|
|
 |
|
Calanoida ( Order ) |
|
|
|
Diaptomoidea ( Superfamily ) |
|
|
|
Centropagidae ( Family ) |
|
|
|
Centropages ( Genus ) |
|
|
| |
Centropages chierchiae Giesbrecht, 1889 (F,M) | |
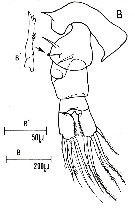
| | | | | | | Syn.: | Centropages typicus : Tanaka, 1960 (p.46, figs.F,M) | | | | Ref.: | | | Giesbrecht, 1892 (p.304, 320, 771, figs.F,M); Giesbrecht & Schmeil, 1898 (p.55, Rem. F,M); Thompson & Scott, 1903 (p.234, 246); Wolfenden, 1911 (p.356); Brady, 1914 (p.5, figs.F,M, Rem.); Pesta, 1920 (p.520); Farran, 1926 (p.269, Rem.); Candeias, 1926 (1929) (p.38, figs.F,M); Rose, 1929 (p.32); Rose, 1933 a (p.187, figs.F,M); Massuti Alzamora, 1942 (p.94); Lysholm & al., 1945 (p.34); Farran, 1948 (n°11, p.3, figs.F,M); Marques, 1953 (p.116); 1958 (p.215); Crisafi, 1960 (p.484, figs.F,M, juv.); Tanaka, 1963, p.7, Rem.); 1964 (p.9); Vervoort, 1964 b (p.308); 1965 (p.90, Rem.); Vilela, 1965 (p.8); Neto & Paiva, 1966 (p.25); Vilela, 1968 (p.21); Corral Estrada, 1970 (p.181); Lee, 1972 (p.8, figs.F,M, spermatophore and coupling device, P6 male biometry, Text-fig.13); Razouls, 1972 (p.94, Annexe: p.64, figs.F); Binet & al., 1972 (p.71); Marques, 1974 (p.16); Sazhina, 1982 (p. 1157, 1158, fig.N, Rem.); Schnack, 1989 (p.137, tab.1, fig.6: Md); Sazhina, 1985 (p.59, figs.N); Bradford-Grieve & al., 1999 (p.884, 951, figs.F,M); Vives & Shmeleva, 2007 (p.469, figs.F,M, Rem.); Blanco-Bercial & al., 2014 (p.5: Rem.: problematical for barcoding) |  issued from : C. Razouls in Th. Doc. Etat Fac. Sc. Paris VI, 1972, Annexe. [Fig.47, A, A', A'', D, G]. Female (from Banyuls, G. of Lion): D, P5; A, right P5; A', exopod 1 and 2 of left P5; A\", proximal segments of A1.
|
 issued from : C. Razouls in Th. Doc. Etat Fac. Sc. Paris VI, 1972, Annexe. [Fig.48, B]. Female: B, last thoracic segment and urosome (rwisted dorsal); B', process on genital segment.
|
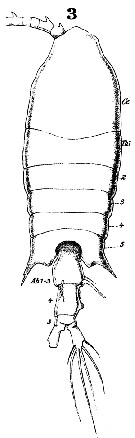 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.38, Fig.3]. Female: 3, habitus (dorsal).
|
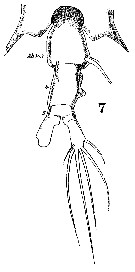 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.38, Fig.7]. Female: 7, urosome (dorsal).
|
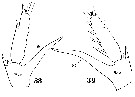 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.17, Figs.38, 39]. Female: 38, exopodal segments 2 and 3 of left P5; 39, exopodal segments 2 and 3 of right P5.
