|
|
 |
|
Calanoida ( Order ) |
|
|
|
Diaptomoidea ( Superfamily ) |
|
|
|
Candaciidae ( Family ) |
|
|
|
Candacia ( Genus ) |
|
|
| |
Candacia pachydactyla (Dana, 1849) (F,M) | |
| | | | | | | Syn.: | Candace pachydactyla Dana, 1849; Giesbrecht, 1892 (p.424, 439, 771, figs.F,M); Brady, 1883 (p.68, figs.F,M); T. Scott, 1894 b (p.60);
Ifionyx typicus Kröyer,1845; 1849 (in Damkaer & Damkaer, 1979, p.11, 35, figs.F) | | | | Ref.: | | | Giesbrecht & Schmeil, 1898 (p.128, Rem. F); Thompson, 1888 d (p.140); Thompson & Scott, 1903 (p.235, 251); A. Scott, 1909 (p.153, Rem.); Wolfenden, 1911 (p.357); Sewell, 1914 a (p.230); Sars, 1925 (p.351); Rose, 1929 (p.40); Farran, 1929 (p.210, 272); Sewell, 1932 (p.337); Wilson, 1932 a (p.141, figs.F,M); Dakin & Colefax, 1933 (p.206); Rose, 1933 a (p.254, figs.F,M); Tanaka, 1935 a (p.211, figs.F,M, juv.); Mori, 1937 (1964) (p.85, figs.F,M); Dakin & Colefax, 1940 (p.105, figs.F); Wilson, 1942 a (p.175, fig.F); Sewell, 1947 (p.247); Chiba, 1956 (p.62, figs.F); Chiba & al., 1957 (p.310); 1957 a (p.11); Tanaka, 1960 (p.55); Fish, 1962 (p.16); Brodsky, 1962 c (p.136, figs.F,M); Grice, 1962 (p.231, figs.F,M); 1963 (p.178, figs.F,M, Rem.); Grice & Vervoort, 1963 (p.150, Rem.); Paiva, 1963 (p.65, figs.F,M); Kasturirangan, 1963 (p.44, 47, figs.F,M); Tanaka, 1964 c (p.244); Vervoort, 1965 (p.172, Rem.); Chen & Zhang, 1965 (p.90, figs.F,M); Jones, 1966 b (p.406, figs.F,M, Rem.); Saraswathy, 1966 (1967) (p.81); Owre & Foyo, 1967 (p.93, figs.F,M); Vidal, 1968 (p.41, figs.F,M); Shih & al., 1971 (p.37); Ramirez, 1971 (p.86, fig.F); Björnberg, 1972 (p.52, figs.N, Rem.N & Juv.); Kos, 1972 (Vol. I, figs.F, M, Descr.); Marques, 1973 (p.244, figs.F,M); 1976 (p.995); Lawson, 1977 (p.71, tab.2,3,4, fig.4, 5); Björnberg & al., 1981 (p.657, figs.F,M); Zheng & al., 1982 (p.65, figs.F,M); Sazhina, 1985 (p.67, figs.N); Baessa de Aguiar, 1987 (p.42, figs.F,M, Rem.F,M); Chihara & Murano, 1997 (p.754, Pl.75,78: F,M); Mulyadi, 1997 a (p.97, Redescr.F,M, figs.F,M); Bradford-Grieve & al., 1999 (p.885, 956, figs.F,M); Bradford-Grieve, 1999 b (p.173, figs.F,M, fig.193); Conway & al., 2003 (p.111, figs.F,M, Rem.); G. Harding, 2004 (p.26, figs.F,M); Boxshall & Halsey, 2004 (p.85: fig.F); Mulyadi, 2004 (p.93, figs.F,M, Rem.); Vives & Shmeleva, 2007 (p.453, figs.F,M, Rem.); Phukham, 2008 (p.63, figs.F,M); | 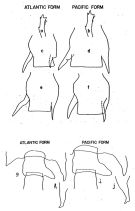 issued from : E.C. Jones in Proc. Sympos. Crustacea, ser.2, Mar. Biol. Ass. India, 1966. [Fig.2, p.408; Fig.3,p.409]. Female: a, P5 (Atlantic form); b, P5 (Pacific form); c, genital segment (Atlantic form); d, genital segment (Pacific form); e and f: genital segments showing intermediate forms of right spine. Male: g, genital segment (Atlantic form); h, thoracic process, lateral view (Atlantic form); i, genital segment (Pacific form); j, thoracic process, lateral view (Pacific form).
