|
|
 |
|
Calanoida ( Order ) |
|
|
|
Clausocalanoidea ( Superfamily ) |
|
|
|
Clausocalanidae ( Family ) |
|
|
|
Pseudocalanus ( Genus ) |
|
|
| |
Pseudocalanus elongatus (Boeck, 1865) (F,M) | |
| | | | | | | Syn.: | Clausia elongata Boeck,1864; Bourne, 1889 (p.146, Rem.);
Calanus clausii Boeck,1865;
Pseudocalanus minutus elongatus Farran & Vervoort, 1951 e (n°37, p.3, figs.F,M); Hernroth, 1978 (p.1, Rem.: p.5);
Pseudocalanus minutus : Conover, 1959 (p.259, Table 1, 2, respiration, Rem.: p.260);
Non Lucullus acuspes Giesbrecht,1881; Grice, 1962 a (p.101, 102); Marques, 1966, p.3);
Non Pseudocalanus elongatus : Brodsky, 1950 (1967) (p.112, figs.F,M); ? Nakai, 1955 (p.12, chemical composition); ? Minoda, 1958 (p.253, Table 1, 2, abundance); ? Kolosova, 1975 (p.92, fig.3, Table 1); Geletin, 1977 (p.82, figs.F,M); ? Morris, 1970 (p.2300); ? Morioka, 1972 a (p.314); ? Shih & Young, 1995 (p.72);
Pseudocalanus : Harris & al., 1986 (p.845, Table 2, comparison pump vs net);
Pseudocalanus elongatus minutus : Bathmann & Liebezeit, 1986 (p.59, faecal pellets- chlorophyll incorporated);
Paracalanus elongatus : Kürten & al., 2013 (p.167, Table 1, C:N, fatty acid) lapsus calami. | | | | Ref.: | | | Brady, 1878 (figs.F,M, Rem.); Giesbrecht, 1892 (p.197, figs.F,M); Karavaev, 1894 (figs.F,M, Rem.); Sars, 1901 a (1903) (p.20, figs.F,M); Mràzek, 1902 (p.507, figs.F, Rem.); Thompson & Scott, 1903 (p.233, 244); Farran, 1908 b (p.28); Lysholm, 1913 (p.5); With, 1915 (p.57, Rem.); Pesta, 1920 (p.504); Adler & Jespersen, 1920 (p.5); Sars, 1925 (p.27); Farran, 1926 (p.242); Rose, 1929 (p.16); 1933 a (p.79, figs.F,M); Massuti Alzamora, 1942 (p.88, figs.F, Rem.); Sewell, 1951 (p.351, Rem.: parasites); Corkett, 1968 (p.51, fig.N, Rem.N, juv.); Kos, 1976 (Vol. II, figs.F,M); Corkett & McLaren, 1978 (p.6, Rem); Vives & al., 1981 (p.337); Klein Breteler, 1982 (p.1, figs. juv.); Schnack, 1982 (p.89, figs.Mx2, Md, Mxp); Sazhina, 1985 (p.43, figs. N); Frost, 1989 (p.535, Redescr., figs.F,M); McLaren & al., 1989 a (p.566); Sévigny & al., 1989 (p.321 & suiv.: genetic); Ferrari & Benforado, 1998 a (p.209, figs. juv., F,M); Bucklin & al., 2003 (p.335, tab. 2, fig.3, Biomol.); Boxshall & Halsey, 2004 (p.93: figs.F,M); Conway, 2006 (p.10, 25, copepodite stages 1-6, Rem.); Unal & al., 2006 (p.1961, genetic analysis, Rem., phylogeography); Ferrari & Dahms, 2007 (p.34, 35, Rem. N); Avancini & al., 2006 (p.74, Pl. 43, figs.F,M, Rem.); Vives & Shmeleva, 2007 (p.635, figs.F,M, Rem.); Aarbakke & al., 2011 (p.187, Table III, fig.2, genetic analysis); Laakmann & al., 2013 (p.862, figs.1, 2, 3, 4, 5, Table 1, 2, 3, mol. Biol.) | 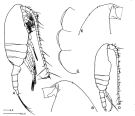 issued from : B.W. Frost in Can. J. Zool., 1989, 67. [Fig.10, p.535]. Female: A, habitus (lateral view fom right side); B, anterior portion of cephalosome (lateral view from left side); C, Th1-Th3 (posteroventral margins from left side). Male: habitus (lateral view); E, anterior portion of ephalosome (lateral view from right side). All scale bars = 0.1 mm.
|
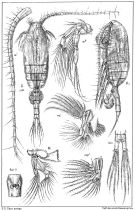 issued from : G.O. Sars in An Account of the Crustacea of Norway. Vol. IV. Copepoda Calanoida. Published by the Bergen Museum, 1903. [Pl. X]. Female. M = Md; m = Mx1; mp1 = Mx2; Gen. S = genital segment (ventral view).
|
 issued from : G.O. Sars in An Account of the Crustacea of Norway. Vol. IV. Copepoda Calanoida. Published by the Bergen Museum, 1903. [Pl. XI]. Female & Male. mp1 = Mx2; mp2 = Mxp.
|
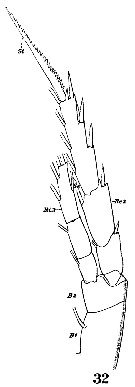 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf. 10, Fig.32]. Female: 32, P3 (anterior surface). B1 = coxa; B2 = basis; Ri3 = endopodal segment 3; Re2 = exopodal segment 3.
|
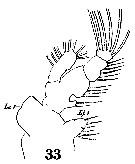 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf. 10, Fig.33]. Male: 33, Mx1. Li 1= inner lobe; (= arthrite) Le 1= outer lobe (= epipodite).
|
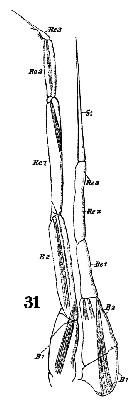 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf. 10, Fig.31]. Male: 31, P5 (anterior surface).
|
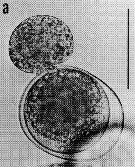 issued from : G. Drebes in Helgoländer Meeresunters., 1984, 37. [p.612, Fig.4]. Trophont of Dissodinium pseudocalani (Dinophytes Blastodiniales) sucking out egg of Pseudocalanus elongatus. Scale bar: 100 µm.
