro Muños, 1974 (tab.I); Vives & al., 1975 (p.35, tab.II); Boucher & al., 1975 (p.85, nutrition/enzyme); Petit & Courties, 1976 (p.177, annual cycle); Tranter, 1977 (p.596, 601: Rem., fig.2); Grindley, 1977 (p.341, Table 2, fig.2); Tomasini & Petit, 1977 (p.1, development time); Carter, 1977 (1978) (p.35); Courties, 1978 (p.1, figs., seasonal abundance); Dessier, 1979 (p.53, 204); Andronov & Maigret, 1980 (p.71, Table 1, 2, 3); Brenning, 1981 (p.1, spatial distribution, T-S diagram, Rem.); Vives, 1982 (p.290); Smith S.L., 1982 (p.1331, abundance, monsoon effect, feeding); S. B. Schnack, 1982 a (p.303, feeding); De Decker, 1984 (p.315, 329: chart); Guangshan & Honglin, 1984 (p.118, tab.); Greze & al., 1985 (p.9); Brenning, 1985 a (p.24, Table 2); Valentin & al., 1986 (p.117, temporal variations); Madhupratap & Haridas, 1986 (p.105, tab.1); Kosobokova & al., 1988 (p.375, biochemical characteristics); Verheye, 1989 (p.1, dynamic and production); Lozano Soldevilla & al., 1988 (p.57); Timonin, 1990 (p.479); Valdes & al., 1990 (tab.2); Perterson & Painting, 1990 (p.283, population dynamic); Peterson & al., 1990 (p.85, feeding rates: methods); Peterson & al., 1990 (p.259, Table V, feeding); Othman & al., 1990 (p.568, Rem.); Fernandez Araoz, 1991 (p.575); Santos & Ramirez, 1991 (p.79, 80, 82, 83); Huntley & Lopez, 1992 (p.201, Table A1, egg-adult weight, temperature-dependent production); Seguin & al., 1993 (p.26, Rem.); Klein Breteler & al., 1994 (p.1039, fig. 5, 6, 8, Rem.: p.1052, stage duration); Hays & al., 1994 (tab.1); Heinrich, 1995 (tab.1); Shih & Young,1995 (p.68); Arashkevich & al., 1996 (p.197, diapause); Timonin, 1997 (p.83, vertical migration, Rem.); Stephen, 1998 (p.342, 343, chart, Rem.); Verheye & al., 1998 (p.317, Table II); Lopes & al., 1999 (p.215, tab.1); Seridji & Hafferssas, 2000 (tab.1); Smith S. & al., 1998 (p.2369, Table 6, moonsoon effects); Lapernat, 2000 (tabl.3, 4); Razouls & al., 2000 (p.343, Appendix) ; d'Elbée, 2001 (tabl. 1); Holmes, 2001 (p.35); Viñas & al., 2002 (p.1031); Beaugrand & al., 2002 (p.1692); Beaugrand & al., 2002 (p.179, figs.5, 6); Bode & al., 2003 (p.85, Table 1, abundance); Hsiao & al., 2004 (p.325, tab. 1); Lan & al., 2004 (p.332, tab.1); Marrari & al., 2004 (p.667, tab.1); CPR, 2004 (p.49, fig.136); Berasategui & al., 2005 (p.313, fig.2); Koppelmann & Weikert, 2005 (p.1173, vertical distribution); Prusova & Smith, 2005 (p.76, tab.3); Auel & al., 2005 (p.653, metabolic adaptations, respiration); Hwang & al., 2006 (p.943, tab.I) ; Dias & Araujo, 2006 (p.31, Rem., chart); Ceballos & Alvarez-Marqués, 2006 (p.1, dynamic of population); 2006 a (p.189, reproduction); Hwang & al., 2007 (p.23); Dur & al., 2007 (p.197, Table IV); Albaina & Irigoien, 2007 (p.433, tab.1); Valdés & al., 2007 (p.103: tab.1); Auel & Verheye, 2007 (p.234, hypoxia tolerance); Cabal & al., 2008 (289, Table 1); Wishner & al., 2008 (p.163, Table 2, fig.8, vertical zonation-oxycline); Muelbert & al., 2008 (p.1662, Table 1, 3); Lan Y.C. & al., 2008 (p.61, Table 1, % vs stations, Table 2: indicator species); Miyashita & al., 2009 (p.815, Tabl.II); Lan Y.