|
|
 |
|
Calanoida ( Order ) |
|
|
|
Calanoidea ( Superfamily ) |
|
|
|
Calanidae ( Family ) |
|
|
|
Neocalanus ( Genus ) |
|
|
| |
Neocalanus tonsus (Brady, 1883) (F,M) | |
| | | | | | | Syn.: | Calanus tonsus Brady, 1883 (p.34, figs.F); Giesbrecht, 1892 (p.92); Giesbrecht & Schmeil, 1898 (p.19); Wolfenden, 1908 (p.11, figs.F); Farran, 1929 (p.207, 216, fig.F); Wilson, 1932 a (p.20, fig.F); Campbell, 1934 (p.1, life history); Wilson, 1942 a (p.173); Sewell, 1948 (p.390, 513, 516, 544, 549, 555, 559, 565); C.B. Wilson, 1950 (p.178); Vervoort, 1951 (p.41, 56, Rem.); Tanaka, 1956 a (p.49, figs.F, Rem.); Chiba & al., 1957 (p.306); Vervoort, 1957 (p.27, figs.F, Rem.); Tanaka, 1960 (p.13, Rem., juv.); Brodsky, 1962 b (p.709); Tanaka, 1964 (p.5); De Decker & Mombeck, 1964 (p.11); Vervoort, 1965 b (p.394, figs.F); Jillett, 1968 (p.19, 27, figs.M); Bradford, 1970 a (p.352, fig.M); Marshall & Orr, 1972 (p.8); Bradford, 1972 (p.32, figs.F); Brodsky, 1972 (1975) (p.9, 69, 85,121, figs.F,M); Vyshkvartzeva, 1972 (1975) (p.188, figs.); Rudyakov, 1972 (p.886, Table 1, 2, 3: sinking rate); Heinrich, 1973 (p.95, fig.1); Björnberg, 1973 (p.287, 384); Heinrich, 1974 (fig.3); Vyshkvartzeva, 1976 (p.14); 1977 a (p.125, figs.); Tranter, 1977 (p.596, 601, 602); Carter, 1977 (1978) (p.35); Arashkevich, 1978 (p.118, Table: diets); Voronina & al., 1978 (p.512, distribution); Björnberg & al., 1981 (p.618, figs.F,M); Biggs, 1982 (p.55, Table 2: NH4 excretion, O2 consumption); De Decker, 1984 (p.332: carte); Fleminger, 1975 (p.275, 283, Table 1, Fig.M); Santos & Ramirez, 1991 (p.80); Pakhomov & al., 2000 (p.1663, Table 2, transect Cape Town-SANAE antarctic base);
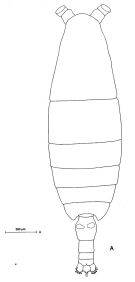
no Calanus tonsus : Cf. in Minoda, 1971 (p.12) ; Campbell, 1929 (p.1930; 1934 (figs.7 b, 7 c); Brodsky, 1938 (form typica ); 1948 (Pl.III, figs.1, 2, 5, 6); 1950 (fig.22: F,M, Ur from form typica ); Tanaka, 1954 (figs.1, 4); 1956 (fig.2); ? Park & al., 1991 (p.203) | | | | Ref.: | | | Wilson, 1942 a (p.173); Tanaka,1956 (p.257, Rem.); 1956 a (p.49, figs.F, Rem.); Bradford & Jillett, 1974 (p.6); Fleminger, 1985 (p.285, fig.M); Bradford, 1988 (p.74, 76, 79); Bradford & al., 1988 (p.301, Developement., figs.N, juv.); Nishida, 1989 (p.173, table 2, 3, dorsal hump); Razouls, 1994 (p.33, figs.F,M); Miller, 1988 (p.232, 247: Rem. synonymies); Bradford-Grieve, 1994 (p.42, figs.F,M, fig.98); Mazzochi & al., 1995 (p.105, fig.F, Rem.); Atkinson & Sinclair, 2000 (p.50, 51, 55); Bradford-Grieve & al., 1999 (p.877, 908, figs.F,M); Ramirez & Sabatini, 2000 (p.26, figs. C5); Bucklin al., 2003 (p.335, tab.2, fig. 2, Biomol.); Machida & al., 2006 (p.1071, tab.1,3, fig.3,4, molecular phylogeny); Ferrari & Dahms, 2007 (p.61, Rem.) | 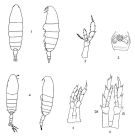 Female: 1, habitus (dorsal, lateral); 2, P5; Issued from : Brodsky K.A. in Calanoida of the Far Eastern seas and polar basin of the USSR (Israel Program scient. Trans., 1967, 1-440 ). 3, genital segment; Issued from : Brodsky K.A. in Issled. Fauny Morei, 1972, 12 (20): 5-110. Male: 4, habitus (dorsal, lateral; 5, P5; issued from Brodsky, 1967. 6, P5 (G: left, Dt: right); issued from : Brodsky, 1972. As .