|
 Issued from M. Rose in Résult. Camp. scient. Prince Albert I, 1929, 78, p.17. Remarques concernant Pseudocalanus elongatus identifié lors des campagnes de 1903, 1906, 1907, 1908, 1909, 1910, 1912, 1913 et 1914. Nota: Depuis 1929, d'autres espèces de Pseudocalanus ont été reconnues comme des espèces différentes dans les mers arctiques et subarctiques.
| | | | | Compl. Ref.: | | | Thompson, 1888 d (p.140); Gadeau de Kerville, 1894 (p.81); T. Scott, 1902 (p.450, Rem.); Pearson, 1906 (p.10, Rem.); Carazzi & Grandori, 1912 (p.8, 38); ? Wilson, 1932 (p.29); Fleury, 1950 (p.47, fig.2); Dimov, 1964 b (p.33, Tableau 1); Urry, 1965 (p.49, feeding); Bodo & al., 1965 (p.219, annual cycle); Marshall & Orr, 1966 (p.513, 521, fig. I, 2, Table 1, 2, 3, 4, 6, 7, feeding, respiration); Mazza, 1966 (p.70); 1967 (p.344, 356); Matthews, 1967 (p.159, Table 1, Rem.); Pertsova, 1967 (p.240); Porumb, 1968 (p.417); 1968 a (p.509); Corkett & Urry, 1968 (p.97, survival); Corner & Cowey, 1968 (p.393, Table I, amino acid composition); Greze & al., 1968 (p.1066, annual variation); in Oceanology, 1968 p.839, P/B quotient); Dimov, 1968 (p.506); Margineanu, 1968 a (p.421); Vinogradov, 1968 (1970) (p.44, 50, 61, 111, 243, 244, 266, 278); Kovalev, 1968 a (p.441, fig.1); 1969 a (p.159); Corkett & McLaren, 1969 (p.90, 101, egg production, , survival vs diet); Dowidar & El-Maghraby, 1970 (p.269); Paulmier, 1971 (p.168); Lefèvre-Lehoërff, 1972 (p.1681); Martens, 1972 (p.35, fecal pellets); Eriksson, 1973 (p.37, fig.10-13, annual cycle); 1973 b (p.113, 117); Corkett & Zillioux, 1975 (p.13, temperature vs egg laying); Porumb, 1976 (p.91); Paffenhöfer & Harris, 1976 (p.327, feeding); 1976 a (p.875, food concentration); Ciszewski & Witek, 1977 (p.449, P/B quotient); Colebrook, 1978 (tab.1); Comaschi Scaramuzza, 1978 (p.13, vertical distribution); Longhurst & Williams, 1979 (p.1, Table IVb, V, vertical distribution); Spooner & Corkett, 1979 (p.197, feeding rates vs oil effects); Hure & al., 1980 (p.297, 304); Pipe & Coombs, 1980 (p.223, figs. 1, 2, 4, table 1, vertical distribution); Pertsova, 1982 (p.67)3; Kovalev & Schmeleva, 1982 (p.83); Castel & Courties, 1982 (p.417, Table II, spatial distribution); Thompson B.M., 1982 (p.35, growth, development); Hernroth, 1983 (p.835, Rem.: p.840); Chojnacki, 1983 a (p.435, length-weight, calculations); Chojnacki & Hussein, 1983 (p.53, Table 1, length-weight); Pasternak & Kopylov, 1983 (p.240, parasitism); Baars & Fransz, 1984 (p.120, Table 1, grazing); Baars & Oosterhuis, 1984 (p.97, diurnal feeding rhythms); Fransz & al., 1984 (p.86); Drebes, 1984 (p.603, 611, 619: parasitic dinophytes); Prygunkova, 1985 (p.9); Williams & Collins, 1985 (p.28); Colebrook, 1985 (p.261, tab.1, fig.2); Oosterhuis & Baars, 1985 (p.89, digestive enzymes); Groendahl & Hernroth, 1986 (tab.1); Brylinski, 1986 (p.457, spatial variations); Robinson & al., 1986 (p.201, seasonal distribution); Robinson & Hunt, 1986 (p.791, Table 1, 2, fig.2); Mikhailovsky, 1986 (p.83, Table 1, ecological modelling); Comaschi Scaramuzza, 1987 (tab.1); Vuorinen & Ranta, 1987 (tab.2, 4); Tiselius, 1988 (p.215, grazing); Brylinski & al., 1988 (p.503, size/spatial distribution); Aksnes & Magnesen, 1988 (p.57, population dynamic, production); Hay & al., 1988 (p.431, enclosed population dynamic); Kattner & Krause, 1989 (p.261, Table 6, lipids); Kosobokova, 1989 (p.27); Klein Breteler & al., 1990 (p.177, food vs length); Tiselius & Jonsson, 1990 (p.23, table 1-4, feeding behaviour); Shushkina & Vinogradov, 1991 (p.719); Hay & al., 1991 (p.1453, Table 2); Voss, 1991 (p.217, faecal pellets); Green & al., 1992 (p.1631, Nauplii-fecal pellets); Huntley & Lopez, 1992 (p.201, Table 1, A1, eggs, egg-adult weight, temperature-dependent production); Bautista & Harris, 1992 (p.41, ingestion rate, gut contents); Viitasalo, 1992 (tab.2); Mumm, 1993 (tab.1, fig.2); Munk & Nielsen, 1994 (p.1225, fig.4, predation); Hays & al., 1994 (tab.1); Harris, 1994 (p.431, ingestion & egestion); Klein Breteler & al. 1994 (p.1039, Table I, fig. 3, 6, 7, duration life stages); 1995 (p.99, Table 2, generation times); Hajderi, 1995 (p.542); Krause & al., 1995 (p.81, Fig.31, 32, abundance, Rem.: p.133, 137); Fernandez de Puelles & al., 1996 (p.97, occurrence: p.100); Park & Choi, 1997 (Appendix); Mauchline, 1998 (tab.8, 19, 21, 24, 25, 26, 33, 45, 46, 47, 48, 51, 58, 61, 63, 64); Hure & Krsinic, 1998 (p.100); Koski & al., 1998 (p.169, growth, development, egg production vs food quality); Vigoni & al., 1998 (tab.2); Suarez-Morales & Gasca, 1998 a (p.111); Harvey & al., 1999 (p.1, 49: Appendix 5, in ballast water vessel); Klein Breteler & al., 1999 (p.191, feeding vs protozoans, fatty acids, development time); Selifonova, 