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.18, Fig.5]. Male: 5, right A1 (segments 15 and 16).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.17, Fig.45]. Male: 45, exopod of right P5 (posterior view). Re 2, Re3 = exopodal seggments 2 and 3.
|
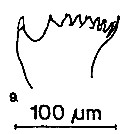 issued from : S.B. Schnack in Crustacean Issue, 1989, 6. [p.143, Fig.6: 9]. 9, Centriopages chierchiae (from off NW Africa, upwelling region): Cutting edge of Md.
|
 issued from : S.B. Schnack in Crustacean Issue, 1989, 6. [p.146, Fig.8]. Schematic diagram of Mx2. Distance between setae and length of setule not considerd. (Herbivore mode)) Nota: The intersetule spacing on the Mx2 may be used as an index for the feeding habit. Compare with Mx2 of the herbivore Calanoides carinatus.
|
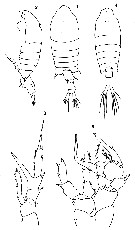 Issued from : O. Tanaka in Spec. Publs. Seto mar. biol. Lab., 10, 1960 [Pl. XX]. As Centropages typicus. Female (from 35°020°13'9'S): 1-2, habitus dorsal and lateral, respectively); 3, P5. Nota: Cephalothorax and abdomen in the proportional lengths 71 to 29. Head with a small process on the mid-dorsal line. Last thoracic segment sharply produced and slightly asymmetrical.. Abdomen 3-segmented. Genital segment with 2 spines on the left, one on the dorsal, and one on the ventral. 2nd abdominal segment produced on the right distal corner; P5: 2nd exopodal segment has a long and stout process on the inner distal corner, exceeding the distal end of the 3rd exopodal segment. Male: 4, habitus (dorsal); 5, P5.<
|
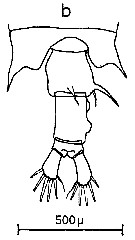 Issued from : C.M. Lee in Bull. mar. Ecol., 1972, 8. [p.4, Text-Fig. 1 b]. Dorsal view of the fifth metasome segment and the urosome of females of Centropages chierchiae (from Algiers, Mediterranean Sea).
|
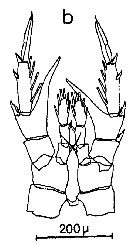 Issued from : C.M. Lee in Bull. mar. Ecol., 1972, 8. [p.5, Text-Fig. 2 b]. P5 female of Centropages chierchiae.
|
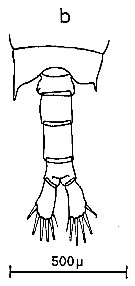 Issued from : C.M. Lee in Bull. mar. Ecol., 1972, 8. [p.4, Text-Fig. 1 b]. Dorsal view of the fifth metasome segment and the urosome of males of Centropages chierchiae (from Algiers, Mediterranean Sea).
|
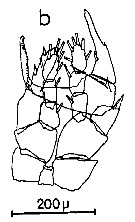 Issued from : C.M. Lee in Bull. mar. Ecol., 1972, 8. [p.5, Text-Fig. 2 b]. P5 male of Centropages chierchiae.
|
 Issued from : G.S. Brady in Ann. Durban Mus., 1914, Vol. I. [Pl. II, figs. 1-6]. Female (from Durban Bay, Natal): 1, posterior metasome and urosome; 2, outer branch of P5; 3, forehead and base of right anterior antenna (A1) of male; 4, part of anterior antenna (A1) of female; 5, P5 of male; 6, urosome and last segment of metasome male. Nota Female: - Forehead prominent in the middle, obtusely angular. - Metasome abruptly truncated behind, ite hinder margin deeply excavated in the middle and strongly produced at the lateral angles, forming two long, acutely pointed spines. - Urosome much narrower than the metasome, often more or less swollen and distorted. - Genital segment very tumid and bearing 2, or sometimes 3, long curved spines. - Urosomal segments following also tumid and produced laterally on the right side, forming a prominent mimillar protuberance. Caudal rami short, broad and somewhat divergent. A1 long and slender, reaching to the extremity or slightly beyond the extrrmity of the tail segments. - Outer branch of P5 has its middle segment produced, into a long sharp spine. Nota male: - Body somewhat more slender than that of the female, but nearly of the same length. - Posterior extremity of metasome, however, much narrower, lateral spines much smaller and sometimes absent altogether. - Urosome 4-segmented, very slender. - Right A1 geniculated, the first two segments bearing short tooth-like spines. Forehead with 2 tentacular processes. - Outer branch (= exopod) of the left P5 bi-articulate, that of yje right forming a strongly prehensile chelate limb, the longer claw of which is subsigmoid in shape with a crenulated, imperfectly ringed extremity.
