|
|
 |
|
Calanoida ( Order ) |
|
|
|
Clausocalanoidea ( Superfamily ) |
|
|
|
Clausocalanidae ( Family ) |
|
|
|
Clausocalanus ( Genus ) |
|
|
| |
Clausocalanus furcatus (Brady, 1883) (F,M) | |
| | | | | | | Syn.: | Drepanopus furcatus Brady, 1883 (p.77, Descr.F, figs.F);
Clausocalanus pergens : Tanaka, 1960 (part., p.34, Pl.12 , fig.9) | | | | Ref.: | | | Giesbrecht, 1892 (p.186, 194, 771, figs.F,M); Giesbrecht & Schmeil, 1898 (p.27, Rem. F,M); Thompson & Scott, 1903 (p.233, 243); A. Scott, 1909 (p.32, Rem.); Wolfenden, 1911 (p.203); Esterly, 1911 b (p.223, figs.F,M); Sewell, 1914 a (p.215); Pesta, 1920 (p.506); Früchtl, 1924 b (p.44); Sars, 1925 (p.28); Sewell, 1929 (p.93); Farran, 1929 (p.208, 225); Rose, 1929 (p.18); Rose, 1933 a (p.82, figs.F,M); Dakin & Colefax, 1933 (p.205); Farran, 1936 a (p.81); Mori, 1937 (1964) (p.35, figs.F); Dakin & Colefax, 1940 (p.97, figs.F,M); Wilson, 1942 a (p.178, figs.F,M); Vervoort, 1946 (p.144, Rem.); Sewell, 1947 (p.55, Rem.); Brodsky, 1950 (1967) (p.119, fig.M, Rem.F,M)); Sewell, 1951 (p.352, figs.F,M, Rem.: parasites); Carvalho, 1952 a (p.144, figs.F); Tanaka, 1956 c (p.383); Chiba & al., 1957 (p.306); 1957 a (p.11); Tanaka, 1960 (p.31); Heinrich, 1961 b (p.93); Fagetti, 1962 (p.16); Grice, 1962 (p.189, figs.F); Brodsky, 1962 c (p.116, figs.F); Björnberg, 1963 (p.31); ; Vervoort, 1963 b (p.118, Rem.); Paiva, 1963 (p.28, figs.F); Vilela, 1965 (p.6, figs.F); Gonzalez & Bowman, 1965 (p.246, figs.F, Rem.); Chen & Zhang, 1965 (p.48, figs.F,M); Owre & Foyo, 1967 (p.41, figs.F,M); Vidal, 1968 (p.27, figs.F,M); Vilela, 1968 (p.16); Frost & Fleminger, 1968 (p.76, figs.F,M, Rem.); Corral Estrada, 1970 (p.143); Björnberg, 1972 (p.21, figs., Rem.N); Kos, 1972 (Vol. I, figs.F,M, Rem.); Razouls, 1972 (p.94, Annexe: p.36, figs.F); Marques, 1973 (p.238); Chen & Zhang, 1974 (p.103); Marques, 1974 (p.14); Williams & Wallace, 1975 (p.177, fig.F, Rem.); Dawson & Knatz, 1980 (p.7, figs.F,M); Alvarez-Marques, 1981 (p.157, figs.F, Rem.); Björnberg al., 1981 (p.628, figs.F,M, Rem.); Marques, 1982 (p.757); Sazhina, 1982 (p.1156, fig., Rem.N); Brodsky & al., 1983 (p.232, figs.F,M, Rem.); Ferrari, 1984 (p.177, Rem: asymétrie); Sazhina, 1985 (p.45, figs.N); Lin & Nakamura, 1993 (p.449); Bradford-Grieve, 1994 (p.113, figs.F,M, fig.101); Chihara & Murano, 1997 (p.777, Pl.89,92: F,M); Bradford-Grieve & al., 1999 (p.878, 915, figs.F,M); Bucklin & al., 2003 (p.335, tab.2, fig.1, Biomol); Conway & al., 2003 (p.182, figs.F,M, Rem.); Mulyadi, 2004 (p.183, figs.F,M, Rem.); Avancini & al., 2006 (p.76, Pl. 45, figs.F,M, Rem.); Vives & Shmeleva, 2007 (p.616, figs.F,M, Rem.); Phukham, 2008 (p.129, figs.F); Uttieri & al., 2008 (p.535, sensory structure of A1). | 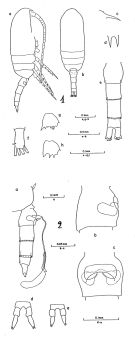 issued from : B. Frost & A. Fleminger in Bull. Scripps Inst. Oceanogr. Univ. California, San Diego, 1968, 12. [Pl.64, p.228-229; Pl.65, p.230-231]. Female: 1 a, habitus (right lateral view); 1 b, idem (dorsal view); 1 c, rostrum (right lateral); 1 d, rostrum (anteroventral); 1 e, urosome (dorsal); 1 f, right caudal ramus (dorsal); 1 g, B2 of P2; 1 h, B2 of P3; 1 a,d,f taken from same specimen; 1 b from another; 1 c from a third; 1 e from a fourth; 1 g-h from a fifth; 2 a, Th.4-5 (posterior part) and urosome with spermatophore attached (right lateral); 2 b, genital segment (right lateral); 2 c, genital segment (ventral view); 2 d-e, P5; 2 a taken from one specimen; 2 b-c from another; 2 d-e from a third specimen. Nota: Genital segment length about equal to urosomal segment 3 length.
|
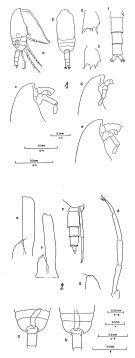 issued from : B. Frost. & A. Fleminger in Bull. Scripps Inst. Oceanogr. Univ. California, San Diego, 1968, 12. [Pl.66, p.232-233; Pl.67, p.234-235]. Male: 1 a, habitus (right lateral view); 1 b, idem (dorsal view); 1 c-e, frontal region (right lateral); 1 f, urosome (armature of caudal rami incomplete) (dorsal view); 1 g, B2 of P2; 1 h, B2 of P3; 1 a-e from different specimens; 1f-h from a sixth specimen; 2 a-b, middle region of body (posterior part of Th.2 to anterior part of urosome3) (dorsal view) (specimen a with longer ramus of P5 and genital pore on right side; specimen b with longer ramus of P5 and genital pore on left side); 2 c, Th.4-5 (posterior part) and uerosome (right lateral); 2 d, P5 (left posterolateral); 2 e-f, left 1P5 and right P5 (anterior view); 2 g, right P5; 2 a-c taken from different specimens; 2 d and g from a fourth; 2e and f from fifth and sixth specimens, respectively. Nota: Rostrum in lateral view not knoblike and not protruding ventrally. Longer ramus of P5 usually on right side.
|
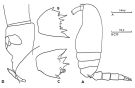 issued from : C. Razouls in Thèse Doc. ès Sciences, Univ. P & M. Curie, Paris , Tome annexe, 1972, Fig.38 (unpublished). Female (from Banyuls): A, habitus (lateral left side); B, basipod 2 of P2; C, basipod 2 of P3; D, genital somite and P5 (lateral right side).