|
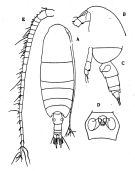
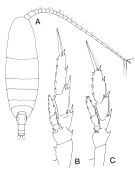 issued from : J.M. Bradford-Grieve in The Marine Fauna of New Zealand: Pelagic Calanoid Copepoda. National Institute of Water and Atmospheric Research (NIWA). New Zealand Oceanographic Institute Memoir, 102, 1994. [p.43, Fig.17]. Female: A, habitus (dorsal); B, P2; C, P5.
|
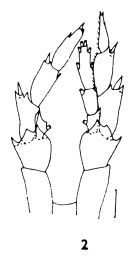
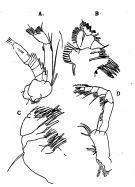
 issued from : J.B. Jillett in Aust. J. Freshwat. Res., 1968, 19. [p.28, Fig.3]. As Calanus tonsus. Male (from Otago Peninsula, SE New Zealand):A, habitus (dorsal); B, A1; C, A2; D, Md; E, Mx1; F, Mx2; G, Mxp; H, P1; I, P2; J, P3; K, P5. Nota: Urosome length about 1/4 of the total body length. - Head and 5 thoracic segments. - The 4th thoracic segment bears a small pair of dorso-lateral spines. - Postero-lateral corners of the 5th thoracic segment broadly rounded (when viewed laterally). - Rostrum with 2 slender filaments. - Urosome is 5-segmented, having the proportional lengths as 17 : 36 : 21 : 13 = 100. - The furcal rami are about 1.5 times as long as broad, and are strongly divergent. - A1 25-segmented, distinctly separate from one another; reaches just beyond the end of the caudal rami; proportional lengths of segments 98 : 30 :38 : 34 : 38 ; 38 : 30 : 27 : 34 : 42 : 45 : 45 : 45 : 45 : 45 : 49 : 45 : 45 : 38 : 38 : 34 : 30 : 27 : 15 = 1000. All segments bear setae on their anterior border, on e on the 2nd segment being particularly large. - P1 with a small blunt projection on the posterior face of the basal segment of exopodite (this projection is also present in the females but appears yo have been overlooked by other workers). - P2 with a recurved hook just inside the distal outer corner of the basal exopodite segment (this hook was figured for the female by Tanaka (1986) but not by Vervoort (1957). - P2-P4 with a row of short spines on the inner distal posterior corner of the 2nd basal segment (there are also present on the P5 of the female, but not in the male). Usually there are 3 but sometimes 4 spines in this row and in some specimns the number differ on each leg in the same pair. - P5 are modified by comparison with those of the female but are very similar to those of the male of C. plumchrus (= Neocalanus plumchtus). The 2nd and 3rd exopodite segments both lack setae on their inner margins. The left exopodiyr differs from the right in having a larger basal segment, the inner margin of all three segments is finely setose, and the terminal segment bears much smaller spines and has a concave inner margin.
|
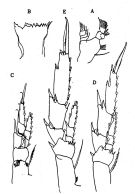
 issued from : J.M. Bradford in N.Z. Jl Mar. Freshw. Res., 1970, 4 (4). [p.352, Fig. 2]. As Calanus tonsus. Male (off Kaikoura, New Zealand): 2, P5 Scale bar represents 0.1 mm.
|
 Issued from: M.G. Mazzocchi, G. Zagami, A. Ianora, L. Guglielmo & J. Hure in Atlas of Marine Zooplankton Straits of Magellan. Copepods. L. Guglielmo & A. Ianora (Eds.), 1995. [p.106, Fig.3.15.1]. Female: A, habitus (dorsal). Nota: Proportional lengths of urosomites and furca 42:22:11:11:14 = 100.Basipod of P5 with smooth inner margins without teeth; basipod 2 with 4 spines on distal inner surface.