-C. & al., 2009 (p.1, Table 2, % vs hydrogaphic conditions); Park & Ferrari, 2009 (p.143, fig.1, biogeography); Eloire & al., 2010 (p.657, Table II, temporal variability); Lidvanov & al., 2010 (p.356, Table 3); Viñas & al., 2010 (p.177, body dimensions vs bivolume); Dias & al., 2010 (p.230, Table 1, fig.6 a); Schnack-Schiel & al., 2010 (p.2064, Table 2: E Atlantic subtropical/tropical); Ji & al., 2010 (p.1355, Rem.: p.1360); Vogedes & al., 2010 (p.1471, lipid sac area vs lipid content); Di Mauro & al., 2011 (p.69, biovolume); Hsiao S.H. & al., 2011 (p.475, Appendix I); Hsiao & al., 2011 (p.317, Table 2, indicator of seasonal change); Padovani & al., 2011 (p.205, Table 1, 2, abundance, predation); Glushko & Lidvanov, 2012 (p.138, Tableau 1, 3); Zizah & al., 2012 (p.79, Tableau I, Rem.: p.89); Bode & al., 2012 (p.108, spatial distribution vs time-series, % biomass); Menéndez & al., 2012 (p.389, Table 1: seasonal abundance); Salah S. & al., 2012 (p.155, Tableau 1, Rem.); Spinelli & al., 2012 (p.39, potential prey for fish); Miyashita & al., 2012 (p.1557, Table 2: occurrence); Alvarez-Fernandez & al., 2012 (p.21, Rem.: Table 1); Gusmao & al., 2013 (p.279, Table 4, sex ratio); Belmonte & al., 2013 (p.222, Table 2, abundance vs stations); Teuber & al., 2013 (p.1, Table 1, 2, abundance vs oxygen minimum zone, respiration rates); Tseng & al., 2013 (p.507, seasonal abundance); Sobrinho-Gonçalves & al., 2013 (p.713, Table 2, fig.8, seasonal abundance vs environmental conditions); Bode M. & al., 2013 (p.1, Table 1, 3, respiration rate & ETS activity); Schukat & al., 2013 (p.1, Table 1, 2, fig.2, respiration, ingestion); Lidvanov & al., 2013 (p.290, Table 2, % composition); Julies & Kaholongo, 2013 (p.78, fig.4, Rem, %); Hwang & al., 2014 (p.43, Appendix A: seasonal abundance); Bonecker & a., 2014 (p.445, Table II: frequency, horizontal & vertical distributions); Bode & al., 2014 (p.1, fig.4, 5, 10, 11, abundance vs long-term 2005-2011); Zaafa & al., 2014 (p.67, Table I, occurrence): Bode & al., 2015 (p.268, Table 1, 2, figs.3, 4, chemical components, trophic level, geographic zone); Dias & al., 2015 (p.483, Table 2, abundance, biomass, production, Table 4: % vs. season); Araujo & al., 2016 (p.1, Table 3, abundance, %); Rosa J.C.L. & al., 2016 (p.71, Rem.Benedetti & al., 2016 (p.159, Table I, fig.1, functional characters); Bonecker C.T. & al., 2017 (p.247, fig.10: abundance day/night vs. vertical types of mass water); El Arraj & al., 2017 (p.272, table 2, spatial distribution, Rem.: p.281); Record & al., 2018 (p.2238, Table 1: diapause); Belmonte, 2018 (p.273, Table I: Italian zones); Chaouadi & Hafferssas, 2018 (p.913, Table II: occurrence); Dias & al., 2018 (p.1, Tables 2, 4: vertical distribution, abundance vs. season); Dias & al., 2018 a (p.189, Rem.: p.196); Acha & al., 2020 (p.1, Table 3: occurrence % vs. ecoregions, Table 5: indicator ecoregions). | | | | NZ: | 3 + 2 doubtful | | |
|
Distribution map of Calanoides carinatus by geographical zones
|
| | | | | | | | | | | |  issued from : F.C. Ramirez & M.E. Sabatini in Hydrobiologia, 2000, 439. [p.35, Fig.11]. issued from : F.C. Ramirez & M.E. Sabatini in Hydrobiologia, 2000, 439. [p.35, Fig.11].