|
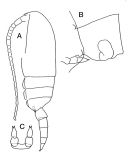 issued from : J.M. Bradford-Grieve in The Marine Fauna of New Zealand: Pelagic Calanoid Copepoda. National Institute of Water and Atmospheric Research (NIWA). New Zealand Oceanographic Institute Memoir, 102, 1994. [p.113, Fig.63]. Female: A, habitus (lateral left side); B, genital segment (lateral); C, P5.
|
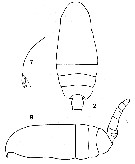 issued from : C.O. Esterly in Proc. Am. Acad. Arts Sci., 1911, 47 (7). [Pl.1, Figs.2, 7, 9]. Female (from Bermuda Is.): 2, cephalothorax and part of abdomen; 7, forehead (lateral); 9 habitus (lateral).
|
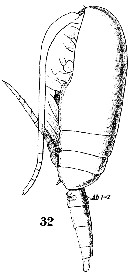
 issued from : C.O. Esterly in Proc. Am. Acad. Arts Sci., 1911, 47 (7). [Pl.2, Fig.11]. Male: 11, part of last thoracic segment and urosome (on left side; dorsal).
|
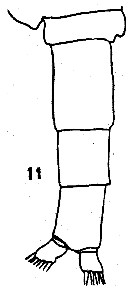
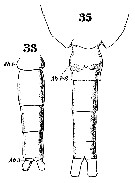
 issued from : C.O. Esterly in Proc. Am. Acad. Arts Sci., 1911, 47 (7). [Pl.3, Fig.33]. Female: basipodite 2, 1st and 2nd segments of exopod, and endopod of P3.
|
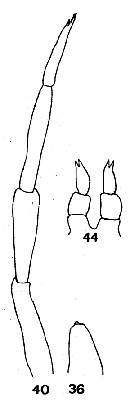 issued from : C.O. Esterly in Proc. Am. Acad. Arts Sci., 1911, 47 (7). [Pl.4, Figs.36, 40, 44]. Female: 44, P5. Male: 36, right P5; 40, left P5.
|
 issued from : B. Frost & A. Fleminger in Bull. Scripps Inst. Oceanogr. Univ. California, 1968, 12. [p.78, Table 4a]. Clausocalanus furcatus Females: Measurements and ratios . TL = total body length ; SL = spermatophore length ; P :U = ratio of prosome length to urosome length ; U :U1 = ratio of total urosome length to length of 1st urosomal segment (genital segment) ; S :U1 = ratio of spermatophore sac length to U1 length of female on which spermatophore is attached ; r = sample range; m = sample mean; n = number of specimens measured; s = sample standard deviation.
|
 issued from : B. Frost & A. Fleminger in Bull. Scripps Inst. Oceanogr. Univ. California, 1968, 12. [p.79, Table 4b]. Clausocalanus furcatus Males: Measurements and ratios . TL = total body length; P :U = ratio of prosome length to urosome length ; P:U2 = ratio of prosome length to length od 2nd urosomal segment; U2:2P5 = ratio of U2 length to length of 2nd segment of longer ramus of P5; U2:3P5 = ratio of U2 length of 3rd segment of longer ramus of P5; 3P5:2P5 = ratio of length of 3P5 of longer ramus to length of 2P5 of longer ramus; r = sample range; m = sample mean; n = number of specimens measured; s = sample standard deviation.
|
 issued from : B. Frost & A. Fleminger in Bull. Scripps Inst. Oceanogr. Univ. California, 1968, 12. [p.235, Pl.67, c, d, e-f, g]. Male: c, posterior part of last thoracic segment and urosome (right lateral); d, P5 (left posterolateral); e-f, 1st segment of left P5 and right P5 (anterior); g, right P5.
|
 issued from : C. Razouls in Th. Doc. Etat Fac. Sc. Paris VI, 1972, Annexe. [Fig.38]. Female (from Banyuls, G. of Lion): A, habitus (lateral); B, basipod 2 of P2; C, basipod 2 of P3; D, P5 and genital segment (lateral).
|
 Issued from : G.S. Brady in Rep. Scient. Results Voy. Challenger, Zool., 1883, 8 (23). [Pl.XXIV, Figs.12-15]. Female: 12, P2; 13, P4; 14, termoinal spines of swimming legs; 15, P5.
|
 Issued from : G.S. Brady in Rep. Scient. Results Voy. Challenger, Zool., 1883, 8 (23). [Pl. IV, Figs.1, 2]. As Drepanopus furcatus. Female: 1, habitus (lateral); 2, A1.
|
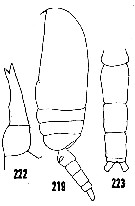 issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1, Crustacea, 1: Copepoda. Copepods of the Florida Current. [p.41, Figs.219, 222, 223]. Female: 219, habitus (lateral); 222, P5. Male: 223, urosome (dorsal).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf. 36, Fig.32]. Female: 32, habitus (lateral).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf. 36, Figs.33, 35]. Female: 35, urosome (ventral). Male: 33, urosome. 33 and 35 at the same scale.
|
 issued from : N. Phukham in Species diversity of calanoid copepods in Thai waters, Andaman Sea (Master of Science, Univ. Bangkok). 2008. [p.210, Fig.84]. Female (from W Malay Peninsula): a-b, habitus (doirsal and lateral, respectively); c, urosome; d, P5. Body length after drawing (a): F = 0.969 mm.
|
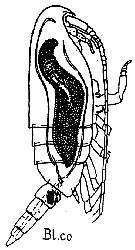 issued from : G. Trégouboff & M. Rose in Manuel de planctonologie méditerranéenne, 1957, CNRS, Paris. [Pl. 125]. Clausocalanus furcatus female (from Mediterranean Sea). Blastodinium contortum (Bl.co) parasit in the copepod's gut.
|
 issued from : A. Ianora, B. Scotto di Carlo, M.G. Mazzocchi & P. Mascellaro in J. Plankton Res., 1990, 12 (2). [p.251, Fig.1, C-D]. Clausocalanus furcatus (from Gulf of Naples, Italy) parasitized by Syndinium sp. (C) and the discharge of mature dinospores from an infested copepod. Scale bar = 0.100 mm. Nota: The most devastating form of infestion in coastal copepods is due to coelomic parasites such as the dinoflagellate Syndinium. This desease always induced gross changes in the external morphology of the host and affected its reproductive capacity. Infected specimens were dark and dilated in appearance, and often showed intersex characters which consisted in the development of an asymmetrical fifth pair of legs resembling the male condition (see Ianora & al., 1987).
|
 issued from : A. Ianora, B. Scotto di Carlo, M.G. Mazzocchi & P. Mascellaro in J. Plankton Res., 1990, 12 (2). [p.256, Fig.8]. Longitudinal section (A) of Clausocalanus furcatus (from Gulf of Naples) infested by bacteria cells (bc) and B close-up of previous section (oc = oocytes). TEM micrograph of (C) bacteria cells. Scale bar = 0.100 mm.