| | | | | Compl. Ref.: | | | Cleve, 1904 a (p.187); Pearson (1906, p.22); Rose, 1924 d (p.479); 1925 (p.152); 1927 e (p.543, Rem.); Wilson, 1942 a (p.177); Massuti Alzamora, 1942 (p.94, Rem.); Duran, 1963 (p.21, Rem.); Giron-Reguer, 1963 (p.51); Unterüberbacher, 1964 (p.28); Neto & Paiva, 1966 (p.24, Table III), annual cycle); Mazza, 1966 (p.71); 1967 (p.327, 329, 367, fig.65); Ehrhardt, 1967 (p.740, 887, figs.16, 51-53, geographic distribution, Rem.); De Decker, 1968 (p.45); Séguin, 1968 (p.488); Champalbert, 1969 a (p.620); Casanova, 1970 (p.30: Rem.); Carli, 1971 (p.372, tab.1); Bainbridge, 1972 (p.61, Appendix Table I: vertical distribution vs day/night, Table IV: seasonal abundance); Binet, 1973 (p.77); Corral Estrada & Pereiro Muñoz, 1974 (tab.I); Vives & al., 1975 (p.48: Rem.); Carter, 1977 (1978) (p.36); Binet, 1979 (p.400); Dessier, 1979 (p.89, 201, 205); Vaissière & Séguin, 1980 (p.23, tab.1); Andronov & Maigret, 1980 (p.71, Table 2, 3); Schnacck & Elbrächter, 1981 (p.433, fig.3, gut contents); Casanova, 1981 (p.509, Rem.: p.526, Bergmann's rule, Jordan's rule, size v.s. latitude); Vives, 1982 (p.293); Kovalev & Shmeleva, 1982 (p.84); De Decker, 1984 (p.316, 321: Rem., 340: carte); 1984 a (p.155); Brenning, 1985 (p.5, spatial distribution/TS); 1985 a (p.28, Table 2); Diouf & Diallo, 1987 (p.260); Lozano Soldevilla & al., 1988 (p.59); Samba Diouf, 1991 (p.104); Seguin & al., 1993 (p.23, Table 2: abundance, %), p.26: Rem.); Hure & Krsinic, 1998 (p.53, 101); Mauchline, 1998 (tab.47); Gilabert & Moreno, 1998 (tab.1, 2); Verheye & al., 1998 (p.317, Table II); Seridji & Hafferssas, 2000 (tab.1); Lapernat, 2000 (tabl.3, 4); d'Elbée, 2001 (tabl.1); Batten & al., 2001 (p.385, grazing vs microzooplankton); Holmes, 2001 (p.20); Pardal & Azeiteiro, 2001 (p.25); Zerouali & Melhaoui, 2002 (p.91, Tableau I, fig.5); Bode & al., 2003 (p.85, Table 1, abundance); Daly Yahia & al., 2004 (p.366, fig.4, tab.1); CPR, 2004 (p.52, fig.146); Queiroga & al., 2005 (p.195, table 1); Lindley & Daykin, 2005 (p.869, occurrence between 1959 to 2000); Marques & al., 2006 (p.297, tab.III); Varela & al., 2006 (p.272, Table 3, oil spill effects); Valdés & al., 2007 (p.104: tab.1); Khelifi-Touhami & al., 2007 (p.327, Table 1); Cabal & al., 2008 (289, Table 1); Rossi, 2008 (p.90: Tableau XII); Eloire & al., 2010 (p.657, Table II, temporal variability); Lidvanov & al., 2010 (p.356, Table 3); Mazzocchi & Di Capua, 2010 (p.424); S.C. Marques & al., 