Seasonal relative abundance and distribution of adults in water off Argentina. |
 issued from : C. de O. Dias & A.V. Araujo in Atlas Zoopl. reg. central da Zona Econ. exclus. brasileira, S.L. Costa Bonecker (Edit), 2006, Série Livros 21. [p.31]. issued from : C. de O. Dias & A.V. Araujo in Atlas Zoopl. reg. central da Zona Econ. exclus. brasileira, S.L. Costa Bonecker (Edit), 2006, Série Livros 21. [p.31].
Chart of occurrence in Brazilian waters (sampling between 22°-23° S) |
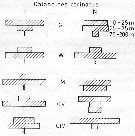 issued from : U. Brenning in Wiss. Z. Wilhelm-Pieck-Univ. Rostock - 30. Jahrgang 1981. Mat.-nat. wiss. Reihe, 4/5. [p.9, Fig.11]. issued from : U. Brenning in Wiss. Z. Wilhelm-Pieck-Univ. Rostock - 30. Jahrgang 1981. Mat.-nat. wiss. Reihe, 4/5. [p.9, Fig.11].
Vertical distribution for Calanoides carinatus from 8° S - 26° N; 16°- 20° E.
T: daylight; N; night; G: all copepodis stages; W; females; M; males. = C. natalis |
 Issued from : M.D. Viñas, N.R. Diovisalvi & G.D. Cepeda in J. Braz. Oceanogr., 2010, 58 (2). [p.178, Table 1]. Issued from : M.D. Viñas, N.R. Diovisalvi & G.D. Cepeda in J. Braz. Oceanogr., 2010, 58 (2). [p.178, Table 1].
Body dimensions and estimated biovolume of Calanoides carinatus samples on October 18th and November 11th at the permanent coastal station (38°28'S, 57°41'W).
Body wet weight can be derived from measurements of body volume by applying a factor of 1 for specific gravity. Dry weight can be obtained by mulitplyng the wet weight by 0.20 and the carbon content as 40% of the dry weight.
C: copepodite stages C1 to C5; F: female; M: male.
Prosome length, width and height, as well as urosome length and width, were measured, in order to apply the model of Chojancki & Hussein (1983) slightly modified (antenna and leg volume excluded) by Fernadez Araoz (1991). |
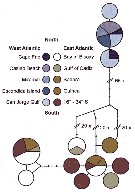 Issued from : M.D. Viñas, L. Blanco-Bercial, A. Bucklin, H. Verheye, J.G.F. Bersano & S. Ceballos in J. Exp. Mar. Biol. Ecol., 2015, 468. [p.101, Fig.2]. Issued from : M.D. Viñas, L. Blanco-Bercial, A. Bucklin, H. Verheye, J.G.F. Bersano & S. Ceballos in J. Exp. Mar. Biol. Ecol., 2015, 468. [p.101, Fig.2].
TCS Network diagram showing frequencies, relationships and source locations for the 12 haplotypes observed for Calanoides carinatus s.l.
The size of the pie indicates relative frequency; color of the slice indicates the collection area (see also Fig.1); samples in legend are ordereds from North to South.
Due to the low number of individuals, the SE Atlantic samples from 16° to 34°S (Benguela Current) were pooled.
Nota: C. carinatus s.l. collected at 13 sampling locations was analysed (Table 1: localities, , date, number of individuals, sequence identification and GenBank). The TCS network diagram indicated complete isolation between the SW and NE/SE Atlantic populations, with no shared haplotypes between them. In addition, TCS analysis indicated no differentiation between NE and SE populations, with 2 groups of haplotypes found in both regions. Several individuals from the NE Atlantic, at the northern edge of the species distribution, showed very divergent haplotypes.
C. carinatus s.s. (SW Atlantic) and C. carinatus s.l. (NE/SE Atlantic) are clearly genetically differentiated from both C. macrocarinatus and C. acutus. Accirdingly, Sabatini & al. (2007) found morphological differences between specimens of C. carinatus s.s. and C. macrocarinatus from new Zealand and concluded that they were different species. They also found that both species may be distinguished from C. acutus by several morphological characters of the female and male. |
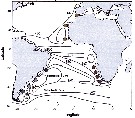 Issued from : M.D. Viñas, L. Blanco-Bercial, A. Bucklin, H. Verheye, J.G.F. Bersano & S. Ceballos in J. Exp. Mar. Biol. Ecol., 2015, 468. [p.100, Fig.1]. Issued from : M.D. Viñas, L. Blanco-Bercial, A. Bucklin, H. Verheye, J.G.F. Bersano & S. Ceballos in J. Exp. Mar. Biol. Ecol., 2015, 468. [p.100, Fig.1].