|
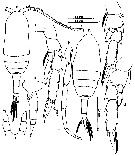 issued from : Mulyadi in Published by Res. Center Biol., Indonesia Inst. Sci. Bogor, 2004. [p.104, Fig.38]. Female (from 03°40'S, 128°10'E): a, habitus (dorsal); b, last thoracic segment and urosome (lateral left side); c, P2; d, P3; e, P4; f, P5. Male: g, habitus (dorsal); h, P5.
|
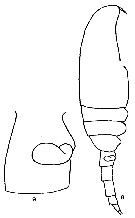 Issued from F. Alvarez-Marques in Rev. Fac. Cienc. Univ. Oviedo (Ser. Biologia), (1979-80), 20-21, 1981. [p.165, Pl. II, Figs.8-9]. Female (from Gijon, NW Spain): 8, habitus (lateral); 9, genital segment and spermatheca (lateral).
|
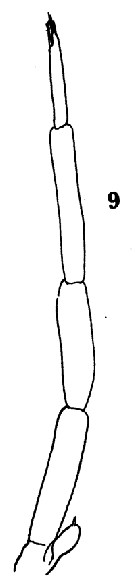 Issued from : O. Tanaka in Spec. Publs. Seto mar. biol. Lab., 10, 1960 [Pl. XII, 9]. As Clausocalanus pergens. Male (from Indian Ocean): 9, P5.
|
 Issued from : C. Alves-da-Souza, C. Cornet, A. Nowaczyk, S. Gasparini, A. Skovgaard & L. Guillou inBiogeosciences, 2011, 8. [p.2130, Fig.2 E]. Observation of Blastodinium sp. located inside the gut of their host: Clausocalanus furcatus.
|
 Issued from : E. Chatton in Arch. Zool. Exp. & Gen., 1920, 59, [Pl. IV, 37]. Clausocalanus furcatus female , with one individual of Blastodinium contortum var. hyalinum (Dinoflagellate), which distends intestinal stomach, appearing to show all general cavity filled in.
t.d: gut; Bl. h: Blastodinium hyalinum
|
 Issued from : E. Chatton in Arch. Zool. Exp. & Gen., 1920, 59, [Pl. IV, 38]. Clausocalanus furcatus female (from Banyuls-sur-Mer), with in the stomach about twenty individuals of Blastodinium spinulosum (Dinoflagellate).
|
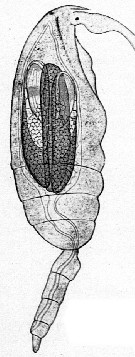 Issued from : E. Chatton in Arch. Zool. Exp. & Gen., 1920, 59, [p.150, Fig.1]. Clausocalanus furcatus female parasited by Blastodinium pruvoti in the gut.
|
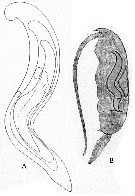 Issued from : E. Chatton in Arch. Zool. Exp. & Gen., 1920, 59, [p.189, Fig.93 & 94]. Clausocalanus furcatus female (from Banyuls) parasited by Blastodinium contortum (Dinoflagellate): A, triblastic slender form; B, super-twisted form pigmented of yellow-green colour inside the copepod's gut.
|
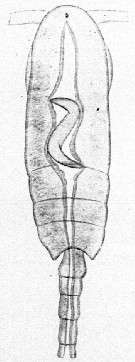 Issued from : E. Chatton in Arch. Zool. Exp. & Gen., 1920, 59, [p.176, Fig.79]. Clausocalanus furcatus female (from Banyuls) parasited by Blastodinium contortum inside the copepod's gut.
|
 Issued from : M. Uttieri, E.R. Brown, G.A. Boxshall & M. G. Mazzocchi in J. Mar. Biol. Ass. U.K., 2008, 88 (3). [p.537, Fig.1]. Alignment of setae and aesthetascs along the margins of the A1 of Clausocalanus furcatus female (from the Gulf of Naples). Camera lucida drawning shows the setation pattern and the arrangement of aesthetascs (cylindrical-like structure) and modified setae in black) along the segments.
|
 ssued from : M. Uttieri, E.R. Brown, G.A. Boxshall & M. G. Mazzocchi in J. Mar. Biol. Ass. U.K., 2008, 88 (3). [p.536, Table 1]. The distribution of sensory structures along the antennules of Clausocalanus furcatus female obtained by comparative analysis of camera lucida drawings, scanning electron microscopy micrographs and laser scanning confocal microscopy reconstructions. Nota: The A1 had aesthetascs and 3 different kinds of setae: simple, serrulate and modified. All sensory structures were aligned along the margin of the A1; only segments 22, 23 and compound segments 24-25 had 1 seta each on the posterior margin. Following the scheme applied by Lenz & Yen (1993), the A1 could be divided into 3 sectors: the basal sector (segments 1 to 8-9), where setae were more closely spaced; the median sector (segments 10 to 18), where setae became sparse; the distal sector (segments 19 to 24-25) bearing a reduced number of setae. A1 also carried single aesthetascs on all segments except 1, 20, 21 and 23; on segments 3, 7, 14 and 16 aesthetascs were arranged as part of a trithek (the basic antennulary armature unit comprising 2 setae and 1 aesthetasc) typical of female copepods (Boxshall, 1983). All tritheks were arranged as described by Giesbrecht (see in Boxshall, 1983) with 1 seta almost in the middle of the segment, and another seta close to the aesthetasc on the distal part. When modified setae were present on a segment, it was always the seta closer to the aesthetasc that was modified. The sensory arrangement on the distal tip comprised: 2 serrulate setae and 1 aesthetasc on the anterior margin, 1 seta on the posterior edge and a tuft of 3 outwardly projecting setae sharing a common origin. Simple setae were pure mechanoreceptors, with a smooth external surface and a tapered tip. They originated from a symmetrical circular (about 4 µm in diameter) socket which, coupled with the lack of any one-point innervation at the base of the sensor, provided indirect evidence of the absence of directional sensitivity (see in Bush & Laverack, 1982). The simple setae had the typical characteristics of the copepod mechanosensor (i.e a thick cuticle enveloping a large proliferation of microtubules). Segment 1 and compound 24-25 carried 2 setae ornamented with short setules (1-2 µm) (serrulate setae) each. In addition , segment 1 had a pore between 2 neighbouring setae. Another pore was sometimes visible close to the socket of the seta on the anterior margin of segment 23, but this character was not discernible in all individuals investigatede. The ultrastructure of serrulate setae was similar to that of simple setae (thick cuticle, presence of microtubules). In addition, small setules did not have any sort of innervation, indicating that they were just chitinous extensions of the mechanosensor. Modified setae were presumed to be chemosensory or dual-function sensors. They were characterized by a smooth texture, as a simple setae, whilst the median and distal part of the sensor possessed a spongy-like structure typical of copepod aesthetascs. Modified setae were attached to the A1 via a socket larger (5-7 µm) than that of simple setae. Their base was characterized by a clearly recognizable neck constriction, and the tip lacked any apical pore. The internal structure of modified setae possessed characters typical of simple setae: the cuticle was rather thick and consisted of consecutive layers of chitin, while the internal cavity was pazrtly filled with a relatively large number of microtubules. The aesthetascs had the characteristic bulbous shape with a spongy external texture. The root of the sensor protruded directly from the chitinous surface of the A1, the base having a diameter of nearly 2 µm. Unlike the same receptors found in other species (see Kurbjeweit & Buchholz, 1991; Bundy & Paffenhöfer, 1993), the aesthetascs of C. furcatus did not show any neck constriction.The tip lacked a pore. The base of the aesthetasc on segment 2 was always accompanied by a pore; this association was occasionally noted also for other aesthetascs (segments 14, 16, 19, and 24-25), but did not represent a common feature in all specimens analysed. All pores reported for the A1 of C. furcatus were circular shaped, as described for other calanoids (see Blades & Youngbluth, 1979; Gill, 1986). From an ultrastructural point of view, the aesthetascs of C. furcatus were characterized by the lack of a cluster of microtubules and a very thin and apparently perforated cuticle, through which chemical odorants might permeate, coming into contact with the nervous structures of the sensor. The dark circular profiles evident in the TEM section might be the ends of forked dendrites whose presence further supports the chemosensory function of this structure (see lenz & al., 1996). Compiled confocal images showed brightly fluorescent aesthetascs and weakly non-uniformly fluorescent modified setae, while simple setae did not fluorescence at all. Aesthetascs fluoresced as a consequence of the absorption of DiI (sensillae were stained by a fluorescent carbocyanine dye, which penetrates into the lipid bilayer of neuronal cell membranes; see Honig & Hume, 1986; Carotenuto, 1999) through their spongy cuticle; the assimilation of the dye occurred somewhat uniformly along the entire length of the sensor. Simple setae did not fluoresce at all indicating that their thick chitinous cuticle was almost impermeable to DiI. Modified setae showed spots of fluorescence localized on the median/distal part, corresponding to the spongy texture noted using SEM. By contrast, no fluorescence was noticed at the base of the modified seta, where the cuticle was thick.