|
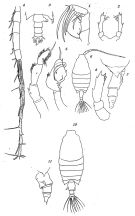 issued from : O. Tanaka in Suisan Gakkai Ho, Tokyo, 1935, 6 (4). [Pl.I, p.217; Pl.II, p.219]. Female: 1, Mx2; 2, P5; 10, habitus (dorsal view). Female juv.: 6, habitus (dorsal view); 7, last thoracic segment and urosome (gight lateral view); 8, P5. Male: 3, last thoracic segment and urosome (dorsal view); 4, right A1 (distal part with hinge segments); 5, P5 (left and right).
|
 issued from : Giesbrecht in M. Rose, 1933. Faune de France, 26 Copépodes pélagiques. Paul Lechevalier, ed., Paris. [Fig.321, p.254].
|
 issued from: Q.-c Chen & S.-z. Zhang in Studia Marina Sinica, 1965, 7. [Pl.36, 1-2]. Male (from E China Sea): 1, habitus (dorsal); 2, P5 (posterior).
|
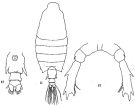 issued from: Q.-c Chen & S.-z. Zhang in Studia Marina Sinica, 1965, 7. [Pl.35, 11-13]. Female: 11, habitus (dorsal); 12, urosome (ventral); 13, P5 (posterior).
|
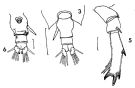 issued from : F.C. Ramirez in Revta Mus. La Plata, Seccion Zool., 1971, XI[Lam.II, figs.3, 5, 6]. Female (from off Mar del Plata): 3, urosome (dorsal); 5, P5; 6, urosome (ventral).
|
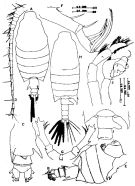 issued from : Mulyadi in Treubia, 1997, 31 (2). [p.98, Fig.11]. Female (from Flores Sea): A, habitus (dorsal); B, last somites of metasome and urosome (dorso-lateral right view); C, urosome (ventral); D, A1; E, Md; F, Mx2; G, P5 (anterior view). Nota: The female is identifiable by the 2 large spinous processes of urosomite 1 Male: H, habitus (dorsal); I, angles of the 5th metasomal somite, genital somite and urosomal somite 2 (dorsal); J, P5 (posterior view). Nota: The male is identifiable by the bifurcate prolongation of right posterolateral end of 5th metasomal somite and the great process of the right side of urosomite 1.
|
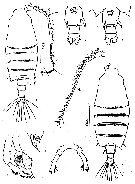 issued from : Z. Zheng, S. Li, S.J. Li & B. Chen in Marine planktonic copepods in Chinese waters. Shanghai Sc. Techn. Press, 1982 [p.66, Fig.37]. Female: a, habitus (dorsal); b, urosome (ventral); c, idem (another specimen); d, A1; e, P5. Male: f, habitus (dorsal); g, distal segment of exopod of P3; h, P5. Scale bar in mm.
|
 issued from : T. Mori in The Pelagic copepoda from the neighbouring waters of Japan, 1937 (1964). [Pl. 58, Figs.1-5]. Female: 1, habitus (dorsal); 3, P5. Male: 2, habitus (dorsal); 4, P4; 5, right A1.
|
 issued from : G.D. Grice in Fish. Bull. Fish and Wildl. Ser., 1962, 61. [p.232, Pl.30, Figs.1-4]. Female (from equatorial Pacific): 1, habitus (dorsal); 2, posterior part of thorax and urosome (ventral); 3, Md (biting edge); 4, P5. Nota: Genital segment with the coarse spine-like protrusions. Basal tooth of Md ends in 3 very unequal cusps. Distal segment of P5 ends in 3 points, the innermost one of which is curved.