2000 (p.68, tab.1); Sautour & al., 2000 (p.531, Table II, abundance); Dippner 1 al., 2000 (p.23, long-term variability vs. climate); d'Elbée, 2001 (tabl.1); Cotonnec & al., 2001 (p.693, Table III, IV, food selectivity); Fransz & Gonzalez, 2001 (p.255, tab.1); Holmes, 2001 (p.43, Rem.); Bressan & Moro, 2002 (tab.2); Kovalev, 2003 (p.47); Bode & al., 2003 (p.85, Table 1, abundance); Zagorodnyaya & al., 2003 (p.52); Shushkina & al., 2004 (p.524, tab.2); Eiane & Ohman, 2004 (p.183, mortality rates); Rawlinson & al., 2005 (p.205, tidal exchange); Veistheim & al., 2005 (p.382, tab.2, fig.1); ? Ashjian & al., 2005 (p.1380: tab.2); Dzierzbicka-Glowacka, 2005 (p.19, modelisation: population dynamic); Uriarte & Villate, 2005 (p.863, tab.I); Kiørboe & al., 2005 (p.117, mating behaviour); Marques & al., 2006 (p.297, tab.III); De Olazabal & al., 2006 (p.966); Deibel & Daly; 2007 (p.271, Table 1, Rem.: Arctic polynyas); Albaina & Irigoien, 2007 (p.435: Tab.1); Chojnacki & al., 2007 (p.38, Table 2); Valdés & al., 2007 (p.103: tab.1); Busatto, 2007 (p.26, Tab.3); Stegert & al., 2007 (p.214); Moll & Stegert, 2007 (p.35, modelling); Titelman & al., 2007 (p.1023, Table I, mate-behaviour); Iversen & Poulsen, 2007 (p.79, coprophagy, clearance, ingestion); Isinibilir & al., 2008 (p.745: Tab.1); Cabal & al., 2008 (p.289, Table 1); Kiørboe, 2008 (p.155, Table 2, swimming speed); Selifonova & al., 2008 (p.305, Tabl. 2); Shmeleva & al., 2008 (p.31, Table 1); Calliari & Tiselius, 2009 (p.111); Brylinski, 2009 (p.253, Tab.1); Stegert & al., 2009 (p.1, population dynamics); Eloire & al., 2010 (p.657, Table II, temporal variability); Drif & al., 2010 (p.159, Rem.: egg production); Hirst & al., 2010 (p.2193, sex ratio); Zenetos & al., 2010 (p.397); Mazzocchi & Di Capua, 2010 (p.425); Dvoretsky & Dvoretsky, 2010 (p.991, Table 2); Yen & Lasley, 2010 (p.177, Rem.: p.183 as P. elongates, mating): Isinibilir, 2010 (p.233, abundance %); Ji & al., 2010 (p.1355, Rem.: p.1360); S.C. Marques & al., 2011 (p.59, Table 1); Tutasi & al., 2011 (p.791, Table 2, abundance distribution vs La Niña event); Fileman & al., 2011 (p.403, abundance vs bloom condition); Amin & al., 2011 (p.1965, clearance, feeding, egg production vs diets); Van Ginderdeuren & al., 2012 (p.3, Table 1); Zizah & al., 2012 (p.79, Tableau I); Stefanova & al., 2012 (p.403, Table 2, interannual abundance); Aubry & al., 2012 (p.125, table 3, fig. 6, 8 a, interannual variation); McGinty & al., 2012 (p.122, time series abundance); Renz & al., 2012 (p.1, life history vs population model); Gonçalves & al., 2012 (p.321, abundance vs climatic variability); Salah S. & al., 2012 (p.155, Tableau 1); Bode & al., 2012 (p.108, spatial distribution vs time-series, % biomass); Barton & al., 2013 (p.522, Table 1: metabolism, feeding mode, biogeo); Gusmao & al., 2013 (p.279, fig.2, sex ratio vs predators); ; Belmonte & al., 2013 (p.222, Table 2, abundance vs stations); Gubanova & al., 2013 (in press, Table 1, 4, fig.2); Palomares-Garcia & al., 2013 (p.1009, Table I, V, abundance vs environmental factors); in CalCOFI regional list (MDO, Nov. 2013; M. Ohman, comm. pers.); Heuschele & al., 2013 (p.399, Table 2, mate behavior); Dippner & Krause, 2013 (p.263, Rem.: p.266); Zaafa & al., 2014 (p.67, Table I, occurrence); Zakaria & al., 2016 (p.1, Table 1); Benedetti & al., 2016 (p.159, Table I, fig.1, functional characters); El Arraj & al., 2017 (p.272, table 2, spatial distribution); Hansen B.W. & al., 2017 (p.984, pH effects); Record & al., 2018 (p.2238, Table 1: diapause); Benedetti & al., 2018 (p.1, Fig.2: ecological functional group); Belmonte, 2018 (p.273, Table I: Italian zones); Camatti & al., 2019 (p.1, fig. 6, Table 3, 4); Richirt & al., 2019 (p.3, Table 1, fig.2, 3, 4, 5, abundance changes vs years 1998-2014, table 2: diversity index) | | | | NZ: | 7 + 6 doubtful | | |
|
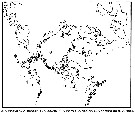
Distribution map of Pseudocalanus elongatus by geographical zones
|
| | | | | | | | | | | | | | |  issued from : B.W. Frost in Can. J. Zool., 1989, 67. [p.529, Fig.3]. issued from : B.W. Frost in Can. J. Zool., 1989, 67. [p.529, Fig.3].
Geographical distribution of Pseudocalanus spp. |
 issued from : P.S.B. Digby in J. Mar. Biol. Ass. U.K., 1950, 29. [p.398, Fig.2]. issued from : P.S.B. Digby in J. Mar. Biol. Ass. U.K., 1950, 29. [p.398, Fig.2].
Life history of Pseudocalanus elongatus at station L (5 miles from Plymouth, English Channel).
A: abundance of copepodite stages; B: percentage distribution of stages; C: size-groups of adult females; D: suggested interpretation of generations (or cohorts) succession during the year (1947).
Correlate the size variations with the water temperature at station L4 (p.397, Fig.1).