Sampling locations of Calanoides carinatus s.l. for molecular analysis in the Atlantic Ocean, with diagrammatic representation of oceanographic currents (modified from Peterson & Stramma, 1991).
Abbreviations are: AC = Agulhas Current; ACC = Antarctic Circumpolar Current; BC = Brasil Current; CC = Canary Current; MC = Malvinas (= Falkland) Current; NBUC = North Brazil Undercurrent; NEC = North Equatorial Current; SAC = South Atlantic Current; SEC = South Equatorial Current; SECC = South Equatorial Countercurrent;
Number refer to sampling localities (data in Table 1). Color of the circles indicates the source locations for the TCS analysis. For interpretation of the references to color , the reader is referred to the web version of the publication.
Nota: Variation in morphological characters (e;g. male P5) and/or differences in life cycle between C. carinatus s;l. from diverse regions suggests the existence of more than one species. Sabatini & al. (2007) analysed the historical taxonomy of C. carinatus s.l. and produced a precise morphological redescription of C. carinatus sensu stricto, based on collections from a location near the species' putative type locality. In addition, female and male specimens collected off Brazil and Argentina were compared morphologically and no significant differences were found. Sabatini & al. (2007) concluded that specimens collected from the various South American locations were conspecific.
The reproductive isolation and genetic divergence of populations of C. carinatus s.l. from coastal regions of th SW and SE Atlantic Ocean are not easy explained by the present-day circulation patterns of the major oceanographic currents in the South Atlantic. Major current systems connect the margins of the ocean both on the surface (Peterson & Stramma, 1991) and in deeper levels of the water column (Stramma & England, 1999). Close to South Africa, the Benguela Current flows northwest around the Subtropical Gyre, and then feeds the South Equatorial Current. The SEC transports near surface water onto the Brazilian shelf near 16°S, and then separates into the northward-flowing Brasitl Undercurrent and the southward-flowing Brazil Current, with the latter mixing with the Malvinas Current and leaving the shelf to flow eastward as the South Atlantic Current, near South Africa a branch of this current flows eastward to the Indian Ocean, while the other turns North into the Benguela Current and finally into the South Equatorial Current.
Although it is not possible to accurately estimate the likelihood and timing of transport through this series of currents, it seems possible- if not likely- that isolation by distance and limited advective transport may allow genetic divergence between copepod populations in the NE/SE versus SW Atlantic regions.
Active individuals of C. carinatus s.l. would not be expected to survive transport across the South Atlantic Ocean, because of food limitation in the oligotrophic central gyre (Moreno-Ostos & al., 2011). Verheye & al. (2005) found that active, surface-dwelling juveniles (copepodite stage V) and adults in the SE Atlantic required a regular food supply, and that their limited lipid reserves would not sustain their survival for more than 10 days. The maximum survival time of diapausing CV copepodites of C. carinatus s.l. was estimated to be 149-192 days, suggesting that it unlikely that diapausing individuals could be transported alive across the basin.
Analysis of samples of C. carinatus s.l. collected along the west coast of Africa, near Cadiz (Spain), and in the Bay of Biscay suggests a single, genetically-cohesive species population along the margins of the NE and SE Atlantic Ocean. A seasonal ontogenetic migration strategy associated with complex oceanographic processes may contribute to the species' dispersion throughout this extended region ( John & al., 1998; Peterson, 1998).
In the NE Atlantic, C. carinatus s.l. has been recorded along the west coast of the Iberian Peninsula, in the Bay of Biscay and in waters southwest of the British Isles (Ceballos & al., 2004; John & al., 1998). Diapausing juveniles (copepodite stage V) are transported from off the west coast of Africa to the Bay of Biscay by along-slope undercurrents (John & al., 1998), in which the species has established a permanent population (Ceballos & Alvarez-Marqués, 2006). Although the species has been considered an African upwelling specialist (Peterson, 1998), data concerning its reproductive rate in the NW Iberian coast and the Bay of Biscay show that C. carinatus s.l. can adopt different life strategies to cope with unusual or rare environmental conditions (Ceballos & Alvarez-Marqués, 2006; Ceballos & al., 2004).