|
 issued from : G.D. Grice in Fish. Bull. Fish and Wildl. Ser., 1962, 61. [p.232, Pl.30, Figs.5-9. Male: 5-6, habitus (dorsal and lateral, respectively); 7, urosome (dorsal); 8, left P5; 9, right P5. Right posterior margin of thorax similar to that of C. ethiopica but the protrusion of the genital segment is quite large. The thumb of the chela on the right P5 ends in a long spine as does the distal segment of the left P5.
|
 issued from : G. Harding in Key to the adullt pelagic calanoid copepods found over the continental shelf of the Canadian Atlantic coast. Bedford Inst. Oceanogr., Dartmouth, Nova Scotia, 2004. [p.26]. Female & Male. Nota: Male right A1 with segments 17 and 18 fused (arrowed).
|
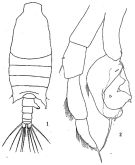
 issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1, Crustacea, 1: Copepoda. Copepods of the Florida Current. 1967. [p.28, Fig.133]. Female: 133, P5.
|
 issued from : N. Phukham in Species diversity of calanoid copepods in Thai waters, Andaman Sea (Master of Science, Univ. Bangkok). 2008. [p.146, Fig.20]. Female (from W Malay Peninsula): a, habitus; b, urosome; c, P5. Male: d, habitus (dorsal); e, last thoracic segment and urosome (dorsal); f, P5. Body length after the drawings: F: = 2.452 mm; M = 2.361 mm.
|
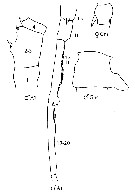 Issued from : J.M. Bradford-Grieve, E.L. Markhaseva, C.E.F. Rocha & B. Abiahy in South Atlantic Zooplankton, edit. D. Boltovskoy. 1999, Vol. 2, Copepoda; [p.1065, Fig. 7.371: Candacia pachydactyla ]. Gns = genital segment; r = right. Female characteristics (from key, p.956) : - In dorsal view, 1 robust spine-like process extending obliquely posteriad from left side, and 1 robust spine extending posteriad from right side of genital segment; both surpass posterior margin of genital segment. - Spines or spine-like processes on dorsal, lateral or ventral margins of genital segment. - 3 inner edge setae present on terminal segment of P5. - Urosomal segment 2 without lateral or ventral protrusion and without spine-like process arising from ventral surface. - Posterior corners of prosome pointed. Male (from key, p.956) : - In dorsal view, process on right side of genital segment large, consisting of single broad and rounded projection. - Right A1with segments 17 and 18 fused. - Right A1 with segments 2 and 3 fused. - In dorsal view, genital segment with large process. - Left posterior corner of the prosome pointed.
|
 Issued from : J.M. Bradford-Grieve in NIWA Biodiversity Memoir 111, 1999. [p.174, Fig.124]. After Giesbrecht 1893 ['1892'] Female (from SW Pacific): A, urosome (ventral view); B, same (left side); C, P5. Male (from SW Pacific): D, anterior urosome (dorsal view); E, same (right side); F, P5.
|
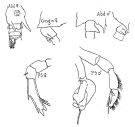
 Issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf. 39, figs. 30, 33, 31, 32. As Candace brachydactyla. Female: 30, urosome (ventral); 33, thoracic segment 5 and abdominal segments 1 and 2 (lateral). Male; 31, thoracic segment 5 and abdominal segments 1 and 2 (dorsal); 32, corner of posterior prosome and abdominal segments 1 and 2 (lateral, right side).
|
 Issued from : M.C. Kos in Field guide for plankton. Zool Institute USSR Acad., Vol. I, 1972. After Brodsky, 1962. Female: 1, habitus (dorsal); 2, A1; 3, corner of the last thoracic segment and abdomen (dorsal); 4, abdomen (ventral); 5, P. Male: 6, habitus (dorsal); 7, corner of the last thoracic segment and abdomen (dorsal); 8, P5.