The author deduces the succession of 5 generations. |
 issued from : P.S.B. Digby in J. Mar. Biol. Ass. U.K., 1950, 29. [p.397, Fig.1]. issued from : P.S.B. Digby in J. Mar. Biol. Ass. U.K., 1950, 29. [p.397, Fig.1].
Temperature of the water at 1 and 30 m depth at station L4 (5 miles from Plymouth, English Channel) during 1947. x: surface readings from closer in-shore. |
 issued from : J.-M; Brylinski, D. Bentley & C. Quishoudt in J. Plankton Res., 1988, 10 (3). [p.507, Fig.5]. Lenth prosome histogramm (in mm) of Pseudocalanus elongatus females (F) in relation with the transect from Boulogne-sur-Mer to Dover, through the Dover Strait (stations 3, 5, 7, 10, 12). issued from : J.-M; Brylinski, D. Bentley & C. Quishoudt in J. Plankton Res., 1988, 10 (3). [p.507, Fig.5]. Lenth prosome histogramm (in mm) of Pseudocalanus elongatus females (F) in relation with the transect from Boulogne-sur-Mer to Dover, through the Dover Strait (stations 3, 5, 7, 10, 12).
Stations 3, 5 off Gris-nez cape, stations 7 and 10 in axis of the Dover Strait, station 12 off Dover.
The dotted line is arbitrary reference. Each stations equidistant. |
 issued from : S. Eriksson in ZOON, 1976, 4. [p.157, Fig.2]. issued from : S. Eriksson in ZOON, 1976, 4. [p.157, Fig.2].
Seasonal distribution of neritic copepod Pseudocalanus elongatus off Gothenburg, The Skattegatt. (Monthly means for adult specimens during 1968-1973; point : inshore, depth = 10 m; x: offshore, depth > 40 m.The species is very numerous from April to October, i.e. during the whole period when the water mass is stalilized both temperature and salinity stratifications. In concordance with this the species displays a wide optimum temperature range (4 to 17°C). This species is a spring to automn form on the Swedish west coast (the earlier mistake arising from the author erroneously judging the early occurrence of the species during 1968-69 to be normal). |
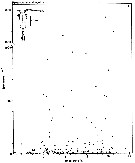 issued from : S. Eriksson in ZOON, 1976, 4. [p.158, Fig.3, a]. issued from : S. Eriksson in ZOON, 1976, 4. [p.158, Fig.3, a].
Temperature occurrence of neritic copepod adult specimens Pseudocalanus elongatus off Gothenburg (Göteborg), The Skattegatt.
.Surface salinity of the investigation area varies around 25 p.1000 and the deep water slinity around 34 p.1000. There is a temperature stratification with surface water warmer than 10°C from May to October with maximum of 20°C in August. The coldest period is January to March with surface temperatures of 1-2°C. The deep water ranges between 5 and 10°C.
The hauls were horizontal at 2, 20, and 40 m.
Limits subjectively regarded as the optimum temperature range: 4-17°C. |
 issued from : S. Eriksson in ZOON, 1973, 1. [p.50, Fig.13]. issued from : S. Eriksson in ZOON, 1973, 1. [p.50, Fig.13].
Size distribution of adult females of Pseudocalanus elongatus (offshore station H6:11°30'N, 57°40'.5 E, The Kattegatt) during 1969-70 in the main series.
|
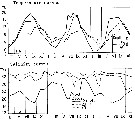 issued from : S. Eriksson in Mar. Biol., 1974, 26. [p.320, Figs. 2-3] issued from : S. Eriksson in Mar. Biol., 1974, 26. [p.320, Figs. 2-3]
Salinity and temperature curves for main series at offshore station H6 (11°30'N, 57°40'.5 E, The Kattegatt) from March 1968 to November 1970. |
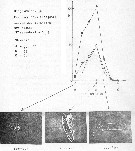 issued from : P. Martens in M.S. Inst. Meeresk. Christian-Albrechts Univ., 1972. [p.39]. issued from : P. Martens in M.S. Inst. Meeresk. Christian-Albrechts Univ., 1972. [p.39].
Numbers of fecal pellets (n) by width classes (Breite) (interval from 0.005 mm).
Nota: individuals in culture on Chaetoceros decipiens, C. socialis, Detonula cystifera, Skeletonema costatum and flagellates.
Mean size of fecal pellets: length = 143 ± 57 micrometers; width = 30 ± 8 micrometers; volume male = 120282 µ3; female = 118663 µ3 |
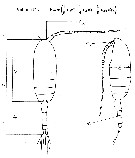 issued from : J. Chojnacki in Int. Revue ges. Hydrobiol., 1983, 68 (3). [p.436, Fig.1]. issued from : J. Chojnacki in Int. Revue ges. Hydrobiol., 1983, 68 (3). [p.436, Fig.1].
Length-weight relationship determined by geometrical method.
Lc = cephalothorax length; La = urosome length; Lan = antennula length; h : cephalothorax height; Ra = diameter of urosome cross-section; Ran = diameter of antennula cross-section.
A conversion factor of 1.025, reflecting the density (g per cm3) of the coastal Baltic water.
The correlation coefficient for the relationship studied was found to be about 0.96. |
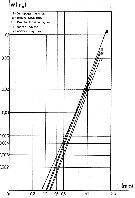 issued from : J. Chojnacki & M.M. Hussein in Zesz. nauk. Akad. Roln. Szczec., 1983, 103. [p.55, Fig.1]. issued from : J. Chojnacki & M.M. Hussein in Zesz. nauk. Akad. Roln. Szczec., 1983, 103. [p.55, Fig.1].
Total length - weight relationship in selected copepod species (copepodites I to V and adults) from the eastern sector of the Southern Baltic Sea.
Animals preserved in 4 % formalin and lengths in different copepodites and adults stages measured under stereomicroscope. Volume et weight calculated according to the simplified formula (Chojnacki & al., 1980). The results in fig. 1 present mean Lt (total length values for different developmental stades (nauplii not examined) by season and area. |
 issued from : J. Chojnacki & M.M. Hussein in Zesz. nauk. Akad. Roln. Szczec., 1983, 103. [p.55, Fig.1]. issued from : J. Chojnacki & M.M. Hussein in Zesz. nauk. Akad. Roln. Szczec., 1983, 103. [p.55, Fig.1].
Total length - weight relationship in selected copepod species (copepodites I to V and adults) from the eastern sector of the Southern Baltic Sea.