|
 issued from : O. Tanaka in J. oceanogr. Soc. Japan, 1956, 12 (2). [p.49, Fig.1]. Female (from subantarctic Indian Ocean): A, habitus (dorsal); B, forehead (lateral); C, 5 th thoracic segment and urosome (lateral left side); D, genital segment (ventral); E, right A1. Nota: Cephalothorx and urosome in the proportional lengths 128:35. Head and 1st thoracic segment separate, 4th and 5th separate. Rostrum with 2 slender filaments. Proportional lengths of urosomites and furca 40:26:11:3:20 = 100.Genital segment laterally produced, about as long as wide in dorsal aspect (14:15). Genital opening produced slightly below, genital flap rather small. Furcal rami 1.5 times as long as wide. A1 (25-segmented)extends about to the distal end of the anal segment.
|
 issued from : O. Tanaka in J. oceanogr. Soc. Japan, 1956, 12 (2). [p.50, Fig.2]. Female: A, A2; B, Mx1; C, Mx2; D, Mxp. Nota: Endopod of A2 slightly longer than exopod (47:44); inner marginal setae on 1st and 2nd exopodal segments not feeble; 2nd exopodal segment with 6 + 1 setae on the external lobe. Mx1 with a small 1st inner lobe furnished with 10 strong and 4 slender spines; 2nd and 3rd lobe with each 4 setae, 1st outer lobe with 9 setae; exopod with 11 setae; 2nd basal segment with 4 setae; 1st endopodal segment with 4, 3rd with 7 setae. mx2 with a plumose seta on the outer margin of the 1st basal segment.
|
 issued from : O. Tanaka in J. oceanogr. Soc. Japan, 1956, 12 (2). [p.51, Fig.3]. Female: A, Md (mandibular palp); B, Md (masticatory edge); C, right P1 (posterior); D, right P2; E, right P3.
|
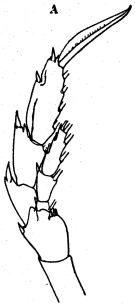 issued from : O. Tanaka in J. oceanogr. Soc. Japan, 1956, 12 (2). [p.51, Fig.4, A]. Female: A, right P5 (posterior). Nota: Inner distal corner of 2nd basal segment with 3 spines on the right leg, and 4 on le left leg; the short spines appear to be variable in number; they varied from 2 to 4 in the specimens observed (Wolfenden's specimen had 5 spines on the 2nd basal segment of right leg).
|
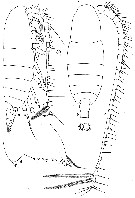 issued from : W. Vervoort in B.A.N.Z. Antarct. Res. Exped., Rep.-Ser. B, III, 1957. [Fig.3]. As Calanus tonsus. Female from 44°05'S, 147°35'E): a-b, habitus (lateral and dorsal, respectively); c, left A1; d, left Md (cutting edge).
|
 issued from : W. Vervoort in B.A.N.Z. Antarct. Res. Exped., Rep.-Ser. B, III, 1957. [Fig.4]. As Calanus tonsus. Female: a, left Mx1; b, left Md (mandibular palp); c-f, P2 to P5 (posterior aspect). Nota: Mx1 differs from that of Calanus propinquus by the presence of 5 (instead of 4) setae on the 3rd inner lobe.The P5 differs from that of C. propinquus by the total absence of spinules along the internal border of the 1st basal segment: moreover, there are 2 setae along the external border of the 3rd endopodal segment (only 1 in C. propinquus.
|
 issued from : W. Vervoort in B.A.N.Z. Antarct. Res. Exped., Rep.-Ser. B, III, 1957. [Figs.5-6]. As Calanus tonsus. Female: 5 (above): a, left A2; b, left Mx2; c, Mxp.; 6 (below): a, c, posterior part cephalothorax and urosome (dorsal and lateral, rescetively); b, left P1 (anterior aspect). Nota: The seta on the ventral border of Mx2 is delicate and may easily be overlooked.
|
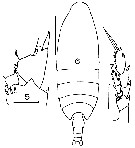 issued from : J.M. Bradford in Mem. N. Z. Oceonogr. Inst., 1972, 54. [p.33, Fig.4 (5-7)]. As Calanus tonsus. Female (from Kaikoura, New Zealand): 5, P2 (basipod and part exopod, endopod segments 1); 6, habitus (dorsal); 7, P5. Scale bars: 1 mm (6); 0.1 mm (5, 7). Nota: The 1st exopod segment of the female P2 is similar in form to that of Neocalanus.
|
 issued from : R.J. Machida, M.U. Miya, M. Nishida & S. Nishida in Mar. Biol., 2006, 148. [p.1077, Table 5]. Comparisons of selected biological characteristics of 6 species olus 1 subspecies of Neocalanus.
|
 issued from : N.V. Vyshkvartzeva in Issled. Fauny Moreï, 1972, 12 (20). [p.168, Fig.7, 3a, 3b, 3g). As calanus tonsus. Female stage V: 3a, Md (masticatory edge, lateral view); 3b, left Md (above view); 3g, right Md (above view).
|
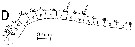 issued from : A. Fleminger in Mar. Biol., 1985, 88. [p.283, Fig.5 D]. As Calanus s.l. tonsus.