| | | | | Compl. Ref.: | | | Cleve, 1904 a (p.187); Carl, 1907 (p.17); Sewell, 1912 (p.353, 368); 1948 (p.346, 391, 408, 414, 422, 443, 450, 457, 460, 463, 465, 490); C.B. Wilson, 1950 (p.182); Krishnaswamy, 1953 (p.128); King & Hida, 1955 (p.11); Yamazi, 1958 (p.151, Rem.); Cervigon, 1962 (p.181, tables: abundance distribution); Ganapati & Shanthakumari, 1962 (p.9, 15); Senô & al., 1963; Björnberg, 1963 (p.55, Rem.); Grice, 1963 a (p.496); Gaudy, 1963 (p.27, Rem.); De Decker & Mombeck, 1964 (p.11); Park, 1970 (p.478); Itoh, 1970 (tab.1); Timonin, 1971 (p.281, trophic group); Deevey, 1971 (p.224); Roe, 1972 (p.277, tabl.1, tabl.2); Bainbridge, 1972 (p.61, Appendix Table I: vertical distribution vs day/night, Table II: %, Table IV: seasonal abundance); Brodsky, 1972 (p.256); Björnberg, 1973 (p.349, 385); Patel, 1975 (p.659); Deevey & Brooks, 1977 (p.156, tab.2, Station "S"); Tranter, 1977 (p.596, 600); Carter, 1977 (1978) (p.36); Dessier, 1979 (p.206); Svetlichnyi, 1980 (p.28, passive submersion); Madhupratap & al., 1981 (p.266, fig.2k: abundance vs. geographic transect); Haury & Wiebe, 1982 (p.915, horizontal micro-distribution); Vives, 1982 (p.294); Kovalev & Shmeleva, 1982 (p.85); Gaudy & Boucher, 1983 (p.37, tab.1, 2, 3, 5, fig.1, 2, Rem.: metabolism); Dessier, 1983 (p.89, Tableau 1, Rem., %); Tremblay & Anderson, 1984 (p.4); Guangshan & Honglin, 1984 (p.118, tab.); De Decker, 1984 (p.317); Cummings, 1984 (p.163, Table 2); Sazhina, 1985 a (p.491, tab.3); Petipa & Borichenko, 1985 (tab.1, 2); Kinzer & Schulz, 1985 (tab.4); Brenning, 1985 a (p.28, Table 2); Brinton & al., 1986 (p.228, Table 1); Chen Y.-Q., 1986 (p.205, Table 1: abundance %, Table 2: vertical distribution); Madhupratap & Haridas, 1986 (p.105, tab.1); Renon, 1987 (tab.2); Brenning, 1987 (p.29, spatial distribution, T-S diagram, Rem.); Jimenez-Perez & Lara-Lara, 1988; Lozano Soldevilla & al., 1988 (p.60); Williams D. & al., 1988 (p.580); Heinrich, 1990 (p.19); Suarez & al., 1990 (tab.2); Schulz, 1990 (p.188); Ohtsuka & Kubo, 1991 (p.541); Arinardi, 1991 (p.295); Yoo, 1991 (tab.1); Samba Diouf, 1991 (p.104); Suarez & Gasca, 1991 (tab.2); Suarez, 1992 (App.1); Shih & Young, 1995 (p.69); Webber & al., 1996 (tab.1); Suarez-Morales & Gasca, 1997 (p.1525); Mauchline, 1998 (p.508, tab.30, 64); Padmavati & al., 1998 (p.347); Hwang & al., 1998 (tab.II); Alvarez-Cadena & al., 1998 (tab.1,2,3,4); Smith S. & al., 1998 (p.2369, Table 6, moonsoon effects); Noda & al., 1998 (p.55, Table 3, occurrence); Suarez-Morales, 1998 (p.345, Table 1); Suarez-Morales & Gasca, 1998 a (p108); Neumann-Leitao & al., 1999 (p.153, tab.2); Fernandez-Alamo & al., 2000 (p.1139, Appendix); Suarez-Morales & Gasca, 2000 (1247, tab.1); Suarez-Morales & al., 2000 (p.751, tab.1); Lopez-Salgado & al., 2000 (tab.1); Madhupratap & al., 2001 (p. 1345, vertical distribution vs. O2, figs.4, 5: clusters, p.1353); Holmes, 2001 (p.30); Beaugrand & al., 2002 (p.179, figs.5, 6); Hsiao & al., 2004 (p.325, tab.1); Wang & Zuo, 2004 (p.1, Table 2, dominance, origin); Razai al., 2004 (p.490, tab.2); Lo & al.