Animals preserved in 4 % formalin and lengths in different copepodites and adults stages measured under stereomicroscope. Volume et weight calculated according to the simplified formula (Chojnacki & al., 1980). The results in fig. 1 present mean Lt (total length values for different developmental stades (nauplii not examined) by season and area. |
 issued from : C.J. Corkett & I.A. McLaren in J. exp. mar. Biol. Ecol., 1969 , 3. [p.102, Table I]. issued from : C.J. Corkett & I.A. McLaren in J. exp. mar. Biol. Ecol., 1969 , 3. [p.102, Table I].
Egg production by Pseudocalanus elongatus (from outside Plymouth); in each experiment 10 dishes with 2 females in each dish were used.
Nota: Outside Plymouth, the females are not commonly found with attached eggs although these may have been detached by collecting methods. Females kept in the laboratory, in the same food and conditions used with the Halifax (Nova Scotia) populations of P. minutus, only rarely had egg sacs and masses attached ; 80 % of their eggs being found singly on the bottom of the culture dishes. Eggs had to be removed after counting, and there was the possibly that some eggs were eaten by females.
Eleven females from Plymouth kept in the laboratory and fed Isochrysis at 2 x 10 5 cells/ml of a positive correlation between female size and total numbers of egg laid in a lifetime ; it is not possible to say that the smaller number of females from Plymouth had achieved their maximum potential fecundity. The maximum number of eggs produced by a female of 0.66 mm lengthis 105 eggs, and another of 0.97 mm length produced 106 eggs. This is lower than the potential number estimated for a female from Halifax (Nova Scotia) producing 10 sacs of 18 eggs each. However, eggs from Plymouth females were 129.3 ± 1.7 µ, larger than the ± 120 µ estimated for females of the same size from Halifax. If volume corrections are taken into account, the two Plymouth females may have laid the equivalent of 7 sacs from Halifax P. minutus. |
 Issued from : N.M. Pertsova in Zool. Zh., 1981, 60 (5). [Canadian translat.Fish. Aquat. Sc, N°4826, 1982, p.9, Fig.2]. Issued from : N.M. Pertsova in Zool. Zh., 1981, 60 (5). [Canadian translat.Fish. Aquat. Sc, N°4826, 1982, p.9, Fig.2].
Seasonal changes in the average length of the cephalothorax in Pseudocalanus elongatus from Velikaya Salma in Kandalaskaya Bay (White Sea).
Nota: It should be noted that the increase in length in all stages of the immature portion of the wintered population begins simultaneously in the spring and coincides with the period of spring bloom. A sequence of attainment of maximum size in individuals of successive age stages is observed: maximum length of the cephalothorax in copepodite stages I - II is noted in May-June, in stages III - IV in June, and in stage V in July. |
 Issued from : N.M. Pertsova in Zool. Zh., 1981, 60 (5). [Canadian translat.Fish. Aquat. Sc, N°4826, 1982, p.5, Fig.1]. Issued from : N.M. Pertsova in Zool. Zh., 1981, 60 (5). [Canadian translat.Fish. Aquat. Sc, N°4826, 1982, p.5, Fig.1].
Changes in the age composition of the population of Pseudocalanus elongatus in Velikaya Salma (White Sea) ; 1 -Copepodite stage VI (adults); 2 - Copepodite stage 5; 3- Copepodite stage IV; 4 - Copepodite stage III; 5 - Copepodite stage II; 6 - Nauplii I, 7 - Nauplii (other stages).
Nota: In the White Sea, each of the determined groups of females occur in the plankton about 3 months and can deposit several consecutive clutches. A single fertilization for a female can deposit up to 9 egg secs.
According to the calculations, the duration of development of the eggs at a temperature of -1.2°C is 13 days |
 Issued from : N.M. Pertsova in Zool. Zh., 1981, 60 (5). [Canadian translat.Fish. Aquat. Sc, N°4826, 1982, p.7, Table 1]. Issued from : N.M. Pertsova in Zool. Zh., 1981, 60 (5). [Canadian translat.Fish. Aquat. Sc, N°4826, 1982, p.7, Table 1].
The relative number of males per female in P. elongatus from Velikaya Salma (White Sea) at various stages of development in 1976-1977.
Nota: Changes in the sex ratio in the population can be traced, beginning with copepodite stage IV, when the sex becomes discernible. In stages IV and V, the sex ratio during the greater part of the year (June to February) is equal, close to unity, or the number of males exceeds that of females, but not by more than 2.3 times. In spring, in April in copepodite stage IV and in May stage V, the number of males decreases sharply and becomes 10 times fewer than that females. In copepodite stage VI, the females predominate in all seasons.
The greatest number of males per female among sexually mature individuals occurs in February prior to the beginning of the propagation season, and the smallest number of males per female occurs from July to October, during which period it does not change. |
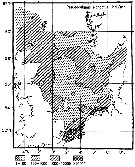 Issued from : M. Krause, J.W. Dippner & J. Beil in Prog. Oceanog., 1995, 35. [p.118, Fig.31]. Issued from : M. Krause, J.W. Dippner & J. Beil in Prog. Oceanog., 1995, 35. [p.118, Fig.31].
Horizontal distribution pattern of Pseudocalanus elongatus (individuals per m2) in the winter North Sea.
Collected by WP2-net. Numbers (ind/m3) depth-integrated, extrapoled to the bottom (but at a maximum to a depth of 500 m) and expressed as ind per m2 of water surface. |
 Issued from : T. Kiørboe in Oecologia, 2008, 155. [p.185, Table 2]. Issued from : T. Kiørboe in Oecologia, 2008, 155. [p.185, Table 2].
Grand average swimming speeds (±SD) and cephalothorax lengths of males and females of Pseudocalanus elongatus.
Nota: Experimental animals were reared in the laboratory at 16°C. |
 Issued from : J. Renz, C. Stegert, J. Peters, A. Moll & H.-J. Hirche in J. Plankton Res. , 2012, 34 (1). [p.11, Fig.9]. Issued from : J. Renz, C. Stegert, J. Peters, A. Moll & H.-J. Hirche in J. Plankton Res. , 2012, 34 (1). [p.11, Fig.9].
Spatial distribution of the secondary production of P. elongatus (mg C per m2 per day) estimated from field data (redrawn after Renz & al., 2008) (left) and from model results (right) in the German Bight (North Sea).