Male (from S Pacific, subtropical): Left A1 proximal segments (ventral view);
Nota: see remarks in Calanus s.l. pacificus californicus (Fleminger, 1985, p.275) concerning the dimorphism in the female A1.
|
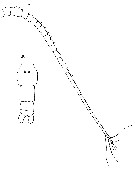 Issued from : G.S. Brady in Rep. Scient. Results Voy. Challenger, Zool., 1883, 8 (23). [Pl. IV, Figs.8, 9]. As Calanus tonsus. Female: 8, A1; 9, urosome.
|
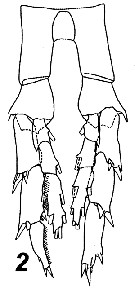 Issued from : V.N. Andronov in Russian Acad. Sci. P.P. Shirshov Inst. Oceanol. Atlantic Branch, Kaliningrad, 2014. [p.73, Fig.19, 2]. As Calanus tonsus after Brodsky, 1964 . Male P5
|
 Issued from : C. Razouls in Ann. Inst. océanogr., Paris, 1994, 70 (1). [p.33]. Caractéristiques morphologiques de Neocalanus tonsus femelle et mâle adultes. Terminologie et abbréviations: voir à Calanus propinquus.
| | | | | Compl. Ref.: | | | Ohman, 1987 (p.1317, feeding, egg production); Ohman & al., 1989 (p.1309, lipids); Heinrich, 1995 (tab.1); Atkinson, 1996 (p.85, feeding); Atkinson & al., 1996 (p.1387, diel periodicity, feeding); Mauchline, 1998 (tab.21, 22, 46, 64); Bradford-Grieve & Jillett, 1998 (p.65, Southern distribution); Stephen, 1998 (p.341, 342, chart); Onishchik, 1999 (p.76, figs.2, 5); Atkinson & Sinclair, 2000 (p.46, fig.2, 3, Table 4, 5, zonal distribution); Razouls & al., 2000 (p.343, tab. 5, Appendix); Ward & al., 2003 (p.121, tab.4); Hunt, 2004 (p.1, 74, Table 4.7); ? Ashjian & al., 2005 (p.1380: tab.2, as tonsa); Berasategui al., 2005 (p.313, fig.2); Hunt & Hosie, 2006 (p.1203, tab.2, fig.8); Park & Ferrari, 2009 (p.143, Table 2, fig.2, Appendix 1, biogeography); Takahashi & al., 2010 (p.317, Table 3, 4); Takahashi & al., 2011 (p.134, distribution); Fierro Gonzalvez, 2014 (p.1, Tab. 3, 5, occurrence, abundance); Acha & al., 2020 (p.1, Table 3: occurrence % vs ecoregions, Table 5: indicator ecoregions). | | | | NZ: | 13 | | |
|
Distribution map of Neocalanus tonsus by geographical zones
|
| | | | | | | | |  issued from : R.J. Machida, M.U. Miya, M. Nishida & S. Nishida in Mar. Biol., 2006, 148. [p.1077, Fig.4]. issued from : R.J. Machida, M.U. Miya, M. Nishida & S. Nishida in Mar. Biol., 2006, 148. [p.1077, Fig.4].
Relationship between the phylogeny and distribution pattern of the Neocalanus species.
Shaded portions of the maps represent distributions based on the litterature. Clades A-E correspond to those in Fig.3, p.1075. |
 issued from : R. Stephen in Pelagic Biogeography ICoPB II. Proc. 2nd Int. Conf. Final report of SCOR/IOC working group 93. 9-14 July 1995. Workshop Rep. No.142. UNESCO, 1998. issued from : R. Stephen in Pelagic Biogeography ICoPB II. Proc. 2nd Int. Conf. Final report of SCOR/IOC working group 93. 9-14 July 1995. Workshop Rep. No.142. UNESCO, 1998.