*, 2004 (p.218, fig.6); Gallienne & al., 2004 (p.5, tab.3); CPR, 2004 (p.51, fig.144); Lo & al., 2004 (p.89, tab.1); Osore & al., 2004 (p.195); Berasategui & al., 2005 (p.313, fig.2); Zuo & al., 2006 (p.162: tab.1); Hwang & al., 2006 (p.943, tabl. I); Dias & Araujo, 2006 (p.35, Rem., chart); Rakhesh & al., 2006 (p.93, Table 2, spatial distribution); Hwang & al., 2007 (p.23); Dur & al., 2007 (p.197, Table IV); Jitlang & al., 2008 (p.65, Table 1); Neumann-Leitao & al., 2008 (p.799: Tab.II, fig.6); Morales-Ramirez & Suarez-Morales, 2008 (p.518); Fernandes, 2008 (p.465, Tabl.2); Ayon & al., 2008 (p.238, Table 4: Peruvian samples); Miyashita & al., 2009 (p.815, Tabl.II); Dias & al., 2010 (p.230, Table 1); Cornils & al., 2010 (p.2076, Table 3); Schnack-Schiel & al., 2010 (p.2064, Table 2: E Atlantic subtropical/ tropical); Medellin-Mora & Navas S., 2010 (p.265, Tab. 2); Tutasi & al., 2011 (p.791, Table 2, abundance distribution vs La Niña event); Guo & al., 2011 (p.567, table 2, indicator); Kâ & Hwang, 2011 (p.155, Table 3: occurrence %); Tseng L.-C. & al., 2011 (p.47, Table 2, occurrences vs mesh sizes); Pagano & al., 2012 (p.538, Table 1); Mulyadi & Rumengan, 2012 (p.202, Rem.: p.204); Hidalgo & al., 2012 (p.134, Table 2); Teuber & al., 2013 (p.28, Table 1, respiration rates); Teuber & al., 2013 (p.1, Table 1, 2, 3, abundance vs oxygen minimum zone, respiration rates, enzyme activity); in CalCOFI regional list (MDO, Nov. 2013; M. Ohman, pers. comm.); Tseng & al., 2013 (p.507, seasonal abundance); Jagadeesan & al., 2013 (p.27, Table 3, 5, 6, fig.11, seasonal abundance); Lidvanov & al., 2013 (p.290, Table 2, % composition); Hwang & al., 2014 (p.43, Appendix A: seasonal abundance); Bonecker & a., 2014 (p.445, Table II: frequency, horizontal & vertical distributions); Dias & al., 2015 (p.483, Table 2, abundance, biomass, production); Palomares-Garcia & al., 2018 (p.178, Table 1: occurrence not seen in La Paz); Bode & al., 2018 (p.840, Table 1, respiration & ingestion rates, depth); Acha & al., 2020 (p.1, Table 3: occurrence % vs. ecoregions). | | | | NZ: | 17 | | |
|
Distribution map of Candacia pachydactyla by geographical zones
|
| | | | | | | | | | | |  issued from : T.J. Lawson in Marine Biology, 1977, 43. [Fig.5, p.78]. issued from : T.J. Lawson in Marine Biology, 1977, 43. [Fig.5, p.78].
Distribution map for the Indian Ocean. |
 issued from : Mulyadi in Treubia, 1997, 31 (2). [p.109, Fig.16]. issued from : Mulyadi in Treubia, 1997, 31 (2). [p.109, Fig.16].