2011 (p.59, Table 1); Glushko & Lidvanov, 2012 (p.138, Tableau 1); Salah S. & al., 2012 (p.155, Tableau 1); Bode & al., 2012 (p.108, spatial distribution vs time-series, % biomass); Cruz & al., 2013 (p.1046, egg production, respiration vs. temperature , food); Sobrinho-Gonçalves & al., 2013 (p.713, Table 2, fig.8, seasonal abundance vs environmental conditions); Garrido & al., 2013 (p.843, feeding); Lidvanov & al., 2013 (p.290, Table 2, % composition); Zaafa & al., 2014 (p.67, Table I, occurrence): Benedetti & al., 2016 (p.159, Table I, fig.1, functional characters); Ben Ltaief & al., 2017 (p.1, Table III, Summer relative abundance); El Arraj & al., 2017 (p.272, table 1, 2, spatial distribution); Benedetti & al., 2018 (p.1, Fig.2: ecological functional group); Belmonte, 2018 (p.273, Table I: Italian zones); Chaouadi & Hafferssas, 2018 (p.913, Table II: occurrence). | | | | NZ: | 8 | | |
|
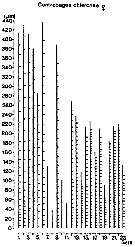
Distribution map of Centropages chierchiae by geographical zones
|
| | | | | | | | |  Chart of 1996 Chart of 1996 | |
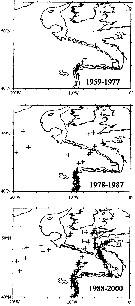 issued from : J.A. Lindley & S. Daykin in ICES Journal. Mar. Sc., 2005, 62. [p.871, Fig.1]. issued from : J.A. Lindley & S. Daykin in ICES Journal. Mar. Sc., 2005, 62. [p.871, Fig.1].
The geographical distribution of occurrences of Centropages chierchiae in CPR samples east of 20°W in the years 1959-1977, 1978-1987, and 1988-2000. Note that the Iberian coastal waters south of 42°N were only sampled from 1978 to 1986 and from 1997 onwards and the Bay of Biscay east of the line between Ushant and Cape Finisterre was sampled only on a few occasions in 1958 and 1959 and regularly from mid-1997 onwards.
See chart in Temora stylifera. |
 issued from : U. Brenning in Wiss. Z. Wilhelm-Pieck-Univ. Rostock - 34. Jahrgang 1985. Mat.-nat. wiss. Reihe, 6. [p.6, Figs.2, 3]. issued from : U. Brenning in Wiss. Z. Wilhelm-Pieck-Univ. Rostock - 34. Jahrgang 1985. Mat.-nat. wiss. Reihe, 6. [p.6, Figs.2, 3].
Spatial distribution and T-S Diagram for Centropages chierchiae from 8° S - 26° N; 16°- 20° W for different expeditions (V1: Dec. 1972-Jan. 1973; V2: Feb./mar. 1973; VI: May 1974; IV: Jun./Jul. 1972).
SO: Southern Surface Water (S °/oo: 34,50; T°C: 29,0); ND: Northern Water of the Surface Layer (S °/oo: 37,5; T°C: 21,0); SD: Southern Deep Water of the surface layer (S °/oo: 35,33; T°C: 13,4). See commentary in Temora stylifera and Brenning (1985 a, p.6).
