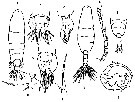|
|
 |
|
Calanoida ( Order ) |
|
|
|
Diaptomoidea ( Superfamily ) |
|
|
|
Acartiidae ( Family ) |
|
|
|
Paracartia ( Genus ) |
|
|
| |
Paracartia latisetosa (Kriczagin, 1873) (F,M) | |
| | | | | | | Syn.: | Dias latisetosus Krizagin, 1873; Karavaev, 1893 (1894) (p.8, figs.F);
Acartia verrucosa Thompson, 1888 d (p.141, figs.F,M); Giesbrecht, 1892 (p.507, 523, 770, figs.F,M); Rose, 1925 (p.305); 1926 (p.47); 1926 g (p.222); 1926 h (p.251); Rose & Vaissière, 1952 a (p.124);
Acartia ( Paracartia ) latisetosa : Pesta, 1920 (p.547); Steuer, 1923 (p.16, figs.F,M); Gurney, 1927 (p.156, fig.M, Rem.); Steuer, 1929, p.505, Rem); Rose, 1933 a (p.273, figs.F,M); Sewell, 1948 (p.441, 472); Belmonte, 1992 (p.363, Table 1, fig.1: eggs, diapause);
Acartia latisetosa : Karavaev, 1894 (1895) (p.30, figs.M); Giesbrecht & Schmeil, 1898 (p.156, Rem.); Rose, 1925 (p.152); 1925 e (p.305); Sars, 1925 (p.362); Massuti Alzamora, 1942 (p.111); Cannicci, 1962 (p.349, Table 3); Blanc & al., 1967 (p.50); Champalbert, 1969 a (p.612); El-Maghraby & Dowidar, 1970 (p.81); Dowidar & El-Maghraby, 1970 (p.267); Salah, 1971 (p.320); Della Croce & al., 1972 (p.1, Rem.); Crisafi & Crescenti, 1972 (1974) (p.229, figs.F,M, Rem. juv.); Desgouille, 1973 (p.1, 131, Rem.: p.138); Guglielmo, 1973 (p.399); Comaschi Scaramuzza, 1978 (p.16); Vaissière & Séguin, 1980 (p.23, tab.1); Kovalev & Shmeleva, 1982 (p.85); Moraitou-Apostolopoulou, 1985 (p.303, occurrence/abundance in E Mediterranean Sea); Ceccherelli & al., 1987 (p.571, fig.5); Jouffre & al., 1991 (p.489, lagoon); Fanelli & al., 1992 (p.295, egg diapause); Patriti & al., 1984 (tab.1); Garcia-Rodriguez, 1985 (p.37, fig.4); Lam Hoi & al., 1985 (p.451, 460); Comaschi Scaramuzza, 1987 (tab.1); Lakkis, 1990 (p.63); 1994 (p.481); Madhupratap & al., 1996 (p.77, table 2: resting eggs); Marcus, 1996 (p.143); Hure & Krsinic, 1998 (p.103); Mauchline, 1998 (tab.40); Siokou-Frangou, 1999 (p.478); El-Serehy & al., 2001 (p.116, Table 1: abundance vs transect in Suez Canal); Bressan & Moro, 2002 (tab.2); Zerouali & Melhaoui, 2002 (p.91, Tableau I); Zagorodnyaya & al., 2003 (p.52); Daly Yahia & al., 2004 (p.366, fig.4, tab.1); Lakkis & al., 2005 (p.152); Gubanova, 2005 (p.146); Khelifi-Touhami & al., 2007 (p.327, Table 1); Zakaria & al., 2007 (p.52, Table 1, vs Salinity); El-Sherbiny & al., 2011 (p.837, seasonal abundance); El-Serehy & al., 2013 (p.2099, Rem.: p.2101) | | | | Ref.: | | | Kiefer, 1978 d (p.59, figs.F,M); Dussart, 1982 a (p.20, figs.F,M); 1989 (p.14, 15, 115: figs.F,M); Belmonte, 1998 a (p.38, fig., Rem.: eggs); Bradford-Grieve, 1999 (n°181, p.14, figs. F,M [not figs.20 but 21]); Avancini & al., 2006 (p.119, Pl. 87, figs.F,M, Rem.); Vives & Shmeleva, 2007 (p.435, figs.F,M, Rem.); Brugnanno & al., 2009 (p.354, Rem.) | 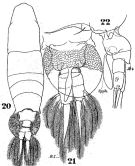 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.43, Figs.10, 21, 22]. As Acartia verrucosa. Female: 20, habitus (dorsal); 21, segment thoracique 5 and urosome (ventral); 22, idem with spermatophore (lateral left side).
|
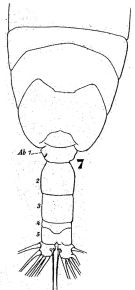 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.43, Fig.7].< As Acartia verrucosa. Male: 7, posterior thoracic segments and urosome (dorsal).
|
 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.30, Fig.3]. As Acartia verrucosa Male: 3, right A1 (distal portion).