Distribution of Candaciidae in Indonesian waters. 10: C. pachydactyla. |
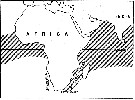 issued from : E.C. Jones in Proc. Symp. Crustacea- Symp. Ser. Mar. biol. Ass. India, (2) 1. [p.407, Fig.1]. The general distribution of Candacia pachydactyla (hatched area). issued from : E.C. Jones in Proc. Symp. Crustacea- Symp. Ser. Mar. biol. Ass. India, (2) 1. [p.407, Fig.1]. The general distribution of Candacia pachydactyla (hatched area).
Nota: Does exist an effect of the southern portion of the continent of South Africa as a barrier to the mixing of warm water Atlantic and Indo-Pacific pelagic copepods ? According to Sewell (1948) two questions seemed to have been considered: 1- Does warm, tropical water from the Aghulhas Stream of the south-western Indian Ocean enter the Atlantic after rounding the Cape of Good Hope, and 2- if this takes place, do the tropical copepods from the Indian Ocean survive and enter the gene pool of their Atlantic counterparts ? Sewell listed 138 species be considered to be "Indo-Pacific species", which also are found in the Atlantic; from this he concluded that the African continent does not form an effective barrier to the mixing of populations of the two oceans. For Steuer (1928) concluded it is likely that Africa forms an effective barrier most the time but occasionally, under certain circonstances, this barrier is broken down. He believed that Indian Ocean populations carried to the Atlantic generally did not survive there. DeDecker (1964) stated that Indo-Pacific copepods carried by the Agulhas Current disappeared over the Agulhas Bank were seldom if ever carried further west.
The evidence of morphological differences between the Atlantic and the Indo-Pacific populations in Candacia pachydactyla suggests a high degree of isolation between those populations. The Benguela Current appears to constitute an additional barrier to Indo-Pacific individuals mixing between the Atlantic and Indo-Pacific populations. No consistent differences were found between samples from the Indian Ocean and the central Pacific; likewise, none were founded between the Atlantic samples; however, differences between Atlantic and Indo-Pacific populations were consistent (see figures). The conclusion that populations in the Atlantic and the Indo-Pacific are separated by the barrier of southern Africa is also supported by distribution records of certain other species within the family Candaciidae. Two other Pacific equatorial species, C. catula and C. truncata, are not found in 1963 (see Grice, 1963), and since exceptionally in the west part of the Cape of Good Hope. |
 issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1, Crustacea, 1: Copepoda. Copepods of the Florida Current. 1967. [p.793, Table 41]. issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1, Crustacea, 1: Copepoda. Copepods of the Florida Current. 1967. [p.793, Table 41].
Vertical distribution of Candacia pachydactyla at the ''40-Mile station'' in the Florida Current (± 25°35'N, 79°27'W).
SL 55: 21 VII 1958. A: during midday; B;: during midnight. |
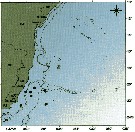 issued from : C. de O. Dias & A.V. Araujo in Atlas Zoopl. reg. central da Zona Econ. exclus. brasileira, S.L. Costa Bonecker (Edit), 2006, Série Livros 21. [p.35]. issued from : C. de O. Dias & A.V. Araujo in Atlas Zoopl. reg. central da Zona Econ. exclus. brasileira, S.L. Costa Bonecker (Edit), 2006, Série Livros 21. [p.35].
Chart of occurrence in Brazilian waters (sampling between 22°-23° S).
Nota: sampling only 8 specimens. |
 issued from : U. Brenning in Wiss. Z. Wilhelm-Pieck-Univ. Rostock - 36. Jahrgang 1987. Mat.-nat. wiss. Reihe, 2. [p.27, Fig.1]. issued from : U. Brenning in Wiss. Z. Wilhelm-Pieck-Univ. Rostock - 36. Jahrgang 1987. Mat.-nat. wiss. Reihe, 2. [p.27, Fig.1].