|
 Issued from : M.S. Kos in Field guide for plankton. Zool Institute USSR Acad., Vol. I, 1972. After Brodsky, 1962. Female: 1-2, habitus (dorsal and lateral, respectively); 3, urosome (ventral); 4, genital segment (ventral); 5, basis of P2; 6, basis of P3; 7, P5.
| | | | | Compl. Ref.: | | | Cleve, 1904 a (p.188); Chatton, 1920 (p.17, parasited); Rose, 1925 (p.152); Hardy & Gunther, 1935 (1936) (p.149, Rem.); Massuti Alzamora, 1942 (p.89); Sewell, 1948 (p.323, 357, 391, 395, 414, 422, 433, 439, 442, 450, 453, 455, 456, 460, 469, 513); C.B. Wilson, 1950 (p.190); Cervigon, 1962 (p.181, tables: abundance distribution); Gaudy, 1962 (p.93, Rem.: p.104); Grice & Hart, 1962 (p.287, table 3, 4: abundance); Duran, 1963 (p.15); V.N. Greze, 1963 a (tabl.2); Shmeleva, 1963 (p.141); Gaudy, 1963 (p.21, Rem.); Ahlstrom & Thrailkill, 1963 (p.57, Table 5, abundance); Björnberg, 1963 (p.34, Rem.); De Decker, 1964 (p.15, 20, 30); De Decker & Mombeck, 1964 (p.12); Grice & Hulsemann, 1965 (p.223); Shmeleva, 1965 b (p.1350, lengths-volume-weight relation); Furuhashi, 1966 a (p.295, vertical distribution in Kuroshio region, Table 10); Neto & Paiva, 1966 (p.22, Table III, annual cycle); Mazza, 1966 (p.70); Pavlova, 1966 (p.43); Grice & Hulsemann, 1967 (p.14); Fleminger, 1967 a (tabl.1); De Decker, 1968 (p.45); Evans, 1968 (p.12); Delalo, 1968 (p.137); Berdugo & Kimor, 1968 (p.447); Vinogradov, 1968 (1970) (p.78, 268); Champalbert, 1969 a (p.635); Dowidar & El-Maghraby, 1970 (p.267); Park, 1970 (p.475); Gamulin, 1971 (p.381, tab.2); Deevey, 1971 (p.224); Timonin, 1971 (p.281, trophic group); Bainbridge, 1972 (p.61, Appendix Table I: vertical distribution vs day/night, Table II: %, Table IV); Apostolopoulou, 1972 (p.327, 343, fig.3); Gaudy, 1972 (p.175, 231, figs.33-35, annual cycle); Valentin, 1972 (p.377, egg diameter = 80 µm); Björnberg, 1973 (p.314, 385); Guglielmo, 1973 (p.399); Corral Estrada & Pereiro Muñoz, 1974 (tab.I); de Bovée, 1974 (p.109, 124); Timonin, 1976 (p.79, vertical distribution); Peterson & Miller, 1976 (p.14, Table 1, abundance vs interannual variations); Timonin & Voronina, 1977 (p.287, fig.6); Peterson & Miller, 1977 (p.717, Table 1, seasonal occurrence); Carter, 1977 (1978) (p.35); Deevey & Brooks, 1977 (p.256, tab.2, Station "S"); Comaschi Scaramuzza, 1978 (p.17); Binet, 1979 (p.400); Star & Mullin, 1981 (p.1322, abundance); Kovalev & Schmeleva, 1982 (p.83); Vives, 1982 (p.290); Smith S.L., 1982 (p.1331, abundance, monsoon effect); Turner & Dagg, 1983 (p.16); Greze & al., 1983 (p.17, Rem.: p.24); Tremblay & Anderson, 1984 (p.6: Rem.); De Decker, 1984 (p.315, 342: chart); Guangshan & Honglin, 1984 (p.118, tab.); Scotto di Carlo & al., 1984 (p.1041); Binet, 1984 (tab.3); 1985 (p.85, tab.3); Brenning, 1985 a (p.23, Table 2); Sazhina, 1985 a (p.491, tab.1); Petipa & Borichenko, 1985 (tab.1); Longhurst, 1985 (tab.2); Moraitou-Apostolopoulou, 1985 (p.303, occurrence/abundance in E Mediterranean Sea); Regner, 1985 (p.11, Rem.: p.29); Jansa, 1985 (p.108, Tabl.V); Almeida Prado Por, 1985 (p.250); Brinton & al., 1986 (p.228, Table 1); Madhupratap & Haridas, 1986 (p.105, tab.1); Renon, 1987 (tab.2); Comaschi Scaramuzza, 1987 (tab.1); Jimenez-Perez & Lara-Lara, 1988; Wiebe & al., 1988 (tab.7); Lozano Soldevilla & al., 1988 (p.58); Herman, 1989 (p.247); Suarez & al., 1990 (tab.2); Echelman & Fishelson, 1990 a (tab.2); Othman & al., 1990 (p.561, 563, Table 1); Hirakawa & al., 1990 (tab.3); Ianora & al., 1990 (p.249, figs., parsitism effects); Yoo, 1991 (tab.1); Hattori, 1991 (tab.1, Appendix); Hirakawa, 1991 (p.376: fig.2); Herman, 1992 (p.395, fig.6 a, size distribution by OPC); Suarez, 1992 (App.1); Seguin & al., 1993 (p.23, Table 2: abundance, %); Verheye & al., 1994 (p.155); Hirakawa & al., 1995 (tab.2); Hajderi, 1995 (p.542); Shih & Young, 1995 (p.72); Roff & al., 1995 (p.165, Table 1; nauplii development times, growth rates); Webber & Roff, 1995 a (p.481, Rem.: p.483: egg production time); Webber & al., 1996 (tab.1); Kotani & al., 1996 (tab.2); Jamet & Ferec-Corbel, 1996 (p.17, tab.1); Go & al., 1997 (tab.1); Suarez-Morales & Gasca, 1997 (p.1525); Park & Choi, 1997 (Appendix); Hure & Krsinic, 1998 (p.100); Alvarez-Cadena & al., 1998 (tab.1, 2, 3, 4); Lopes & al., 1998 (p.195); Hsieh & Chiu, 1998 (tab.2); Suarez-Morales & Gasca, 1998 a (p109); Mauchline, 1998 (tab.30, 58); Noda & al., 1998 (p.55, Table 3, occurrence); Lavaniegos & Gonzalez-Navarro, 1999 (p.239, Appx.1); Lopes & al., 1999 (p.215, tab.1); Neumann-Leitao & al., 1999 (p.153, tab.2); Onishchik, 1999 (p.76); Siokou-Frangou & al., 1999 (p.205, Table 5); Harvey & al., 1999 (p.1, 49: Appendix 5, in ballast water vessel); El-Sherif & Aboul Ezz, 2000 (p.61, Table 3: occurrence); Seridji & Hafferssas, 2000 (tab.1); Fernandez-Alamo & al., 2000 (p.1139, Appendix); Suarez-Morales & Gasca, 2000 (1247, tab.1); Lopez-Salgado & al., 2000 (tab.1); Moraitou-Apostolopoulou & al., 2000 (tab.I, fig.6); Madhupratap & al., 2001 (figs.4, 5); Fragopoulu & al., 2001 (p.49, tab.1); Lo & al., 2001 (1139, tab.I); Bressan & Moro, 2002 (tab.2); Zerouali & Melhaoui, 2002 (p.91, Tableau I); Sameoto & al., 2002 (p.12); Fernandez de Puelles & al., 2003 (p.123, fig.5); Hwang & al., 2003 (p.193, tab.2); Vukanic, 2003 (139, tab.1); Hsiao & al., 2004 (p.325, tab.1); Hsieh & al. 2004 (p.397, tab. 1, p.399, tab.2); Rezai & al., 2004 (p.489, tab.2); Lo & al.