Along the west coast of Africa, C. carinatus s.l. typically occurs in upwelling conditions (Courties, 1978; Thiriot, 1978), which show considerable latitudinal variability in this region in frequency, intensity and seasonality (Verheye & al., 2005). In coastal waters of the Guinea Current, C. carinatus s.l. is found during the short (3-4 months) upwelling season, whereas in the Benguala System (where the upwelling season extends over 6-8 months), the species maintains relatively high densities on the shelf throughout the year. In this region, populations are transported offshore in the wind-driven upwelling plume (Verheye & al., 2005). When productivity of the plume decreases, the juveniles (stage V) descend to depth below 400 m and survive on lipid stores accumulated during the upwelling event (Arashkevich & Drits, 1996; Kosobokova & al., 1988), thereby reducing their metabolic activity and energy demands by as much as 96% (Auel & al., 2005). At the onset of a new upwelling period, the copepods are transported onto the shelf0 where they molt to the adult stage and reproduce, remaining in surface waters and taking full advantage of the phytoplankton bloom to feed and accumulate lipid reserves.
The permanent reservoir of sub-adults of C. carinatus s.l. in deep water along the Benguela System allows reseeding of extensive areas by advection over large spatial scales. The continuous and permanent poleward-flowing undercurrents along the continental slope off Africa may transport C. carinatus s.l. from the subtropical and tropical eastern Atlantic toward British Isles (John & al., 2000), the northern limit of the species' distribution area.. Similarly, the deep (600 m) northward-flowing Mediterranean Outflow Water (off the western Iberian Peninsula) may be a transport vehicle for the species (John & al., 1998). These oceanographic mechanisms- coupled with the vertical migration pattern of the species- may facilitate mixing of NE and SE Atlantic populations.
The molecular phylogeographic results (Viñas & al., 2015) suggest that the distribution of C. carinatus s.s. is restricted of the SW Atlanyic Ocean from 20°S, off Brazil to 47°S off Argentina. near the northern limit of the species distribution, the area around Cape Frio, Brazil is characterized by a coastal upwelling, which occurs primarily during austral summer, when northeast winds drive nutrient-rich bottom South Athantic Central Water (SACW) up onto the shelf. A bend in the coastline generates meanders in the Brazil Current that also drive upwelling and seasonal intrusions of SACW (Campos & al., 1999). C. carinatus s.s. is found throughout the year in the Cape Frio region (Monteiro-Ribas & al., 1979), but its cycle seems to be strongly linked with upwelling events (Lopes & al., 1999). The highest densities (600 to 1000 per m3) have been recorded in summer during periods of strongest upwelling intensity of SACW. The higher abundance levels of C. carinatus s.s. off Cape Frio are similar to those observed for C. carinatus s.l. in the Benguala upwelling region (Lopes & al., 1999). Farther south (23°S to 34°S), the species is found throughout the year, but is typically les abundant (0.1-530 per m3) than in the Cabo Frio region (Valentin & Monteiro Ribas, 1993).
In the Argentina Sea C. carinatuss.s. is widely distributed in coastal and inner shelf waters between 42° and 45°S. Abundance levels are similar to those of the southern Brazilian shelf (up to 500 per m3), with higher values recorded in summer (Ramirez & Sabatini, 2000). The highest abundances of the species are associated with frontal systems, including the estuarine front of the La Plata River (Viñas & al., 2002); the mid-shelf thermal front between coastal and shelf waters off Buenos Aires (Marrari & al., 2004; Santos & Ramirez, 1991); and tidal fronts along northern Patagonia (Sabatini & Martos, 2002). Throughout this distributional range, the species has not been reported in oceanic waters, since sampling has been done only in near-surface (< 200 m) depths of the water column. Thus , the authors do not yet know about the occurrence of diapausing copepods in the deeper strata (below 400 m depth) typical of this and other upwelling species. |
 Issued from : W.C.M. Klein Breteler, N. Schogt & J. van der Meer in J. Plankton Res., 1994, 16 (8). [p.1051, Fig. 8]. Issued from : W.C.M. Klein Breteler, N. Schogt & J. van der Meer in J. Plankton Res., 1994, 16 (8). [p.1051, Fig. 8].