Nota: Sampling with oblique Bongo net hauls (150 µm size mesh) on five cruises between February and October 2004.
Based on field and model data, the annual production estimated for P. elongatus is ca. 10 g C/ m2, which indicates a contribution of at least 1/3 to the copepod production in the southern North Sea. This as well as maximum values up to 120 mg C /m2 /day in late spring and early summer.
Heath (2005) calculated an average secondary production by omnivorous zooplankton in the North Sea of 35 g C / m2 /year based on the temperature relationship of Huntley & Lopez (1992), while annual copepod production in the southern North Sea was estimated to be at least 12 g C /m2 /year in the Dutch coastal zone as opposed to 5-10 g C /m2 /year in the offshore region (Fransz & Gieskes, 1984).
The estimated secondary production of Pseudocalanus sp. Was 52 mg C /m3 /year on Georges Bank (see Davis, 1984) and > 70 mg C /m3 /year in the Gulf of Alaska (see Napp& al., 2005) being comparable with the production of large copepods such as Calanus finmarchicus or Neocalanus sp., respectively. |
 Issued from : J. Renz, C. Stegert, J. Peters, A. Moll & H.-J. Hirche in J. Plankton Res. , 2012, 34 (1). [p.12, Table IV]. Issued from : J. Renz, C. Stegert, J. Peters, A. Moll & H.-J. Hirche in J. Plankton Res. , 2012, 34 (1). [p.12, Table IV].
Generation times of P. elongatus at different mean in situ temperatures as derived from length-frequency data of females at six stations (left column) as given in Renz & al., 2008, from simulations (middle) at station 32 and from data by Klein Breteler & al., 1995 for laboratory reared at fixed temperature/food scenarios (right).
Nota: While the overall similation-derived number of generations (5-6 in the german Bight) resembled those interpreted from length-frequency data of females (4-5), the phenology of generations differed strongly.
The overwintering parameterization together with the general definition of a generation in the simulation led to a fast development of the first generation at the beginning of the year and and the first newly produced adults mid February. However, the onset of the main reproduction in February/March (see Halsband & Hirche, 2001), low food concentration until March and slow development at low temperatures (Klein Breteler & al., 1995), suggests that the first generation occurred more likely in April as also indicated by the field data. |
 Issued from : J. Renz, C. Stegert, J. Peters, A. Moll & H.-J. Hirche in J. Plankton Res. , 2012, 34 (1). [p.6, Table III]. Issued from : J. Renz, C. Stegert, J. Peters, A. Moll & H.-J. Hirche in J. Plankton Res. , 2012, 34 (1). [p.6, Table III].
Mortality rates (per day) of Pseudocalanus elongatus derived from field observations in the German Bight in 2004 (annual mean over all stations), and reported by Eiane & Ohman (2004), as well as parameter values used in Stegert & al. (2009) and this publication.
Nota : Stegert & al. (2009) used mortality values between 0.13 and 0.17, with lowest rates for adults and hifghest for older nauplii. In contrast, mortality rates from the field study were highest for stages CI/II and CV/adults (up to 0.21 per day in Table III), while CIII/IV showed lowest rates (0.07 per day). Hence, mortality rates of nauplii (0.08 per day) and young copepodids (0.07-0.21 per day) from field samples in the southern North Sea were higher than those reported by Eiane & Ohman (2004) but lower than fitted in the model simulation by Stergert & al. (2009).
As in this study adults include male and female abundance, the mortality rate was increased to reflect high male mortality. The estimated mean values from the field were therefore taken for the new parameterization, and the effects on simulated population dynamics.
|
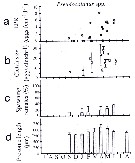 issued from : C. Halsband & H.J. Hirche in Mar. Ecol. Prog. Ser., 2001, 209. [p.223, Fig.4]. issued from : C. Halsband & H.J. Hirche in Mar. Ecol. Prog. Ser., 2001, 209. [p.223, Fig.4].
Pseudocalanus sp. (presumed represented by at least two species: P. elongatus and P. acuspes). Reproductive parameters at Helgoland Roads.
(a) Egg production rate; (b) clutch size, weekly means ± SD; (b) egg production rate; (c) proportion of females spawning; (d) prosome length. |
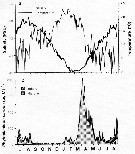 issued from : C. Halsband & H.J. Hirche in Mar. Ecol. Prog. Ser., 2001, 209. [p.221, Fig.2]. issued from : C. Halsband & H.J. Hirche in Mar. Ecol. Prog. Ser., 2001, 209. [p.221, Fig.2].
Seasonal cycles of (a) temperature and salinity at Helgoland Roads. (b) phytoplankton at Helgoland Roads. 'others' = total phytoplankton carbon - diatom carbon. Phytoplankton data smoothed by 3-point running average. |
 issued from : C. Halsband & H.J. Hirche in Mar. Ecol. Prog. Ser., 2001, 209. [p.224, Table 2]. issued from : C. Halsband & H.J. Hirche in Mar. Ecol. Prog. Ser., 2001, 209. [p.224, Table 2].
Pseudocalanus sp. : presumed represented by at least two species: P. elongatus and P. acuspes. Correlation coefficients (r2) between temperature at collection (T), prosome length (PL), clutch size (CL), egg production rate (EPR), diatom carbon (Diat), non-diatom carbon (NonDiat), and total phytoplankton carbon (PPC) at Helgoland Roads.
Clutch size = daily egg production rate of spawning females. Sample size in parentheses. Significance levels = * p<0.01; *** p < 0.0001.
|
 Issued from : L. Dzierzbicka-Glowacka, M. Kalarius & M.I. Zmijewska in Oceanologia, 2013, 55 (2). [p.421, Fig.9]. Issued from : L. Dzierzbicka-Glowacka, M. Kalarius & M.I. Zmijewska in Oceanologia, 2013, 55 (2). [p.421, Fig.9].
Horizontal distribution of the total abundance [indiv. m2] of Pseudocalanus sp. [mainly Pseudocalanus elongatus] in the Gulf of Gdansk in 2006-2007. |
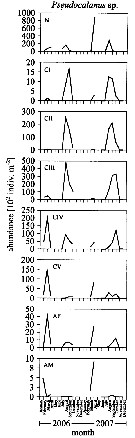 Issued from : L. Dzierzbicka-Glowacka, M. Kalarius & M.I. Zmijewska in Oceanologia, 2013, 55 (2). [p.416, Fig.4]. Issued from : L. Dzierzbicka-Glowacka, M. Kalarius & M.I. Zmijewska in Oceanologia, 2013, 55 (2). [p.416, Fig.4].