*, 2004 (p.218, fig.6); Lo & al., 2004 (p.468, tab.2); Lo & al., 2004 (p.89, tab.1); Wang & Zuo, 2004 (p.1, Table 2, dominance, origin); Daly Yahia & al., 2004 (p.366, fig.4); Lan & al., 2004 (p.332, tab.1, tab.2); Peralba & Mazzocchi, 2004 (p.645, figs.3,6); Vukanic & Vukanic, 2004 (p.9, tab. 2); Fernandez de Puelles & al., 2004 (p.654, fig.7); Kazmi, 2004 (p.229); Dias & Bonecker, 2005 (p.100 + poster); Licandro & al., 2005 (p.153); Lakkis & al., 2005 (p.152); Choi & al., 2005 (p.710: Tab.III); Ashjian & al., 2005 (p.1380: tab.2); Wiggert & al., 2005 (p.1013: feeding behavior); Smith & Madhupratap, 2005 (p.214, tab.4); Prusova & Smith, 2005 (p.78); Obuid Allah & al., 2005 (p.123, occurrence % vs metal contamination); Zuo & al., 2006 (p.159, tab.1, abundance, fig.8: stations group); Isari & al., 2006 (p.241, tab.II); Hwang & al., 2006 (p.943, tabl. I); Sterza & Fernades, 2006 (p.95, Table 1, occurrence); Dias & Araujo, 2006 (p.40, Rem., chart); Bi & Benfield, 2006 (p.1199, egg production rates, stage-specific development times); Mageed, 2006 (p.171, Table 4); Zervoudaki & al., 2006 (p.149, Table I); Hooff & Peterson 2006 (p.2610); Cornils & al., 2007 (p.1261, feeding); Cornils & al., 2007 (p.57, Table I, fig.2, Rem: reproduction; abnormality); Hwang & al., 2007 (p.24); Dur & al., 2007 (p.197, Table IV); Fernandez de Puelles & al., 2007 (p.338, fig.7); Khelifi-Touhami & al., 2007 (p.327, Table 1); Valdés & al., 2007 (p.103: tab.1); Jitlang & al., 2008 (p.65, Table 1); Lan Y.C. & al., 2008 (p.61, Table 1, % vs stations); Tseng L.-C. & al., 2008 (p.153, Table 2, fig.5, occurrence vs geographic distribution, indicator species); McKinnon & al., 2008 (p.844: Tab.1); Ayon & al., 2008 (p.238, Table 4: Peruvian samples); Neumann-Leitao & al., 2008 (p.799: Tab.II, fig.6); Uttieri & al., 2008 (p.925, Rem.: Behaviour); Morales-Ramirez & Suarez-Morales, 2008 (p.513, 519); Fernandes, 2008 (p.465, Tabl.2); Wishner & al., 2008 (p.163, Table 2, fig.8, oxycline); Tseng & al., 2008 (p.402, Table 2); Rossi, 2008 (p.90: Tableau XII); Pagano, 2009 (p.116); C.-Y. Lee & al., 2009 (p.151, Tab.2); Galbraith, 2009 (pers. comm.); Chiba & al., 2009 (p.1846, Table 1, occurrence vs temperature change); Miyashita & al., 2009 (p.815, Tabl.II); Skovgaard & Salomonsen, 2009 (p.425, Table 2); Zhang W & al., 2009 (p.261, table 2); Lan Y.-C. & al., 2009 (p.1, Table 2, % vs hydrogaphic conditions); Brugnano & al., 2010 (p.312, Table 2, 3, fig.8); Hafferssas & Seridji, 2010 (p.353, Table 2, 3); Lidvanov & al., 2010 (p.356, Table 3); Hernandez-Trujillo & al., 2010 (p.913, Table 2); Dias & al., 2010 (p.230, Table 1); Cornils & al., 2010 (p.2076, Table 3); Sun & al., 2010 (p.1006, Table 2); Schnack-Schiel & al., 2010 (p.2064, Table 2: E Atlantic subtropical/tropical, Fig.4, 6, 7, Tabe 4) ; Mazzocchi & Di Capua, 2010 (p.425); Fazeli & al., 2010 (p.153, Table 1); Medellin-Mora & Navas S., 2010 (p.265, Tab. 2); W.-B. Chang & al., 2010 (p.735, Table 2, 3, 4, fig.5, abundance); Hafferssas & al., 2010 (p.1281, Table III, abundance vs spatial distribution); Hsiao S.H. & al., 2011 (p.475, Appendix I); Maiphae & Sa-ardrit, 2011 (p.641, Table 2); Alves-da-Souza & al., 2011 (p.2125, Blastodinium infestation); Magris & al., 2011 (p.260, abundance, interannual variability); Moscatello & al., 2011 (p.80, Table 4); Tseng L.-C. & al., 2011 (p.239, Table 3, abundance %); Andersen N.G. & al., 2011 (p.71, Fig.3: abundance); Tseng L.-C. & al., 2011 (p.47, Table 2, occurrences vs mesh sizes); Kâ & Hwang, 2011 (p.155, Table 3: occurrence %); Hsiao & al., 2011 (p.317, Table 2, indicator of seasonal change);Selifonova, 2011 a (p.77, Table 1, alien species in Black Sea); Mazzocchi & al., 2011 (p.1163, Table II, fig.6, long-term time-series 1984-2006); Isari & al., 2011 (p.51, Table 2, abundance vs distribution); Bi & al., 2011 (p.145, abundance, mortality rates); Mazzocchi & al., 2012 (p.135, annual abundance 1984-2006); Johan & al., 2012 (2013) (p.1, Table 1); Glushko & Lidvanov, 2012 (p.138, Tableau 1); Jang M.-C & al., 2012 (p.37, abundance and seasonal distribution); Uysal & Shmeleva, 2012 (p.909, Table I); Vidjak & al., 2012 (p.243, Rem.: p.254); Tseng & al., 2012 (p.621, Table 3: abundance); Lavaniegos & al., 2012 (p. 11, Appendix); Dorgham & al., 2012 (p.473, Table 3: abundance %); Naz & al., 2012 (p.61, Table 4 as Calusocalanus furcatus, relative abundance); Takahashi M. & al., 2012 (p.393, Table 2, water type index); Gubanova & al., 2013 (in press, p.4, Table 2); Belmonte & al., 2013 (p.222, Table 2, abundance vs stations); in CalCOFI regional list (MDO, Nov. 2013; M. Ohman, comm. pers.); Palomares-Garcia & al., 2013 (p.1009, Table I, fig. 7, abundance vs environmental factors); Tachibana & al., 2013 (p.545, Table 1, seasonal change 2006-2008; Tseng & al., 2013 (p.507, seasonal abundance); Tseng & al., 2013 a (p.1, Table 3, abundance); Hwang & al., 2014 (p.43, Appendix A: seasonal abundance); Bianco & al., 2013 (p.1, swimming behavior); Terbiyik Kurt & Polat, 2013 (p.1163, Table 2, fig. 9, seasonal distribution); Lidvanov & al., 2013 (p.290, Table 2, % composition); Fernandez de Puelles & al., 2014 (p.82, Table 3, seasonal abundance); Ortega & al., 2014 (p.495, Table I); Bonecker & a., 2014 (p.445, Table II: frequency, horizontal & vertical distributions, Rem.: p. 455, 459) ; Pansera & al., 2014 (p.221, Table 2, abundance); Mazzocchi & al., 2014 (p.64, Table 3, 4, 5, spatial & seasonal composition %); Zakaria, 2014 (p.3, Table 1, abundance vs. 1960-2000); Dias & al., 2015 (p.483, Table 2, abundance, biomass, production, Table 4: % vs. season, fig.8); Araujo & al., 2016 (p.1, Table 3, abundance, %); Zakaria & al., 2016 (p.1, Table 1); Benedetti & al., 2016 (p.159, Table I, fig.1, functional characters); Ben Ltaief & al., 2017 (p.1, Table III, Summer relative abundance) ; Ortega & al., 2017 (p.123, fig. 1, 6, table 2, abundance, %); Marques-Rojas & Zoppi de Roa, 2017 (p.495, Table 1); Ohtsuka & Nishida, 2017 (p.565, Table 22.1); Bonecker C.T. & al., 2017 (p.247, fig.7: abundance day/night vs. vertical types of mass water); El Arraj & al., 2017 (p.272, table 2, spatial distribution); Benedetti & al., 2018 (p.1, Fig.2: ecological functional group); Belmonte, 2018 (p.273, Table I: Italian zones); Chaouadi & Hafferssas, 2018 (p.913, Table II: occurrence); Dias & al., 2018 (p.1, Tables 2, 4, 5: vertical distribution, abundance vs. season); Palomares-Garcia & al., 2018 (p.178, fig.3: relative frequency, Table 1)
| | | | NZ: | 20 | | |
|
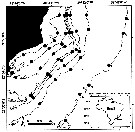
Distribution map of Clausocalanus furcatus by geographical zones
|
| | | | | | | | | | | | | | |  issued from : B. Frost & A. Fleminger in Bull. Scripps Inst. Oceanogr. Univ. California, San Diego, 1968, 12. [p.80, Chart 13, a]. issued from : B. Frost & A. Fleminger in Bull. Scripps Inst. Oceanogr. Univ. California, San Diego, 1968, 12. [p.80, Chart 13, a].
Occurrence of C. furcatus in samples examined; closed circles represent samples examined; open circles represent samples in which adults were found; bars through open circles represent samples from which specimens were removed for measurements. |
 issued from : B. Frost & A. Fleminger in Bull. Scripps Inst. Oceanogr. Univ. California, San Diego, 1968, 12. [p.81, Chart 13, b]. issued from : B. Frost & A. Fleminger in Bull. Scripps Inst. Oceanogr. Univ. California, San Diego, 1968, 12. [p.81, Chart 13, b].
Occurrence of C. furcatus in samples examined; closed circles represent samples examined; open circles represent samples in which adults were found; bars through open circles represent samples from which specimens were removed for measurements. |
 issued from : T.S.K. Björnberg in Bol. Inst. Oceanogr., Sao Paulo, 1963, XIII (1). [p.32, Fig.16]. issued from : T.S.K. Björnberg in Bol. Inst. Oceanogr., Sao Paulo, 1963, XIII (1). [p.32, Fig.16].
Probability of occurrence of Clausocalanus furcatus in different environments.
T: Tropical waters (above 36.00 p.1000 salinity and above 20°C temperature); SST: Surface subtropical waters (salinity around 36 p.1000 and temperature 18°C or less); DST: Deeper shelf waters (salinity between 34 and 36, temperature under 20°C p.1000); SS: Surface shelf waters (same salinity and temperature above 20°C);
C: coastal waters with low salinity and variable temperature.
In each column no shading means no probability of finding the species in the samples from this environment; horizontal shading indicates the probability of finding the sepecies in percentages less than one in samples from this environment; cross shading indicates the probability of finding it in percentages higher than one and black shading represents the probability of finding it in the largest percentages of the total number of copepods.
Nota: The histogram shows this species as a dominant copepod in Tropical Water of the Brazil Current and of the South Equatorial Current. It is usually the dominant species among the copepods of waters of more than 35.00 p.1000 salinity and 25°C temperature. The histogram shows it to be dominant also in waters of lower temperatures (under 25°C), but the number of samples in which it occurs in highest numbers diminishes with the temperature.l |
 issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1, Crustacea, 1: Copepoda. Copepods of the Florida Current. 1967. [p.42, Table 15]. issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1, Crustacea, 1: Copepoda. Copepods of the Florida Current. 1967. [p.42, Table 15].
Vertical distribution of Clausocalanus furcatus at the ''40-Mile station'' in the Florida Current (± 25°35'N, 79°27'W).
SL 53: 18 V 1958. A: during midday; B: during midnight. |
 issued from : A.A. Shmeleva in Bull. Inst. Oceanogr., Monaco, 1965, 65 (n°1351). [Table 6: 14 ]. Clausocalanus furcatus (from South Adriatic). issued from : A.A. Shmeleva in Bull. Inst. Oceanogr., Monaco, 1965, 65 (n°1351). [Table 6: 14 ]. Clausocalanus furcatus (from South Adriatic).
Dimensions, volume and Weight wet. Means for 50-60 specimens. Volume and weight calculated by geometrical method. Assumed that the specific gravity of the Copepod body is equal to 1, then the volume will correspond to the weight. |
 issued from : A. Cornils, B. Niehoff, C. Richter, T. Al-Najjar & B. Schnack-Schiel in J. Plankton Res., 2007, 29 (1). [p.60, Figs.1, 2]. issued from : A. Cornils, B. Niehoff, C. Richter, T. Al-Najjar & B. Schnack-Schiel in J. Plankton Res., 2007, 29 (1). [p.60, Figs.1, 2].
Above: Annual cycle of sea surface temperature and depth -integrated (0-100 m) chlorophyll a (mg/m2)
Below: Relative frequency (%) of the clausocalanid females over the investigation period (March 2002 to December 2003) in the northern Gulf of Aqaba (29°27'87 N, 34°57' 87 E; depth 300 m) for Clausocalanus furcatus, C. farrani, C. minor and Ctenocalanus vanus.
Nota: Collected by vertical hauls from 100 m depth to the surface. Sampling performed between 9 a.m. and 3 p.m. on a monthly basis. |
 issued from : A. Cornils, B. Niehoff, C. Richter, T. Al-Najjar & B. Schnack-Schiel in J. Plankton Res., 2007, 29 (1). [p.65, Fig.8]. issued from : A. Cornils, B. Niehoff, C. Richter, T. Al-Najjar & B. Schnack-Schiel in J. Plankton Res., 2007, 29 (1). [p.65, Fig.8].
Prosome length (mm) displayed with SE bars over the investigation period. |
 issued from : A. Cornils, B. Niehoff, C. Richter, T. Al-Najjar & B. Schnack-Schiel in J. Plankton Res., 2007, 29 (1). [p.65, Table III]. issued from : A. Cornils, B. Niehoff, C. Richter, T. Al-Najjar & B. Schnack-Schiel in J. Plankton Res., 2007, 29 (1). [p.65, Table III].
Percentage of clausocalanid females infected with Blastodinium sp. (dinoflagellate) or showing abnormalities of the P5 (%), found in the preserved females. |
 issued from : R. Gaudy in Tethys, 1972, 4 (1). [p.237, Fig.36]. issued from : R. Gaudy in Tethys, 1972, 4 (1). [p.237, Fig.36].
Schematic quantitative abundance of Clausocalanus furcatus in the Gulf of Marseille (Mediterranean Sea) established from samples during the period between April 1962 to December 1967.
Nota: Gaudy (p.233) points to 5 generations per year. |
 Issued from : H. Bi, K.A. Rose & M.C. Benfield in Mar. Ecol. Prog. Ser., 2011, 427. [p.157, Table 5]. Issued from : H. Bi, K.A. Rose & M.C. Benfield in Mar. Ecol. Prog. Ser., 2011, 427. [p.157, Table 5].
Instantaneous mortality rates (individuals by day) .
N = nauplii; C = copepodite; AD = adult.
Nota: Field sampling: 28°37' N, 90°15' W. Zooplankton collected in 2003 at 12 h intervals from 18 March to 6 April and then from 15 May to 9 June using a 30 l Niskin bottle deployed at deoths of 5, 15 and 25 m..
To identify eggs and other stages for C. furcatus were conducted series of incubation experiments, used as reference specimens.
All stage abundances were reported as number of individuals per cubic meter.
The estimated mortality rates for the field data showed maximum values for the early and late stages and were similar between March-April and May-June periods. |
 Issued from : A.C.T. Bonecker, A. V. de Araujo, C.O. Dias, M.M.S. Castro, P.F. Carvalho, R.M. Lopes & S.C.L. Bonecker in A.P.C. Falcao & D.L. Moreira (ed.) Ambiente pelagico caracterizaçao ambiental regional de Bacia de Campos, Atlantico Sudoeste. Rio de Janeiro. Elsevier. 2017, v.5, p.247-281. [p.259, Fig.7] Issued from : A.C.T. Bonecker, A. V. de Araujo, C.O. Dias, M.M.S. Castro, P.F. Carvalho, R.M. Lopes & S.C.L. Bonecker in A.P.C. Falcao & D.L. Moreira (ed.) Ambiente pelagico caracterizaçao ambiental regional de Bacia de Campos, Atlantico Sudoeste. Rio de Janeiro. Elsevier. 2017, v.5, p.247-281. [p.259, Fig.7]
Abundance (ind. m-3) sampled during night and day into four water masses in the Campos Basin (Brazil).
AT: Tropical water; ACAS: Central water of South Atlantic; AIA: Intermediate water of Antarctica; ACS: Deep water Circumpolar water superior.
Sampling at station c (22°54'30''S, 40°43'W), depth 1900 m (end of the continental slope). |
 Issued from : C.O. Dias, A.V. Araujo, S.C. Vianna, L.F. Loureiro Fernandes, R. Paranhjos, M.S. Suzuki & S.L.C. Bonecker in J. Mar. Biol. Assoc. U.K., 2015, 95 (3). Issued from : C.O. Dias, A.V. Araujo, S.C. Vianna, L.F. Loureiro Fernandes, R. Paranhjos, M.S. Suzuki & S.L.C. Bonecker in J. Mar. Biol. Assoc. U.K., 2015, 95 (3).
[p.490, Table 2].
In list of copepod from The Campos Basin (20.5 and 24°S off the central Brazilian coast. Abundance, biomass and production od species collected by horizontal subsurface hauls ( 1 m depth) , by a Multinet (200 µm mesh aperture) in dry and and rainy seasons.
For calculations see : Undinula vulgaris and map of the sampling stations (Fig.1). |
 Issued from : C.O. Dias, A.V. Araujo, S.C. Vianna, L.F. Loureiro Fernandes, R. Paranhjos, M.S. Suzuki & S.L.C. Bonecker in J. Mar. Biol. Assoc. U.K., 2015, 95 (3). Issued from : C.O. Dias, A.V. Araujo, S.C. Vianna, L.F. Loureiro Fernandes, R. Paranhjos, M.S. Suzuki & S.L.C. Bonecker in J. Mar. Biol. Assoc. U.K., 2015, 95 (3).
[p.484, Fig.1].
Map of the study area showing the location of sampling stations. The Campos Basin (20.5 and 24°S off the central Brazilian coast. |
 Issued from : G.A. Lopez Ibarra in Tesis, Inst. Politec. Nac., CICIMAR, 2008. [p.48, Fig. 22 and p.51, Fig.24] Issued from : G.A. Lopez Ibarra in Tesis, Inst. Politec. Nac., CICIMAR, 2008. [p.48, Fig. 22 and p.51, Fig.24]
Spatial distribution of the isotopic values from the dominant species for localities (see map for the different zones: a to f). A: 15N; B: 13C.
Nota: Sampling in Julio to December 2003, with a Bongo net (333 µm mesh aperture) from obliquely drawing from 200 m depth to surface.
Cf. Map of the 6 zones sampled, legend and remarks by the same author in Subeucalanus subcrassus. |
| | | | Loc: | | | Cosmopolite (equatorial, sub-tropical): Sub-Antarct. (South Georgia-Falkland Is.), South Africa (E & W), Angola, Baia Farta, off Ascension Is., Congo, G. of Guinea, off Lagos, Ivorian shelf, Dakar, Cape Verde Is., Morocco-Mauritania, Canary Is., off Madeira, Portugal, Gijon (NW Spain), Brazil (off Rio de Janeiro, Campos Basin, Vitoria Bay, Vitoria-Cabo de Sao Tomé, Mucuri estuary, Camamu, off Natal, off Amazon), Caribbean Colombia, Cariaco Basin, Bahia de Mochima (Venezuela), Caribbean Sea, Jamaica, Yucatan, E Costa Rica, G. of Mexico, Caribbean, Florida, S Georgia coast, Sargasso Sea, off Bermuda: Station "S" (32°10'N, 64°30'W), , Long Island, Gulf Stream (41°N, 58°W), off Nova Scotia E, off S Newfoundland, Ibero-moroccan Bay, Medit. (Alboran Sea, Habibas Is., Sidi Fredj coast, Algiers, Gulf of Annaba, El Kala shelf, Castellon, Baleares, Banyuls, Marseille, Toulon Harbour, Ligurian Sea, Lake Faro, Napoli, Tyrrhenian Sea, Milazzo, Strait of Messina, Taranto, G. of Gabes, Malta, Adriatic Sea, Island of Pag, Vlora Bay, Venezzia, Po delta, Ionian Sea, Aegean Sea, Thracian Sea, Iskenderun Bay, Lebanon Basin, W Egyptian coast, Alexandria, Bardawill Lagoon, Marmara Sea, Black Sea), G. of Aqaba, off Sharm El-Sheikh, Safaga, Red Sea, Gulf of Oman, G. of Aden, Somalia, Arabian Sea, Karachi coast, Maldive & Laccadive Is., Sri Lanka, Natal, Madagascar, Indian, Bay of Bengal, Australia (North West Cape), Andaman & Nicobar Is., S Burma, W Malay Peninsula (Andaman Sea), Straits of Malacca, Indonesia-Malaysia, Bintulu coast, Ambon Bay (SW Ceram Is.), SW Celebes, Philippines, China Seas (Yellow Sea, East China Sea, South China Sea), Kuroshio Current region, Taiwan Strait, Taiwan (S, E, SW, W, Tapong Bay, NW, NE, N, Mienhua canyon), Okinawa, S Korea, Tsushima Straits, Korea Strait, Japan Sea, Japan: Kuchinoerabu Is., Suruga Bay, Tokyo Bay, off Sanriku, Okhotsk Sea, Vancouver Is., off Washington coast, Oregon (off Newport), Bikini Is., Pacif. (equatorial, subtropical), Guaymas Basin, W Baja California, Bahia Magdalena, G. of California, La Paz, G. of Tehuantepec, Clipperton Is., Costa Rica (Dome), W Costa Rica, off W Guatemala, Pacif. (E & W equatorial), Australia (G. of Carpentaria, Great Barrier, New South Wales), ? S Tasmania, ? Macquarie Is., New Caledonia, NW New Zealand, S Pacif. (NPFZ), Peru, Chile, off Valparaiso | | | | N: | 391 | | | | Lg.: | | | (14) F: 1,2-0,8; M: 0,98-0,814; (28) F: 1,13-0,89; M: 0,72; (30) F: 1,31-0,94; M: 0,92-0,70; (34) F: 1,19-1; (55) F: 1,18-1,08; M: 0,85; (66) F: 1,2-0,96; M: 0,81-0,76; (73) F: 1,19-1,11; M: 0,87-0,84; (101) F: 1,19-1; (104) F: 1,2; M: 1,14; (131) F: 1,31-0,94; M: 0,92-0,7; (135) F: 1,4; (146) F: 1,14; M: 0,8; (150) F: 1,2-1,15; (187) F: 1,36-1,2; M: 1,05-0,95; (202) F: 0,8-1,75; M: 0,7-0,97; (237) F: 1,75-1,25; M: 0,85; (290) F: 1,25-1,1; M: 1-0,95; (327) F: 1,36-1,07; M: 1,06-0,96; (335) F: 1,24-1,12; M: 0,84-0,79; (336) F: 1,2-1,05; (373) F: 1,25-1,08; (374) F: 1,05-0,85; (449) F: 1,2-1,1; M: 0,83; (786) F: 1,25; (864) F: 1-1,05; (920) F: 1,03; (991) F: 0,94-1,31; M: 0,7-0,92; (1122) F: 1,05; M: 0,75; (1138) F: 1,11-0,95; {F: 0,80-1,75; M: 0,70-1,14}
The mean female size is 1.131 mm (n = 52; SD = 0.1656), and the mean male size is 0.867 mm (n = 29; SD = 0.1188). The size ratio (male : female) is 0.75 (n = 18; SD = 0.0876). or ± 75 %. | | | | Rem.: | epipelagic-mesopelagic. Sargasso Sea: 0-500 m.
For Frost & Fleminger (1968, p.80) the females are distinguished from all other Clausocalanus species by the relative lengths of urosomal segmenst 2 and 3, the form of the seminal receptacle in lateral view, and the number of aesthetes on A1. Males differ from all other species of Clausocalanus in the shape of the rostrum and in usually having the longer ramus of the P5 and genital pore on the right side.
After Cornils & al (2007, p.63) the percentage of females infected by Blastodinium sp. was 1.7 %, ±0.4); with impact on P5 (abnormal).
The indication of this species in Antarctica is to be confirmed.
This species is the easiest to distinguish from all the others.
Observed in ballast waters from ships in San Francisco.
Timonin (1971, p.282) considers the trophic interrelations in the equatorial and tropical Indian Ocean, and divides the plankters into 6 trophic groups from the litterature and the results of studies of mouth-parts structure and intestine content. This species is a fine-filter feeder.
After Benedetti & al. (2018, p.1, Fig.2) this species belonging to the functional group 7 corresponding to small filter feeding herbivorous.
See in DVP Conway & al., 2003 (version 1) | | | Last update : 28/10/2022 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed February 20, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |







