Stage duration (days) of C. carinatus at 15°C and excess of food. Data from Peterson & Painting (1990): original result using a linear distribution function after data selection (*), present result by fitting a gamma distribution function (o); bars indicate two times the asymptotic standard error, calculated from N III onwards.
Nauplii: stages 1-6, copepodites 1-5 (7-11). |
 Issued from : A.C.T. Bonecker, A. V. de Araujo, C.O. Dias, M.M.S. Castro, P.F. Carvalho, R.M. Lopes & S.C.L. Bonecker in A.P.C. Falcao & D.L. Moreira (ed.) Ambiente pelagico caracterizaçao ambiental regional de Bacia de Campos, Atlantico Sudoeste. Rio de Janeiro. Elsevier. 2017, v.5, p.247-281. [p.261, Fig.10] Issued from : A.C.T. Bonecker, A. V. de Araujo, C.O. Dias, M.M.S. Castro, P.F. Carvalho, R.M. Lopes & S.C.L. Bonecker in A.P.C. Falcao & D.L. Moreira (ed.) Ambiente pelagico caracterizaçao ambiental regional de Bacia de Campos, Atlantico Sudoeste. Rio de Janeiro. Elsevier. 2017, v.5, p.247-281. [p.261, Fig.10]
Abundance (ind. m-3) sampled during night and day into four water masses in the Campos Basin (Brazil).
AT: Tropical water; ACAS: Central water of South Atlantic; AIA: Intermediate water of Antarctica; ACS: Deep water Circumpolar water superior.
Sampling at station c (22°54'30''S, 40°43'W), depth 1900 m (end of the continental slope). |
| | | | Loc: | | | See in Remaks the geographic distribution between the species sensu stricto and sensu lato. Numerous authors [ ] had confounded this species with Calanoides natalis
Sub-Antarct. (SW Atlant.: 20°S-47°S, Indian), [South Africa (E & W), Saldanha, Namibia, Angola, Baia Farta, Congo, Gabon, G. of Guinea, off Lagos, Ivory coast, Ghana, Dakar, off Morocco-Mauritania, Cap Ghir, Moroccan coast, Canary Is., off Madeira, Portugal, off Coruña, off W Cape Finisterre, off Gijon, S Bay of Biscay, W English Channel (Morlaix estuary)]; Argentina, off Mar del Plata, Peninsula Valdés, Bahia Blanca, Uruguay (continental shelf), Brazil (Campos Basin, Paranagua Bay, Cabo Frio, Vitoria-Cabo de Sao Tomé, off Macaé), [ SW Ireland, Ibero-moroccan Bay, off W Tangier, Gibraltar, Medit. (Alboran Sea, Sidi Fredj coast, Algerian Basin, Castellon, Baleares, Banyuls, G. of Lion, Tyrrhenian Sea, Taranto, Egyptian coast) ] , Somalia, Arabian Sea, Indian, off Madagascar S, Natal, [Andaman Sea, W Australia, Indonesia-Malaysia, Philippines, China Seas (East China Sea, South China Sea), Taiwan Strait, off Taiwan, Taiwan (SW, S, Danshuei Estuary, NE), Kuroshio Current, Japan (Izu), Pacif. (W equatorial), E Australia, S Tasmania, New Zealand (Kaikoura)]
Type locality: SW Atlantic Ocean (30° S, close to the coast of Brazil) | | | | N: | 192 ? | | | | Lg.: | | | Sensu lato : (14) F: 2,32; M: 2,7; (17) F: 2,6; (25) F: 3,15; (35) [Atlant.] F: 2,82-2,36; [N-Z° F: 3,6-3,06; (47) F: 2,85-2,25; M: 2,35; (66) F: 3,94; (75) F: 2,72-2,17; M: 2,25-2,06; (104) F: 3,2; M: 2,4; (114) F: 2,82-2,55; 3,95-3,9; (116) F: 3,18; (180) F: 2,55-2,4; M: 2,68-2,3; (199) F: 2,81-2,05; M: 2,66-2,2; (237) Reexamination necessary. F: 1,6-2,5; M: 2,5; (254) F: ± 3,5; (290) F: 2,25-2,6; (321) F: 2,25-2,05; M: 2,24; (322) F: 2; M: 2; (327) F: 2,88-2,09; M: 2,57-2,12; (786) F: 2,77-2,63; M: 2,5-2,33; (818) F: 3,07-2,93; M: 2,5-2,32; (849) F: 2,93-3,07; M: 2,32-2,5; (861) F: 3,26-3,32; (989) F: 2,63-2,97; M: 2,21-2,59; (1009) F: 2,65-3,05; (1023) F: 2,59; (1037) F: 1,7-2,2; (1107) F: 2,36-3,34; M: 2,36-2,8 [NW Africa]; F: 2,54-3,12; M: 2,4-2,93 [SW Africa: Namibia]; (1137) F: 2,0-3,09; M: 2,69-1,98; (1185) F: 3,24-2,89; M: 2,90-2,57; {F: 1,60-4,00; M: 2,06-3,60}.