Abundance [indiv. m2] of developmental stages of Pseudocalanus sp. [mainly Pseudocalanus elongatus] at station J23 (54°32.0'N, 18*48.2'E) in the Gulf of Gdansk in 2006-2007.
AM: adult male; AF: adult female. |
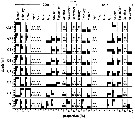
 Issued from : L. Dzierzbicka-Glowacka, M. Kalarius & M.I. Zmijewska in Oceanologia, 2013, 55 (2). [p.418, Fig.6]. Issued from : L. Dzierzbicka-Glowacka, M. Kalarius & M.I. Zmijewska in Oceanologia, 2013, 55 (2). [p.418, Fig.6].
Stage structure of Pseudocalanus sp. [mainly Pseudocalanus elongatus] at station J23 in the Gulf of Gdansk in 2006-2007 (the months without data have been omitted).. |
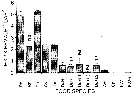
 Issued from : L. Dzierzbicka-Glowacka, M. Kalarius & M.I. Zmijewska in Oceanologia, 2013, 55 (2). [p.424, Fig.12]. Issued from : L. Dzierzbicka-Glowacka, M. Kalarius & M.I. Zmijewska in Oceanologia, 2013, 55 (2). [p.424, Fig.12].
Vertical distribution [%] of nauplii, C1 to C5 and adults (M and F) of Pseudocalanus sp. [mainly Pseudocalanus elongatus] at station J23 in the Gulf of Gdansk in 2006-2007. |
 Issued from : M. Koski, W. Klein Breteler & N. Schogt in Mar. Ecol. Prog. Ser., 1998, 170. [p.178, Fig.2]. Issued from : M. Koski, W. Klein Breteler & N. Schogt in Mar. Ecol. Prog. Ser., 1998, 170. [p.178, Fig.2].
Pseudocalanus elongatus. Egg production of individuals fed with different food species for 5 to 7 days prior maturity.
Rh: Rhodomonas sp.; Th: Thalassiosira weissflogii; Gy: Gymnodinium simplex; Am: Amphidinium sp.; Te: Tetraselmis suecica; Du: Dunaliella sp. (DuL: low dilution; DuH: high dilution rate); Ch: Chrysochromulina polypelis. |
 Issued from : W.C.M. Klein Breteler, N. Schogt, M.B.S. Schouten & G.W. Kraay in Mar. Biol., 1999, 135. [p.195, Fig.1]. Issued from : W.C.M. Klein Breteler, N. Schogt, M.B.S. Schouten & G.W. Kraay in Mar. Biol., 1999, 135. [p.195, Fig.1].
Pseudocalanus elongatus: Cumulative development time (days) of copepod life stages at 15°C using different food sources: Dunaliella sp. (Duna), Oxyrrhis marina grown on Dunaliella sp. [Ox (d)], or Oxyrrhis marina grown on Rhodomonas sp. [Ox (r)];
Egg (0), Nauplius I to VI (1 to 6), Copepodite I to V (7 to 11) and adult (12) stages.
First data point obtained from Klein breteler et al. (1994) at constant conditions of a mixed food used in stock cultures.
Dunaliella sp.: chliorophycean; Oxyrrhis sp.: heterotrophic dinoflagellate. |
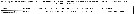 Issued from : H. Bi, K.A. Rose & M.C. Benfield in Mar. Ecol. Prog. Ser., 2011, 427. [p.157, Table 5]. Issued from : H. Bi, K.A. Rose & M.C. Benfield in Mar. Ecol. Prog. Ser., 2011, 427. [p.157, Table 5].
Instantaneous mortality rates (individuals per day) from litterature. |
 Issued from : G.A. Deevey in Bull. Bingham Oceanogr. Coll., 1960, 17 (2); [p.76, Table IX]. Issued from : G.A. Deevey in Bull. Bingham Oceanogr. Coll., 1960, 17 (2); [p.76, Table IX].
Correlation coefficients for mean cephalothorax lengths for Pseudocalanus elongatus with the temperature at sampled and averaged for the previous month from the Rnglish Channel data from Digby, 1950) and the North Sea (Kattegat) (from Adler & Jespersen, 1920). |
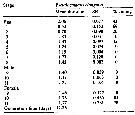 Issued from : W.C.M. Klein Breteler, N. Schogt & J. van der Meer in J. Plankton Res., 1994, 16 (8). [p.1045, Table I]. Issued from : W.C.M. Klein Breteler, N. Schogt & J. van der Meer in J. Plankton Res., 1994, 16 (8). [p.1045, Table I].
The mean duration (days), its standar error (SE) and the among-cultures variance (% of the total variance) of the life stages Pseudocalanus elongatus cultured at 15°C and excess food.
Negative values of among-cultures variance are indicated by 0.
Nauplii (1-6) and copepodites (7-11).
The summed duration of females (generation time.br>
Nota: individuals from the North Sea (Texel, Netherlands Institute for Sea Research).
The brood was raised to maturity at excess food from continuous cultures of Rhodomonas sp. (4.8-8.5 µm ESD) and Isochrysis galbana (3.6-7.0 µm ESD). The heterotrophic dinoflagellate Oxyrrhis marina (10.4-19.0 µm) lived together with the copepods in the culture tank. |
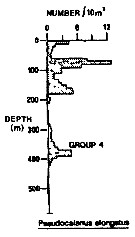 Issued from : R.K. Pipe & S.H. Coombs in J. Plankton Res., 1980, 2 (3). [p.230, Fig.4]. Issued from : R.K. Pipe & S.H. Coombs in J. Plankton Res., 1980, 2 (3). [p.230, Fig.4].
Pseudocalanus elongatus from Wyville Thomson Ridge (60°03.4' N, 07°03.9' W to 60°08' N, 07°02.9' W) on April 1978: Vertical distribution.
Members of this group (Group 4) includes four species confined to the upper 420 m, that is within North Atlantic Oceanic water, in temperatures between 8.0-8.7°C. There were two peaks in the vertical distribution of this group; the first in the upper 180 m and the second between 240-420 m.