Nota: Taking into account the seasonal temperature variations, the number of generations to be expected in a given region when the duration of upwelling in the region concerned is known, we have: 10-11 generations at Nouachott (Mauritania), ± 8 at Cap Vert (Dakar) and 3-4 at Cap Roxo (Casamance). |
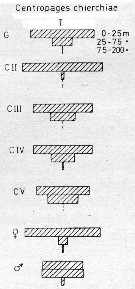 issued from : U. Brenning in Wiss. Z. Wilhelm-Pieck-Univ. Rostock - 34. Jahrgang 1985. Mat.-nat. wiss. Reihe, 6. [p.7, Fig.4]. issued from : U. Brenning in Wiss. Z. Wilhelm-Pieck-Univ. Rostock - 34. Jahrgang 1985. Mat.-nat. wiss. Reihe, 6. [p.7, Fig.4].
Vertical distribution of copepodid stages Centropages chierchiae from NW Africa.
T: daylight |
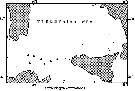 issued from : J.-P. Ehrhardt in Cah. Oceanogr., 1967 (9). [Fig.16]. issued from : J.-P. Ehrhardt in Cah. Oceanogr., 1967 (9). [Fig.16].
Occurrence of Centropages chierchiae collected at 7 stations on 39 stations between the southern Tyrrhenian Sea, Tunisia-Sardinia Strait and Cap Bon-west Sicily , only the might in superficial water.
Nota (p.888, 894): The species represents 19.6 % of adult copepods mainly in the Strait zone, in the waters from salinity between 37.22 and 38.41 p.1000 (more frequently between 37.57-37.67), and between 4.55-5.91 cm3/l oxygen (more frequently between 5.04-5.60 cm3/l), probably under infuence of the atlantic water superficial current. |
 Issued from : S.D. Batten, E.S. Fileman & E. Halvorsen in Prog. Oceanogr., 2001, 51. [p.389, Table 2 (part.)]. Issued from : S.D. Batten, E.S. Fileman & E. Halvorsen in Prog. Oceanogr., 2001, 51. [p.389, Table 2 (part.)].
Mean clearance rates of most abundant prey in Centropages chierchiae (animals sampled off Galician coast, before incubation experiments).
In addition to prosome length the dry weight of about half of the copepod was determined. An allowance was made of 30% weight loss, through effects of preservation in 4% seawater buffered formaldehyde solution, and the ingestion rate calculated as µgC/animal/day.
Nota: For the authors, the accepted 'classical' pelagic food web, where copepods feed on diatoms is a too simple a description of trophic interactions. Even in upwelling areas, where large diatoms may be abundant, protozoan microzooplankton can be an important source of carbon.
The experimental approach was based on that of Gifford (1993) where copepods were incubated with a natural food assemblage.
Measurements of copepod herbivory using fluorescence method, and the number of cells counted before and after experiment.
The microzooplankton carbon ingested was estimated from the prosome length and the proportion of the daily requirement calculate. Table 4 shows the range of this percentage for the species incubated in the experiments. The range is quite large, especially for the bigger species Calanus tenuicornis, owing to the wide difference in the size/ciliate ingestion relationships at the higher end of the prosome length scale.
The estimate for small copepods suggests that they received between ca. 5 and 25% of their daily requirement in the form of microzooplankton, which can also be expressed as between 1.4 and 11.8% of their body carbon (mean 4.4). The community ingestion calculations (Table 3, also see in Halvorsen & al., 2001) resulted in a community daily ingestion rate of microzooplankton of 7 to 15 % of the total carbon ingestion which fits very well with this estimate of 5-25%, given that the community was principally composed of small coppepods. |
 Issued from : S.D. Batten, E.S. Fileman & E. Halvorsen in Prog. Oceanogr., 2001, 51. [p.394, Table 4]. Issued from : S.D. Batten, E.S. Fileman & E. Halvorsen in Prog. Oceanogr., 2001, 51. [p.394, Table 4].