Spatial distribution for Candacia curta, C. armata, C. bipinnata, C. paenelongimana, C. varicans (as varians), C. pachydactyla, C. longimana, C. ethiopica, Paracandacia simplex ( = C. simplex) from 8° S - 26° N; 16°- 20° W, for different expeditions (V1: Dec. 1972- Jan. 1973; V2: Feb/Mar. 1973; VI: May 1974; IV: Jun./Jul. 1972). |
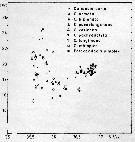 issued from : U. Brenning in Wiss. Z. Wilhelm-Pieck-Univ. Rostock - 36. Jahrgang 1987. Mat.-nat. wiss. Reihe, 2. [p.28, Fig.2]. issued from : U. Brenning in Wiss. Z. Wilhelm-Pieck-Univ. Rostock - 36. Jahrgang 1987. Mat.-nat. wiss. Reihe, 2. [p.28, Fig.2].
T-S Diagram for Candacia curta, C. armata, C. bipinnata, C. paenelongimana, C. varicans, C. pachydactyla, C. longimana, C. ethiopica, Paracandacia simplex ( = C. simplex) from 8° S - 26° N; 16°- 20° W. |
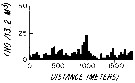 issued from : L.R. Haury & P.H. Wiebe in Deep-Sea Res., 1982, 29 (7A). [p.918, Fig.1, E]. issued from : L.R. Haury & P.H. Wiebe in Deep-Sea Res., 1982, 29 (7A). [p.918, Fig.1, E].
Abundance horizontal distribution of Candacia pachydactyla: non-random spatial structure.
Samples collected with a Longhurst-Hardy Plankton Recorder (LHPR) in the northern Sargasso Sea (34°10'N; 71°35'W) at three depth stata.
Nota: The pattern reveal that dominant variations occurred on scales of tens to hundreds of meters. The fluctuations in abundance are evident on different scales simultaneously.
After the authors, the abundance of species was not significantly nor consistently correlated to either temperature or alinity on the scales resolved by the tows, thus the LHPR data indicate that the physical environment or depth changes along the horizontal tows, was not, in general, a direct causative factor for the patterns observed. |
 Issued from : M. Bode, R. Koppelmann, L. Teuber, W. Hagen & H. Auel inGlobal Biogeochemical Cycles, 2018, 32. [p.844, Table 1). Issued from : M. Bode, R. Koppelmann, L. Teuber, W. Hagen & H. Auel inGlobal Biogeochemical Cycles, 2018, 32. [p.844, Table 1).
Cf. explanations of these measures in Calanoides natalis from the same authors.
Compare with Candacia ethiopica. |
| | | | Loc: | | | off S South Africa, South Africa (E & W), Congo, off St. Helena Is., off Ascension Is., Atlant. (central equatorial), off E Sao Tomé, Angola, G. of Guinea, off Lagos, off NE St. Paul Is., Dakar, Cape Verde Is., off Morocco-Mauritania, Canary Is., Argentina, Brazil (Campos Basin, off Vitoria-Cabo de Sao Tomé, off Natal, off Amazon), Barbada Is., Venezuela, Caribbean Colombia, Cariaco Basin, Caribbean Sea, Jamaica, Yucatan, Caribbean, G. of Mexico, Cuba, Florida, off Bermuda (Station "S"), Sargasso Sea, Cape Hatteras, Woods Hole, off S Nova Scotia, off W Bretagne, Medit. (Alboran Sea, Medit. W & E), G. of Aden, Arabian Sea, Laccadive Is., Maldive Is., Sri Lanka, Madagascar (Nosy-Bé), off Kenya, Rodrigues Is., Seychelles, Natal, Mascarene Basin, Indian (equatorial), India (Saurashtra coast, Lawson's Bay, Madras, G. of Mannar), Bay of Bengal, Nicobar Is. , Nankauri Harbour, Burma, Andaman Sea, W Malay Peninsula (Andaman Sea), Straits of Malacca, Lombok Sea, Flores Sea, Ambon Bay (S Ceram), Indonesia-Malaysia, Java Sea, Flores Sea, SW