Fig.5: Distribution of Neocalanus gracilis and Neocalanus tonsus in the Indian Ocean.
P.341, Fig.5: Distribution of Neocalanus gracilis and Neocalanus tonsus in the Indian Ocean.
|
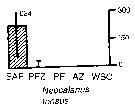 Issued from : A. Atkinson & J.D. Sinclair in Polar Biol., 2000, 23. [p.50, Fig.3] Issued from : A. Atkinson & J.D. Sinclair in Polar Biol., 2000, 23. [p.50, Fig.3]
Neocalanus tonsus from Scotia Sea.
Median and interquartile ranges of copepods (nos /m2) in the five water zones; from north to south these are SAF Subantractic Front area, PFZ Polar frontal Zone, PF Polar Front area, AZ Antarctic Zone, WSC Weddell-Scotia Confluence area/ East Wind Drift.
Numbers on the plots are upper interquartiles where these could not be scaled. |
 Issued from : E.T. Park & F.D. Ferrari in A selection from Smithsonian at the Poles Contributions to International Polar year. I. Krupnik, M.A. Lang and S.E. Miller, eds., Publs. by Smithsonian Institution Scholarly Press, Washington DC., 2009. [p.167, Fig.2]. Issued from : E.T. Park & F.D. Ferrari in A selection from Smithsonian at the Poles Contributions to International Polar year. I. Krupnik, M.A. Lang and S.E. Miller, eds., Publs. by Smithsonian Institution Scholarly Press, Washington DC., 2009. [p.167, Fig.2].
Distribution of selected pelagic calanoids Neocalanus tonsus of the Southern Ocean and the closest relative in the subarctic region of the Arctic Ocean. |
 Issued from : H. Saito & A. Tsuda in Deep-Sea Res. I, 47. [p.2146, Table I]. Issued from : H. Saito & A. Tsuda in Deep-Sea Res. I, 47. [p.2146, Table I].
Egg diameter and prosome length (PL) of females of Neocalanus tonsus afrter Ohman, 1987
Nota: Compare with N. cristatus, N. flemingeri and and N. plumchrus, and others species in genus Calanus. |
| | | | Loc: | | | Antarct. (Indian, SW Pacif., Mc Murdo), sub-Antarct. (South Georgia, Scotia Sea, Indian, SW & SE Pacif.), Agulhas Bank, S & E), South Africa (S), Tristan da Cunha, SW Atlant., G. of Guinea, Argentina (off shore N-S, off Patagonia), off Woods Hole, Indian (Natal, Madagascar, equatorial), Australia (W, SW, SE, Tasmania), Philippines, New Zealand (Otago Peninsula, Kaikoura, SE North Island), off E New Zealand, S Pacif. (NPFZ), Chile (N & S), Straits of Magellan (Pacific & Central area); [ Japan Sea, Okhotsk Sea, SW Bering Is., Alutian, Charlotte Queen Is., Strait of Georgia, Bikini Is. ] | | | | N: | 69 | | | | Lg.: | | | (25) F: 4,05-3,69; (32) F: 3,6-3,5; (35) F: 3,6-3,48; (36) F: 3,92; (47) F: 3,6; (114) F: 4,6-4,35; M: 3,1; (116) F: 4,1-3,8; (124) F: 4,24-4,07; 3,75-3,66; (196) F: 4-3,3; M: 3,9-3,3; (231) M: 3,45-3,25; (313) M: 3,45-3,25; (331) M: 4,4-4,1; {F: 3,30-4,60; M: 3,10-4,40} | | | | Rem.: | Sampling depth (Antarct., sub-Antarct.) : 0-500 m.
After Marshall & Orr (1972, p.8) , the A1 reach the end of the 3rd urosome segment and have setae only on segments 23, 24 and 25. The 24th segment is twice as long as the 25th. In the female the genital segment is broad and swollen. On the coxae of P2 and P3 there are 4 large spines and on the coxa of P4, 1 or 2; on the coxa of P5 there are none.
Tanaka (1956, p;51-52) points to Calanus tonsus of Antarctic distinctly separated from that of the North Pacific reported by the American authors (McMurrich, 1917; Willey, 1920; Wailes, 1929; Johnson, 1932; Campbell, 1930, 1934; Davis, 1949), corresponding well to Calanus plumchrus Marukawa, 1921, a species from northern cold waters of Japan.
Calanus tonsus from the North Pacific is not synonym of Calanus tonsus Brady. A confusion has existed between Calanus tonsus and Calanus plumchrus.