The group 4 includes Sagitta serratodontata, Munida sp. and Maurolicus muelleri.
A computed coefficient of dissimilarity was used in association with a 'group-average' clustering strategy (see Clifford & Stephenson, 1975) to classify the plankton taxa on the basis of their vertical distribution after the indices of similarity and the results arranged diagramatically in the form of a dendogram (Fig.2). Classification was carried out using the Bray-Curtis measure which gives a coefficient of dissimilarity.
Compare with Metridia lucens, Oncaea conifera (= Triconia c.), Scolecithricella minor, Spinocalanus abyssalis. |
 issued from : M.R. Landry & V.L. Fagerness in Bull. mar. Sci., 1988, 43 (3). [p.521, Table 4]. issued from : M.R. Landry & V.L. Fagerness in Bull. mar. Sci., 1988, 43 (3). [p.521, Table 4].
Mean horizontal (H) and vertical (V) components of swimming velocity for naupliar and copepodid 1 stages of Pseudocalanus sp. (as figured to P. elongatus for information, several species of this genus in the NE Pacific, see chart in zone 24). See also to Calanus pacificus, Acartia clausi. |
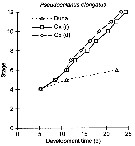
| | | | Loc: | | | Congo (in Marques, 1966), ? Chesapeake Bay, ? Woods Hole, ? G. of Maine, Mauritania-Morocco, Cap Ghir (Morocco), Portugal, Mondego estuary, off Coruña, Bilbao & Urdaibai estuaries, Bay of Biscay, Arcachon Bay, Gironde estuary, Belon estuary, W Norway (Oslo fjord, Raunefjorden: all the year), S Iceland, Scotland, Firth of Forth, Moray Firth, W Ireland, off SW Ireland, Celtic Sea, Lough Hyne, Galway Bay, Bristol Channel, W Norway , Bergen, Skagerrak, Gullmar Fjord, Kattegat, Kosterfjorden, Wyville Thomson Ridge, Arct. (polar Basin, Nansen Basin), White Sea, Kandalaksha Bay, Barents Sea (rare), English Channel, North Sea, Texel, Roscoff, Morlaix estuary, Plymouth, Granville, Southampton, Pas de Calais, North Sea, Dogger Bank, Kiel Bight, Texel, Baltic Sea, off W Tangier, Medit. (Baleares, Taranto, Adriatic Sea, Venice, Po delta, G. of Trieste, Malta, Aegean (coast), Marmara Sea, Black Sea, Sebastopol, Black River estuary, W Egyptian coast, Alexandria), G. of Suez, SE Indian (in Chiba & al., 1957 a, p.11), ? China Seas ( East China Sea), ? Korea, ? Japan Sea, Gulf of California (in Palomares-Garcia & al., 2013), W Baja California, Galapagos-Ecuador, ? Pacif. (sub-Arct.) | | | | N: | 217 ? | | | | Lg.: | | | (38) F: 1,3-1,25; (47) F: 1,63-1,18; M: 1,36-1,25; (65) F: 1,4; M: 1,1; (122) F: 1,77-1,11; M: 1,37-0,97; (354) F: 1,31-0,91; M: 1,2-0,91; (449) F: 1,4; M: 1,1; (796) F: 1,77-1,2; (1302) F: 1,062-0,745; M: 0,837-0,646; (1123) F: 1,05-0,82; M: 1,354-0,514; {F: 0,745-1,770; M: 0,510-1,370} | | | | Rem.: | epi-mesopelagic. 0-420 m (Pipe & Coombs, 1980, Arctic: 60°N, 07°E).
A confusion exists in the literature between the various forms of Pseudocalanus. It is difficult to specify the geographic locations, and in particular those concering the north American coasts (see Bigelow, 1926, p.275 & foll.) and the Pacific (sub-antarctic, China, Korea).
Razouls (1965) observes at Roscoff all year round some female individuals having a rough-shaped P5, as Mrazek had already noted (1902, p.507); for Catley (1948, p.937) there is question of a male individual emasculated by a parasite (Blastodinium).
Ciszewski & Witek (1977) in Gdansk Bay (t°C: 3-7) find a daily ratio P/B = 0.1-0.5 d-1 and an annual P/B yr-1 = 58.
Greze & al. (1968), nearshore in the Black Sea, find a daily ratio P/B = .16 d-1 and an annual P/B yr-1 = 31.
Characteristics according to Frost (1989): See limits of lenght measurements in the figure of the genus Pseudocalanus
Female: frontal part in lateral view rounded anteriad of base of rostrum; mediodorsal surface of one or more of U1-U3 with sensillum; mediodorsal surface of U1-U3 with integumental pore; seminal receptacle small; cephalosome in lateral view strongly tapered anteriorly; ratio sizes Prosome:Urosome = 2,18 (2.03-2.31); ratio lenght:width of U3 = 1.18 (1.02-1.26); ratio lenght of Furca:width of U3 = 1.00 (0.95-1.06).
Male: ratio sizes of P4 B1:B2 < 1.5; ratio sizes of segments of P4 (8-12):(20-21) < 2.0; mediodorsal surface of one or more of U2-U4 with sensillum; ratio sizes U2:U4 < 1,37; ratio sizes Cephalosome:U2 < 5.07; Furca ratio lenght:width < 1.50; length of anterodorsal sensillum ? 15 microns; ratio sizes Cephalosome: U2 = 5,34 (5.13-5.74); ratio sizes U2:U4 = 1.24 (1.13-1.35); ratio Furca length : width = 1.40 (1.31-1.48).
The feeding mode after Barton & al. (2013, Table 1) is 'feeding current' (hovering) (See definition in Rem. Centropages typicus).
After Benedetti & al. (2018, p.1, Fig.2) this species belonging to the functional group 7 corresponding to small filter feeding herbivorous. | | | Last update : 28/10/2022 | |
| | | | Pseudocalanus elongatus was found in the North Aegean Sea (Siokou-Frangou,I. (1985) Sur la presence du copepode calanoide Pseudocalanus elongatus (Boeck) en mer Egee du Nord. Rapp.Comm.int. mer Medit. 29(9): 231-233.) | |
|
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2025. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed December 29, 2025] © copyright 2005-2025 Sorbonne University, CNRS
|
|
 |
 |





