The proportion of an indivudual copepod's daily carbon requirement, calculated from the model of Ikeda and Motoda (1978) which would be met by microzooplankton ingestion, according to the experiments described here, at 18°C. |
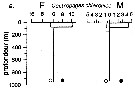 Issued from : P.-E. Lapernat in DEA Océanogr. Biol., Univ. P. & M. Curie, Paris VI. July 5, 2000. [Fig.9 a]. Issued from : P.-E. Lapernat in DEA Océanogr. Biol., Univ. P. & M. Curie, Paris VI. July 5, 2000. [Fig.9 a].
Verical distribution of Centropages chierchiae at an eutrophic site (off Mauritanian coast: 20°32 'N, 18°36' W) in females (F) and males (M) (ind. per m3) in the day (white circle) and night (black circle).
Nota: Sampling in the water column 0-1000 m, one during the day and another during the night with BIONESS multiple-net: 0-75; 75-150; 150-250; 250-350; 350-450; 450-550; 550-700; 700-850; 850-965 m. In May-June 1992. |
| | | | Loc: | | | South Africa (E & W, off Cape of Good Hope), Namibia, Angola, Baia Farta, Congo, G. of Guinea, off Lagos, Ivorian shelf, Casamance, Dakar, Mauritania, Morocco-Mauritania, Cap Ghir, Canary Is., NE Sargasso Sea, Portugal, Mondego estuary, off Coruña, Vigo, Santander, Bay of Biscay, off SW & W Ireland, Ibero-moroccan Bay, off W Tangier, Gibraltar, Medit. (Alboran Sea, Habibas Is., Sidi Fredj coast, Castellon, Baleares, Banyuls, Marseille, Toulon habour, Monaco, Genoa, Tyrrhenian Sea, Strait of Messina, Gulf of Annaba, NW Tunisia, G. of Gabes, Adriatic Sea), Natal Harbor, Natal, Sri Lanka, Indian, Australia (New South Wales) (in Dakin & Colefax, 1933) | | | | N: | 127 (S Atlant.: 17; N Atlant.: 54; Medit.: 43; Red Sea: 1; Indian: 11, Pacif.: 1) | | | | Lg.: | | | (16) F: 1,62-1,48; M: 1,58-1,49; (38) F: 1,65-1,55; M: 1,75-1,68; (47) F: 1,9-1,8; M: 1,75-1,65; (66) F: 1,82-1,72; M: 1,75-1,67; (114) M: 1,6; (327) F: 2,09-1,51; M: 1,92-1,49; (353) F: 1,803-1,618; M: 1,717-1,476; (373) F: 2,02-1,8; M: 1,78-1,67; (432) F: 1,85-1,7; (449) F: 1,9-1,8; M: 1,75-1,65; (1009) F: 1,80-2,10; M: 1,65-1,95; (1308) F: 1,63-1,87; 1,54-2,02; M: 1,6-2,26; 1,73-1,99; (1320) F: 2; {F: 1,48-2,10; M: 1,48-2,26} | | | | Rem.: | epipelagic.
For Brady (1914, p.5) this species agree very accurately with the Giesbrecht's form called C. chierchiae from Gibraltar; in the Durban Bay, this species is the most abundant of all rge copepods.
After Schnack (1989) the Itoh's index value of the mandibular gnathobase = 624.
For Cruz & al. (2013) the abundance of this species has increased at the northern limits of its distribution in recent decades, mainly due to oceanic climate forcing, suggesting this as a key species in monitoring climate change. The present experiments confirm the sensitivity of C. chierchiae to temperature variations and may help in understanding the successful expansion of its distribution towards northern latitudes.
For Blanco-Bercial & al., 2014 (p.6), in light of both morphological and molecular data, there is significant doubt about the validity of the distrinction of C. chierchiae Giesbrecht, 1889 from C. typicus Krôyer, 1849.
After Benedetti & al. (2018, p.1, Fig.2) this species belonging to the functional group 4 corresponding to small filter feeding herbivorous and mixed feeding omnivorous (mostly broadcasters). | | | Last update : 25/10/2022 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed February 03, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |






