Brady's description is so fragmentary that it may well be another species (Vervoort, 1951, suggests that Brady confused C. tonsus and C. acutus which is one of the common species in the Antarctic); but C. tonsus differs from C. acutus in the shape of head and in the simming legs which have no short spine on the inner distal corner of the 2nd basipod.
In the matrix, no account has been taken of the locality records of this species in the North Pacific, for which the references under the ancient denomination of Calanus tonsus are between brackets.
After Ohman (1987, p.1326) adult N. tonsus females from the subantarctic ocean are active suspension feeders, in marked contrast to N. plumchrus and N. cristatus. These results are consistent with interspecific differences in morphology of feeding appendages. Upon molting to the CVI female the mandibular palp, Mx1, Mx2 and Mxp of N. tonsus all enlarge, and the associated setae elongate for both winter and spring females. The mandibular gnathobase females also enlarges and retains crisp dentition. In contrast, the terminal molt to adult female by N. plumchrus and N. cristatus is accompanied by reduction in size and setation of the Mx1, Mx2 and Mxp and complete loss of the cutting edge of the mandibular gnathobase.
Jillett (1968, p.26) points to in the southern New Zealand waters that the species were least abundant inshore stations, where neritic conditions usually prevail. Greatest abundance was at the stations located in the path of the Southland Current, recognized by its high salinity. Greatest abundance was nearly always in the zone between the Southland Curre,t and Circumpolar Subantarctic Water. These conditions typify Australasian Subantarctic Water which lies to the south-west of New Zealand (salinity above 34.5 p.1000, temperature above 8°C) There could well be an affinity between the species and this water mass. In any case, these conditions result from the mixing of Subantarctic and Subtropical Waters at the Subtropical Convergence (see Houtman, 1967). N. tonsus was present in deeper offshore waters when it was absent from surface waters, including the Southland Current which does not extend deeper than above 200 m. The presence of species in deep water during winter months is interesting because Vervoort (1965) lists it as a surface species. Greatest seasonal abundance is attained in the outer portions of the Southland Current. These individuals almost certainly must originate in the colder, less saline Subantarctic Water found further offshore and beneath the surface. N. tonsus also occurs in the central Tasman Sea, to the south of New Zealand and in a zone extending across the southern Pacific, south of the Subtropical Convergence (see Brodsky, 1967). It has also been taken abundantly from the sueface waters off Kaikouraz in the early summer of two consecutive years (Bradford, pers. comm.)The species was also numerous in surface catches taken at the north-east of New Zealand in about latitude 33°40' S. Occurrence to the north of the subtropical Convergence may result from the northward transportation at depth of overwintering Stage V copepodsites in Antarctic Intermediate Water. A similar extension of range has been demonstrated for Neocalanus cristatus off Japan, where it is carried south in cold Otashio Water beneath the warm Kuroshio (see Omori, 1967). Many of the features of the life cycle and the relationships to hydrographic conditions shown by N. tonsus in waters off south-eastern New Zealand are analogous with those of N. plumchrus which is distributed throughout the colder seas of the North Pacific. The similarity is interesting in view of the undoubted close relationship of the two species and past confusion as to their separate identity. Off the Kurile Islands and north-eastern Japan, N. plumchrus accumulates in enormous numbers in surface waters during spring and summer. In late summer and autumn, having developed into the Stage V copepodite and stored up fat, they descend into deeper water where they overwinter (see Bogorov & Vinogradov, 1955). However, while N. plumchrus appears to have only one generation in a year, N. tonsus apparently has two.. In spring and summer N. plumchrus is most abundant near the surface in a band along its southern limit of distribution, indicating the northern frontal zone of the warm Kuroshio (Brodsky, 1955). Furuhashi (1966) considered the presence of N. plumchrus in mid-water beneath the Kuroshio as evidence of a southward extension. of the cold Oyashio at depth. Off southern New Zealand, N. tonsus reaches greatest abundance in a zone at the edge of the cold Subantarctic waters which it originates. It is also very seasonal in surface waters, where it swarms in spring and summer, storing red fat. After reaching the Stage V copepodite in late summer, N. tonsus also disappears from surface waters but is present in deep water throught the winter. The greatzst densities of N. tonsus Stage V (200-450 per m3) are of the same order as equivalent for N. plumchrus (200-500 per m3), as found by Brodsky (1955) in the North Pacific. | | | Last update : 03/04/2020 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2025. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed August 23, 2025] © copyright 2005-2025 Sorbonne University, CNRS
|
|
 |
 |