Celebes, Philippines, Gulf of Tonkin, China Seas (Yellow Sea, East China Sea, Taiwan Strait, South China Sea), Taiwan (S, SW, NE, N: Mienhua Canyon), S Korea, Japan, Kuchinoerabu Is., Sagami Bay, Pacif. (W equatorial), Australia (W & New South Wales, Great Barrier), Bikini Is., Fiji Is., Pacif. (equatorial), off S Hawaii, California, W Baja California, G. of California, La Paz, SW Mexico, G. of Tehuantepec, W Costa Rica, Clipperton Is., Galapagos- Ecuador, off Peru, SW Galapagos, Chile, off Santiago, Tuamotu (Ahe atoll) | | | | N: | 188 (sub-Antarct.: 1; Atlant. S: 28; Atlant. N: 56; Medit.: 2; Red Sea: 1; Indian: 41; Indo-Malaysia: 10; Pacif.: 47) | | | | Lg.: | | | (16) F: 2,9-2,3; M: 2,54-2,45; (45) F: 3-2,4; M: 2,6-2,3; (46) F: 2,8-2,4; M: 2,6-2,3; (66) M: 2,74-2,67; (73) F: 3,4-2,63; M: 2,79-2,63; (91) F: 2,8-2,4; M: 2,6-2,3; (101) F: 2,88-2,48; M: 2,72-2,07; (104) F: 2,25; 2,8; (120) F: 2,95; M: 2,91; (150) F: 2,75; M: 2,7; (187) F: 2,55-2,4; M: 2,5-2,2; (199) M: 3,12-2,28; (237) F: 2,5; M: 2,75; (290) F: 2,5-3; M: 2,6-2,65; (334) F: 3-2,4; M: 2,6-2,3; (530) F: 1,8; M: 1,5; (778) F: 2,38; M: 2,52; (785) F: 2,93-2,56; M: 3,2-2,64; (795) F: 2,5; (864) F: 2,2-2,6; (866) F: 2,5-3,13; M: 2,28-3,12; (991) F: 2,15-3; M: 2,07-2,72; (1023) F: 2,86-3,13; M: 2,80-3,03; (1110) F: 3,17; M: 2,96; (1122) F: 2,38; M: 2,52; (1230) F: 2,1-2,8; M: 2,3-2,7; {F: 1,80-3,40; M: 1,50-3,20}
The mean female size is 2.652 mm (n = 38; SD = 0.3387), and the mean male size is 2.572 mm (n = 37; SD = 0.3336). The size ratio (male : female) is about 0.97. | | | | Rem.: | epi- bathypelagic. Sargasso Sea: 0-1000 m (Deevey & Brooks, 1977, Station "S");
After C.B. Wilson (1950, p.182) Dana's original specimens came mostly from the southern Atlantic, with a few from the China Sea; he gave the color as ''smoky with black bands about the cephalothorax; the extremities of the antennae and some of the natatory legs black. There are still traces of this coloration in the Albatross specimens after 30 years' preservation.
According to Sewell (1912, p.368) this species was rare in the Bay of Bengal)
Jones (1966 b) distinguishes two forms with distinct morphological characters: the first Indo-Pacific, the second Atlantic. This author emphasizes the role of the geographical barrier formed by South Africa. For Sewell (1912, p.368) the species was rare in the Bay of Bengal.
For Yamazi (1958, p.151) this species is very rare, mainly in warm season.
For Björnberg (1963, p.55) this species is euryhaline, but preferring the higher salinities (35 p.1000 and above) and thermophile.
After Itoh (1970 a, fig.2, co-ordinates) the Itoh's index value of the mandibular gnathobase = 2345, and for Gaudy & Boucher (1983) : 2360.
Timonin (1971, p.282) considers the trophic interrelations in the equatorial and tropical Indian Ocean, and divides the plankters into 6 trophic groups from the litterature and the results of studies of mouth-parts structure and intestine content. This species is a piercing and sucking carnivorous.
See in DVP Conway & al., 2003 (version 1) | | | Last update : 14/06/2021 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed February 20, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |




