Sensu stricto :(1319) F: 2,63-2,97; M: 2,9-3,9; {F: 2,63-2,97; 2,21-2,59} | | | | Rem.: | epi-meso-bathypelagic.
Sampling depth (sub-Antarct.): 50 m.
Deep water species, only reaching the surface when the upper layer has cooled down sufficiently. For Acha & al. (2020) this species is observed on the coastal (frequent), shelf and also northern shelfbreak in front of Rio de La Plata (Argentina).
May be confounded with C. philippinensis; according to Kitou & Tanaka (1969, p.74) the main character separating the two species is the structure of the genital opening females. and in the male by the structure of the mandible blade, 1st to 3rd inner lobes of Mx1 and in armature of the lobes on Mx2.
The species is considered as an indicator for the penetration of Atlantic waters in the Mediterranean Sea.
Cf. remarks to Calanoides macrocarinatus.
For Sabatini & al. (2007, p.341-342, 363) a serious doubt exists on the synonymy Calanoides brevicornis (Calanus brevicornis Lubbock, 1856). Later on, Giesbrecht (1892) reported C. brevicornis to be distribued in the Atlantic Ocean from 36°N-41°S, The Indian Ocean (41°S, 45°E) and the Mediterranean Sea (Gibraltar). With (1915) considered Calanus brevicornis to be a junior synonym of Calanus carinatus. Most contemporaneos identifications of Calanoides ( or Calanus) carinatus may have been based on the diagnosis of C. brevicornis as figured by Giesbrecht (1892). The authors note that C. carinatus (Krøyer, 1848) has never been figured from specimens from its type locality off Brazil; The figures of the P5 male presented C. carinatus, have differing proportions in different publications. The wide distribution of C. carinatus and the lack of a formal, detailed description support the view that several species may be referred to this name (see Bradford, 1988; Peterson, 1998). If molecular and/or morphological studies of Calanoides carinatus (sensu lato) indicate that there are species that can be distinguished from Calanoides carinatus (Krøyer, 1848) (sensu stricto) as described by us, the junior synonyms Calanus brevicornis Lubbock, 1856, Calanus frontatus Dahl, 1894, and Calanus natalis Brady, 1914 may be valid species, mainly Calanus (= Calanoides) brevicornis. The identity of C. brevicornis collected by Giesbrecht (1888) from Gibraltar, 20°S, 38°W and Rio de Janeiro, remains uncertain.
Cited for the first time on the Egyptian coast (Matrouh beaches) by Aboul Ezz & al; (2014) as Calanus brevicornis, requires confirmation.
For Itoh (1970 a, fig.2, from co-ordonates) the Itoh's index value from mandibular gnathobase = 382.
After Viñas & al. (2015) phylogeographic analysis of mtCOI sequence variation indicates that C. carinatus s.l. comprises two genetically divergent and geographically distinct species. Since the type locality of the species is in Brazilian waters, the authors consider the SW Atlantic type to represent C. carinatus sensu stricto (s.s.), with a biogeographical range restricted to the SW Atlantic Ocean. Further, the authors consider the NE/SE Atlantic type to be an undescribed, cryptic sibling species. In this condition the species indicated and studied in the STNA and TNA (NE Atlantic) by Bode & al. (2015) requires confirmation, but is probably Calanoides natalis. | | | Last update : 28/10/2022 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed February 10, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |