|
|
 |
|
Calanoida ( Order ) |
|
|
|
Clausocalanoidea ( Superfamily ) |
|
|
|
Clausocalanidae ( Family ) |
|
|
|
Pseudocalanus ( Genus ) |
|
|
| |
Pseudocalanus acuspes (Giesbrecht, 1881) (F,M) | |
| | | | | | | Syn.: | Lucullus acuspes Giesbrecht,1881,1882; ? Thompson, 1888 d (p.142);
no Pseudocalanus elongatus (Boeck, 1864);
Pseudocalanus sp. McLaren, 1965; Conover & al., 1986 (p.1245, distribution, feeding); Conover & al., 1988 (p.267, Table II, IV, grazing respiration, excretion) | | | | Ref.: | | | Corkett & McLaren, 1978 (p.5); Frost, 1989 (p.537, Redescr., figs.F,M); McLaren & al., 1989 (p.552); Sévigny & al., 1989 (p.321 & suiv., genetic); Samatov, 2001 (p.259, fig. F,M: morphometric characters, Rem.); Bucklin & al., 2003 (p.335, tab. 2, fig.3, Biomol.); G. Harding, 2004 (p.48, Rem.); Aarbakke & al., 2011 (p.1487, Table III, fig.2, genetic analysis); Holmborn & al., 2011 (p.507, genetic identification); Markhasea & al., 2012 (p.57, Redescr. F,M, figs.F,M); Questel & al., 2016 (p.610, Fig.3, genetic analyse, Table VII) |  issued from : B.W. Frost in Can. J. Zool., 1989, 67. [Figs.13,16]. Female: A, habitus (lateral view), from Kiel Bay; B, habitus (lateral view), from Beaufort Sea; C, anterior portion of cephalosome; D, thoracic segments 1-3 I (posteroventral margins from left view); D', thoracic segment 3I and urosomal segment 1. Male: E, habitus (lateral view); F, anterior portion of cephalosome. Scale bars = 0.1 mm. (except D')
|
 issued from : E.L. Markhaseva, A.A. Abramova & N.D. Mingazov in Proc. Zool. Inst. RAS, 2012, 316 (1). [p.59, Fig.1]. Female (from 66°19.5'N, 33°39.4'E): habitus (lateral). Scale bar: 0.1 mm. Nota: Prosome 2.06-2.30 times as long as urosome. Rostrum as 2 filaments. Cephalosome and pediger 1, and pedigers 4 and 5 fused.
|
 issued from : E.L. Markhaseva, A.A. Abramova & N.D. Mingazov in Proc. Zool. Inst. RAS, 2012, 316 (1). [p.60, Fig.2]. Female: A, habitus (dorsal); B, rostrum (ventral view); C-F, postero-ventral margins of pedigers 2 and 3, arrows mark spiniform processes; G-H, urosome (dorsal and lateral, respectively); I, posterior prosome and genital double-somite (dorsal); J, A1; K, P4 coxo and basis (protopod), arrows show measurements for their length. Scale bars: 0.1 mm. Nota: A1 of 24 free segments, reaching to about middle of 2-3rd urosomal somite. Caudal rami with 4 terminal plus 1 small dorsolateral and 1 small ventral setae each.
|
 issued from : E.L. Markhaseva, A.A. Abramova & N.D. Mingazov in Proc. Zool. Inst. RAS, 2012, 316 (1). [p.61, Fig.3]. Female: A, A2; B, Md; C, Mx1; D, Mx2; E, Mxp. Scale bars: 0.1 mm. Nota: A2, coxa with 1 seta; basis with 2 setae; endopod 2-segmented with 2 setae and 15 setae, respectively; exopod incompletely 8-segmented with 1-1, 1-1, 1, 1, 1, 1, 1 and 3 setae. Md; gnathobase cutting edge with 8 teeth; exopod 5-segmented with 1, 1, 1, 1, 1, and 2 setae, respectively; endopod 2-segmented with 2 and 11 setae, respectively; basis with 4 setae. Mx1: praecoxal arthrite with 9 terminal spines, 4 posterior setae and 1 anterior seta; coxal endite with 3 setae, coxal epipodite with 9 setae; proximal basal endite with 4 setae, distal basal endite with 5 setae; endopod with 16-17 setae; exopod with 11 setae. Mx2: praecoxal endite bearing 5 setae, coxal (former considered as distal praecixal endite) with 3 setae; basal endites (considered as coxal endites) with 3 setae each; lobe of proximal endopodal segment (considered as proximal basal endite) with 4 setae; endopod with 5 plus 1 setae. Mxp: syncoxa with 1 sclerotized seta on proximal praecoxal endite, 2 sclerotized setae on middle endite, and 3 setae on distal praecoxal endite, coxal endite with 3 setae; basis with 3 medial setae plus 2 setae distally of incorporated endopod segment 1; endopod of 5 free segments with 4, 2, 2, 2+1 and 4 setae.
|
 issued from : E.L. Markhaseva, A.A. Abramova & N.D. Mingazov in Proc. Zool. Inst. RAS, 2012, 316 (1). [p.62, Fig.4]. Female: A-D, P1 to P4, respectively. Scale bars: 0.1 mm.
|
 issued from : E.L. Markhaseva, A.A. Abramova & N.D. Mingazov in Proc. Zool. Inst. RAS, 2012, 316 (1). [p.63, Fig.5]. Male: A, habitus (lateral; B, cephalon (dorsal); C, cephalon and rostrum (lateral); D, posterior prosome and urosome (dorsal); E, same with P5 (lateral); F, A1 (ancestral segments I-XXII-XXIII); G, A1 (ancestral segments XXIV-XXVIII); H, P1; I, P5; J, right P5.. Scale bars: 0.1 mm. Nota: Prosome 1.90-2.13 times as long as urosome. A1 of 23 segments, right and left symmetrical, reaching to anterior 3rd urosomal somite. P5 uniramous, nearly as long as urosome. Right leg protopod of coxo-and basipod nearly completely fused; exopod 2-segmented. Left leg longer than right, with protopod nearly twice as long; exopod 3-segmented, terminal segment small with spine and lateral spinules.
|
 issued from : E.L. Markhaseva, A.A. Abramova & N.D. Mingazov in Proc. Zool. Inst. RAS, 2012, 316 (1). [p.64, Fig.6]. Male: A, A2; B, Md; C, Mx1; D, Mx2; E, Mxp. Scale bar = 0.1 mm. Nota: Md gnathoabse cutting edge with reduced teeth; all setae of appendage rudimentary.
|
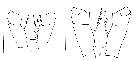 issued from : E.L. Markhaseva, A.A. Abramova & N.D. Mingazov in Proc. Zool. Inst. RAS, 2012, 316 (1). [p.60, Fig.2 K, p.68, Fig.9F]. Female & Male ((66°19.5'N; 33°39.4'E): P4 Coxopod to basipod ratio. K: P4 coxo- and basipod Pseudocalanus acuspes (arrows show measurements for thieir length. Scale bar = 0.1 mm. F: P4 coxo- basipod Pseudocalanus minutus. Scale bar = 0.1 mm. Nota: A key character to distinguish adults of P. minutus from P. acuspes is the length ratio of coxo-to basipod P4 length which is > 1.5 (1.51-1.86) in P. minutus and < 1.5 (1.20-1.45) in P. acuspes.
|
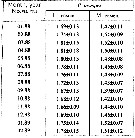
 Issued from : A.D. Samatov in Biologiya Morya, Vladivostok, 2001, 27 (4). [p.262, Tableau 2 and 3]. Female and Male (from Avachinskaya Bay): Morphometric measures. TL = total length; PL : UL = cephalothoracic length: urosomal length; UIIIL : UIIIW = ratio (length : width of urosomal segment 3); CRL : UIIIW = ratio caudal ramus length : width urosomal segment 3; CL : UIII = ratio cephalothoracic length : urosomal segment 3 length; CRL : CRW = ratio caudal ramus length : width; A1 (8-12): A1 (20-21) = ratio length between segments ( 8-12) and (20-21).
See in Pseudocalanus minutus: same table 2 and 3: * = mean; ** = extent sizes; *** = standard deviation.
|
 Issued from : A.D. Samatov in Biologiya Morya, Vladivostok, 2001, 27 (4). [p.265, Fig.3]. Female (from Avachinskaya Bay): 1-2, habitus ( ventral and lateral, respectively); 3, cephalosome (lateral); 4, lasst thoracic segments and uosome (lateral). Male (from Avachinskaya Bay): 5, habitus (lateral); 6, A1; 7, P5; 8, coxo and basis of P4.. Note the characteristic lateral ending of the tergite of the thoracic segments. Compare with P. minutus and P. newmani. The females and males are present all the year and have domination over P. minutus and P. newmani.
| | | | | Compl. Ref.: | | | Conover & al., 1988 (p.650, grazing); McLaren & al., 1989 (p.559, Table 2, 3, 4, development vs. temperature); Bedo & al., 1990 (p.561, nutrition, metabolism); Norrbin & al., 1990 (p.205, lipids: seasonal variation); Conover & al., 1991 (p.177); Norrbin, 1991 (p.421, life history, gonad maturation); 1992 (p.1, life history); 1993 (p.204); Conover & al., 1993 (p.303, Table 2, figs. 7 ,8, dry weight, %); Norbin, 1994 (p.115, gonad maturation, sex ratio); Vinogradov & al., 1994 (tab.1); Petryashov & al., 1995 (tab.1); Pedersen & al., 1995 (p.266, tabl.II); Abramova, 1996 (tab.1); Sirenko & al., 1996 (p.349); Norrbin, 1996 (p.99, life history, timing of diapause); Falkenhaug & al., 1997 (p.449, fig.5, spatio-temporal pattern); Mauchline, 1998 (tab.47, 48, 58); Kosobokova & al., 1998 (tab.2); Conover & Gustavson, 1999 (p.41, tab.6); Halvorsen & Tande, 1999 (p.279, figs.3, 5); White & McLaren, 2000 (p.751, tab.1); Musaeva & Suntsov, 2001 (p.511); Lischka & al., 2001 (p.186); Fortier M. & al., 2001 (p.1263, fig.6, 7, 9, diel vertical migration); Karnovsky & al., 2003 (p.289, Appendix 1, 2 ( auks relation/copepods); Peters & Hagen, 2003 (p.197); Lischka & Hagen, 2005 (p.910, life history); Renz & Hirche, 2006 (p.567, life cycle); Hop & al., 2006 (p.182, Table 4); Hansen F.C. & al., 2006 (p.39, production, Rem.: p.41); Peters & al., 2006 (p.1417, lipids composition, seasonal cycle); Willis & al., 2006 (p.39, Table 2, advection vs changes in community structure); Renz & al., 2007 (p.515, reproduction, growth, secondary production); Margonski & al., 2007 (p.5, figs.4, 5); Schulz J. & al., 2007 (p.47, vertical zonation analysis); Hopcroft & al., 2009 (p.9, Table 3); Dvoretsky & Dvoretsky, 2009 a (p.11, Table 2, abundance); 2010 (p.991, Table 2); Hopcroft & al., 2010 (p.27, Table 2); Hopcroft & Kosobokova, 2010 (p.49, figs.2, 3, 6, 7, 8, Table 1, 2, Rem.: egg production); Dvoretsky & Dvoretsky, 2011 b (p.469, Table A, abundance, biomass); Kosobokova & al., 2011 (p.29, Table 2, 3, fig.4, Rem.: Arctic Basins); Hirche & Kosobokova, 2011 (p.2359, Table 3, abundance, biomass %); Matsuno & al., 2011 (p.1349, Table 1, abundance vs years); Matsuno & al., 2012 (Table 1, 2, fig.6); Postel, 2012 (p.327, Table 1); Dvoretsky & Dvoretsky, 2012 (p.1321, Table 2, 3, abundance, biomass); Questel & al., 2013 (p.23, Table 3, interannual abundance & biomass, 2008-2010); Kwasniewski & al., 2013 (p.83, Table 2, 3, abundance); Dvoretsky & Dvoretsky, 2013 a (p.205, Table 2, % abundance); Record & al., 2018 (p.2238, Table 1: diapause); Dzierzbicka-Glowacka & al. 2019 (p.1, Table 2, 3, 4, 5, fig.5, 6, 8, 12, 13, 14, 16, 20, 21, production, mortality trate) | | | | NZ: | 4 | | |
|
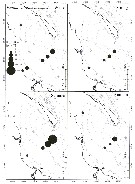
Distribution map of Pseudocalanus acuspes by geographical zones
|
| | | | | | 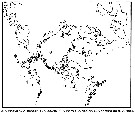 issued from : B.W. Frost in Can. J. Zool., 1989, 67. [p.529, Fig.3]. issued from : B.W. Frost in Can. J. Zool., 1989, 67. [p.529, Fig.3].
Geographical distribution of Pseudocalanus spp. |
 Issued from : M.F. Norrbin in J. Plankton Res., 1994, 16 (2). [p.118, Fig.1]. Issued from : M.F. Norrbin in J. Plankton Res., 1994, 16 (2). [p.118, Fig.1].
Gonad maturation in P. acuspes (lateral view) from Balsfjorden (Tromsø, Norway). A: Stage IV female, immature gonad (G) and barely visible gonoduct (gd); detail gonad from dorsal view. B: Stage V male, immature gonad (G).C: Stage V male, maturing testis (T), with vas deferens (vd), sperm sac (ss) and spermatheca (st). D: Stage V female, maturing ovary (O) with anterior diverticulae (d) forming, oviduct (od). |
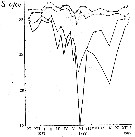
 issued from : R.J. Conover & T.D. Siferd in Arctic, 1993, 45 (4). [p.307, Table 2]. issued from : R.J. Conover & T.D. Siferd in Arctic, 1993, 45 (4). [p.307, Table 2].
Numbers and dry weight biomass for the total water column (frpm Barrow Strait), before, during, and after the dark period 1984-1985 and 1985-1986 (Conover, Sifred and Harris, unpubl.)
Nota: Observations were made in Resolute Passage/Barrow Strait (Northwest Territories, Canada). Zooplankton samples were taken with vertically hauled 0.5 m diameter nets (100, 200, or 800 µm mesh).
Polar night descends about 9 November and the sun does not reappear unyil early February. The sun does not set from 28 April until mid-August. Even during the light period, the average monthly irradiance received in the Resolute area as a percentage of that potentially available is generally less than 50% because of cloud, fog, and polar haze (Atmospheric Environment Service).
The fast-ice season extends from October into the following July. maximum ice thickness is around 2 m in May but varies from season to season, as does snow cover. CTD measurements on the north side of Barrow Strait in late May-early June 1985 suggest that the depth of the mixed layer can be about 20-30 m. Tidal currents under the ice near Resolute can be greater than 25 cm.s-1, with maximum velocity and transport in an easterly direction.
Barrow Strait contains a sill that limits water flow through the Northwest Passage to < 125 m, but it receives a mixture of several water types. Arctic Ocean water passes through the archipelago in a generally easterly direction. That entering from the north is slightly more saline than that entering from the west or south. The warmest, saliest water occurs below 100 m, reflecting some mixing of arctic water.
Compare with Calanus hyperboreus, C. glacialis and Metridia longa in the same area of Barrow Strait.
For the authors, to elaborate on the life cycle, P. acuspes, can complete a generation in one year by overwintering mainly as developmental stages C3-C5, completing maturation using sympagic production. Spawning of newly fertilized females begins in laye spring and continues most of the summer, but its development rate is sufficiently rapid that bot(h structural growth and enough lipid storage take place to permit overwintering of a range of developmental stages. |
 Issued from : A.D. Samatov in Biologiya Morya, Vladivostok, 2001, 27 (4). [p.263, Table 4]. Issued from : A.D. Samatov in Biologiya Morya, Vladivostok, 2001, 27 (4). [p.263, Table 4].
Seasonal total length in female (F) and male (M) in Avachinskaya Bay (SE Kamchatka : ± 53°00' N, 158°30' E). |
 Issued from : A.D. Samatov & I.N. Samatova in Biologiya Morya, Vladivostok, 1996, 22 (1). [p.25, Fig.5]. Issued from : A.D. Samatov & I.N. Samatova in Biologiya Morya, Vladivostok, 1996, 22 (1). [p.25, Fig.5].
Seasonal variation of the salinity (per 1000) in Avachinskaya Bay) during November 1987 to January 1989 .
Seasonal variation of the salinity (per 1000).
1- surface mean; 2- 10 m depth; 3- in the bottom; 4- in surface at the station 2. (western Bay). |
 Issued from : A.D. Samatov & I.N. Samatova in Biologiya Morya, Vladivostok, 1996, 22 (1). [p.25, Fig.5]. Issued from : A.D. Samatov & I.N. Samatova in Biologiya Morya, Vladivostok, 1996, 22 (1). [p.25, Fig.5].
Seasonal variation of the temperature (t°C) in Avachinskaya Bay) during November 1987 to January 1989.
1- surface mean; 2- 10 m depth; 3- in the bottom; 4- in surface at the station 2. (western Bay). |
 Issued from : A.D. Samatov in Biologiya Morya, Vladivostok, 2001, 27 (4). [p.260, Table 1]. Issued from : A.D. Samatov in Biologiya Morya, Vladivostok, 2001, 27 (4). [p.260, Table 1].
Seasonal abundance in female and male (by m3) in Avachinskaya Bay (SE Kamchatka : ± 53°00' N, 158°30' E) from January 1988 ton February 1989.
Compare with P. minutus and P. newmani. |
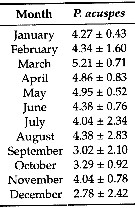 Issued from: L. Dzierzbicka-Glowacka & al. in Appl. Sci., 2019 , 9, 2039 [ p. 7, Table 2]. Issued from: L. Dzierzbicka-Glowacka & al. in Appl. Sci., 2019 , 9, 2039 [ p. 7, Table 2].
Long-term monthly means (± standard deviation) for abundance (In ind. m3) for Pseudocalanus acuspes in the Gulf of Gdansk (S Baltic Sea).
Sampling conduced during three separate projects spanning almost 14 years from 1998 to 2012.
Water temperature ranges from over 20°C at the surface during summer (maximum usually in August, to - 2°C in February. Due to the brackish character of the Baltic Sea, gulf water salinity stays within the range of t to 8. |
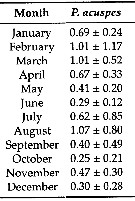 Issued from: L. Dzierzbicka-Glowacka & al. in Appl. Sci., 2019 , 9, 2039 [p.8, Table 3]. Issued from: L. Dzierzbicka-Glowacka & al. in Appl. Sci., 2019 , 9, 2039 [p.8, Table 3].
Long-term monthly means (± standard deviation) for biomass (In mgC. m3) for Pseudocalanus acuspes in the Gulf of Gdansk (S Baltic Sea).
Sampling conduced during three separate projects spanning almost 14 years from 1998 to 2012.
Water temperature ranges from over 20°C at the surface during summer (maximum usually in August, to - 2°C in February. Due to the brackish character of the Baltic Sea, gulf water salinity stays within the range of 7 to 8. |
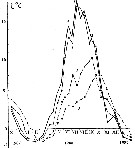
 Issued from: L. Dzierzbicka-Glowacka & al. in Appl. Sci., 2019 , 9, 2039 [p.9.,Fig. 5]. Issued from: L. Dzierzbicka-Glowacka & al. in Appl. Sci., 2019 , 9, 2039 [p.9.,Fig. 5].
Interannual (a) abundance and (b) biomass monthly mean and anomaly of Pseudocalanus acuspes/em> in the Gulf of Gdansk (S Baltic Sea).
Sampling conduced during three separate projects spanning almost 14 years from 1998 to 2012.
Water temperature ranges from over 20°C at the surface during summer (maximum usually in August, to - 2°C in February. Due to the brackish character of the Baltic Sea, gulf water salinity stays within the range of 7 to 8.
Nota: This species was characterized by the lowest production values. In the 1998 to 2000 period, the average values of production rates of the species did not exceed 25 µg C /m2 (summer 2000). During the second period, two production peaks were observed during each year. In 2006, the first was recorded in summer with an average value of ± 175 µg C/m2, and the second in autumn ± 80 µg C/m2. In 2007 these values were similar in the summer the average production was 150 µg C/m2 and in autumn it reached ± 50 µg C/m2. During the last research period exen lower production rate values were observed . In 2010 and 2012 the average values did not exceed 50 µg C/m2, and the maximum was observed in the summer of 2011, reaching 70 µg C/m2. |
 Issued from: L. Dzierzbicka-Glowacka & al. in Appl. Sci., 2019 , 9. [p.22, Fig. 20]. Issued from: L. Dzierzbicka-Glowacka & al. in Appl. Sci., 2019 , 9. [p.22, Fig. 20].
Horizontal distribution of average mortality rates of P. acuspes in the Gulf of Gdansk (Baltic Sea, between Poland and Russia).
The bay is strongly influenced by river waters especially the Vistula (which on average brings 1080 m3per s of fresh water. The average depth of the gulf is 50 m with a maximum depth 115 m. Water temperatures ranges from over 20°C at the surface during summer (August), to - 2°C in February. Water stratification is frequent during the warmer months (thermocline), while during the winter gulf waters become well-mixed. Due to the brackish character of the Baltic Sea, the salinity stays within the range of 7 to 8 per 1000. |
 Issued from: L. Dzierzbicka-Glowacka & al. in Appl. Sci., 2019 , 9, 2039 [p.15, Fig. 12]. Issued from: L. Dzierzbicka-Glowacka & al. in Appl. Sci., 2019 , 9, 2039 [p.15, Fig. 12].
Horizontal distribution of average secondary production rates of P. acuspes in the Gulf of Gdansk. |
| | | | Loc: | | | North Sea, Bay of Kiel, Baltic Sea, Gulf of Gdansk, Bornholm Basin, W Norway, Spitsbergen (Kongsfjorden, Rijpfjord), Malangen fjord, Svartnes, Nordvestbanken, Balsfjorden (Tromsø), Fram Strait, Kongsfjorden, Newfoundland, Nova Scotia, Halifax Harbor, Barrow Strait, Arctic (Resolute Passage, White Sea, Barents Sea, Pechora Sea, Nansen Basin, Amundsen Basin, Pechora Sea, Kara Sea, Laptev Sea, Canada Basin, Chukchi Sea, off NW Alaska), Bering Sea, SE Kamchatka (Avachinskaya Bay) | | | | N: | 62 | | | | Lg.: | | | (122) F: 2,27-1,26; M: 1,74-1,19; (1222) F: 1,45-2,20; M: 1,25-1,70; (1304) F: 1,05-1,45; M: 0,95-1,15; {F: 1,05-2,27; M: 0,95-1,74} | | | | Rem.: | The occurrence of this species pointed to by Thompson (1888) at Malta (Mediterranean Sea) is very doubtful.
Characteristics according to Frost (1989): See limits of lenght measurements in the figure of the genus Pseudocalanus.
Female: frontal part in lateral view rounded; mediodorsal surface of one or more of U1-U3 with sensillum; mediodorsal surface of U1-U3 with integumental pore; seminal receptacle small; cephalosome in lateral view not strongly tapered anteriorly; ratio length:width of U3 usually > 1.26; ratio sizes (dorsal view) Prosome:Urosome = 2.14 (2.00-2.29); ratio lenght of Furca:width of U3 = 1.16 (1.03-1.29).
Male: ratio sizes (internal) B1:B2 < 1.5; ratio sizes of segments of A1 (8-12):(20-21) < 2.0; mediodorsal surface of one or more of U2-U4 with sensillum; ratio sizes (lateral view) U2:U4 < 1.37; ratio sizes Cephalosome: U2 < 5.07; ratio sizes (lateral view) Cephalosome: U2 = 4.82 (4.51-5.06); ratio sizes U2:U4 = 1.27 (1.18-1.35); ratio Furca lenght:width = 1,56 (1.46-1.82).
Characteristic after Markhaseva & al. (2012, p.57) to distinguish the coexistence of P. acuspes and P. minutusin the White Sea (Chupa Inlet) ; P4 coxopod to basipod ratio more than 1.5 in P. minutus and less than 1.5 in P. acuspes.
After Holmborn & al. (2011, p.512) the genetic identification is congruent with the hypothesis that the Baltic Sea population of P. acuspes is a glacial relict. Peters & al. (2006) found that the Baltic population of P. acuspes had a similar life cycle to theArctic population of the same species and hypothesized that the life cycle and lipid storage strategies of the Baltic population of P. acuspes originates from extant adaptations to high latitudinal habitats. Low genetic diversity would be expected in isolated, glacial relict populations, as was observed in P. acuspes by Holmborn & al. (2011).
After Karnovsky & al., 2003 (p.289, Appendix 2) the mean dry mass (mg) female is 0.013. | | | Last update : 21/09/2020 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed February 20, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |






