|
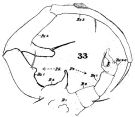 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.30, Fig.33]. As Acartia verrucosa. Male: 33, P5.
|
 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.30, Fig.10]. Female: 10, Md.
|
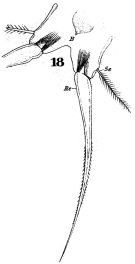 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.30, Fig.18]. Female: 18, P5.
|
 issued from : P. Crisafi & M. Crescenti in Boll. Pesca Piscic. Idrobiol., 1972 (1974), 27 (2). [p.245, Pl.V]. As Acartia latisetosa. Female (from Milazzo, Sicily): f, habitus (dorsal); f ad, posterior thoracic part and urosome (dorsal); f P5, P5. Male: m, habitus (dorsal); m ad, urosome (dorsal); m P5, P5.
|
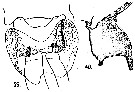 issued from : A. Steuer in Arb. zool. Inst. Innsbruck, 1923, 1 (5). [Taf.IV, Figs.39, 40]. Female: genital segment with spermatophore (ventral); 40, idem (lateral).
|
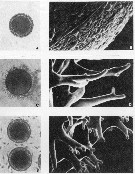 issued from : G. Belmonte in Boll. Zool., 1992, 59. [p.365, Fig.1]. Morphology of three main egg-types in Paracartia latisetosa. A, B: egg with smooth surface. C,D: spiny egg with long spines. E,F: < > egg with short spins on the surface.
A, C, E: Bar, 50 µm; light microscope photographs.B, D, F: Bar, 10 µm; SEM photographs.
Nota: The eggs of P. latisetosa are polymorphic, but it was not possible assign different reproductive meanings to all types. Eggs of three morphological types and at least two biological meanings were obtained from adult specimens under laboratory conditions.
Two types (smooth and brush-like) were subitaneous eggs. A third <> type rested for at least 50 days before hatching and was considered diapausal.
|
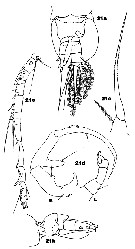 Issued from : J.M. Bradford-Grieve in ICES Identification Leaflets for Plankton N°181, 1999 [p.13, Figs.21 a-e]. Female: a, posterior last thoracic segment and urosome (ventral); b, same (lateral); c, P5. Male: d, P5 (L = left leg; R = right leg); e, distal segments of right A1.
|
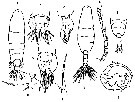 Issued from : M.G. Mazzocchi in Guida al Riconoscimento del plancton dei Mari Italiani, Vol. II, 2006. [p.95, Tav. 87]. After A. Comaschi. Female: a, habitus (dorsal); b-c, urosome (dorsal and lateral, respectively); d, same (ventral); e, P5; f, left P5. Male: g, habitus (dorsal); h, distal part of right A1; i, posterior part of prosome and urosome (dorsal); l, P5 (posterior view).
|
 Issued from : R. Gurney in Trans. zool. Soc. London, 1927, 22. [p.151, Fig. 10 F]. As Acartia latisetosa. Male (from Kabret, Suez Canal): F, P5.
| | | | | Compl. Ref.: | | | Sewell, 1948 (p.378); Gaudy, 1962 (p.93, 99, Rem.: p.115) ; Mazza, 1966 (p.72); Uysal & al., 2002 (p.17, tab.1); Kovalev, 2003 (p.47); El-Shabrawy & Belmonte, 2004 (p.151, Rem.); Pati & Belmonte, 2005 (p.250); Vaglio & al., 2005 (p.163); Smeleva & Selifonova, 2005 (p.57); Mageed, 2006 (p.168, Table 4); De Olazabal & al., 2006 (p.964, Tab.1); Belmonte & Pati, 2007 (p.39, hatching rate, eggs diapause); Drira & al., 2010 (p.145, Tanl.2); Mazzocchi & Di Capua, 2010 (p.423); Mazzocchi & al., 2011 (p.1163, fig.6, long-term time-series 1984-2006); Mazzocchi & al., 2012 (p.135, annual abundance 1984-2006); Boyer & al., 20012 (p.2, fig.2, abundance & annual change, Rem.); Annabi-Trabelsi & al., 2012 (p.445, egg production vs environmental conditions); Rekik & al., 2012 (p.336, Table 1, abundance); Gubanova & al., 2013 (in press, Rem.: p.4, Table 1, fig.2); Belmonte & al., 2013 (p.222, Table 2, abundance vs stations); Terbiyik Kurt & Polat, 2013 (p.1163, Table 2, seasonal distribution); Pansera & al., 2014 (p.221, Table 2, abundance); Benedetti & al., 2016 (p.159, Table I, fig.1, functional characters); Baumgartner & Tarrant, 2017 (p.387, resting eggs); ; Belmonte, 2018 (p.273, Table I: Italian zones); Camatti & al., 2019 (p.1, fig. 3, 9, 10, Table 5, Rem.) | | | | NZ: | 3 | | |
|
Distribution map of Paracartia latisetosa by geographical zones
|
| | | | | | | | |  Chart of 1996 Chart of 1996 | |
 Issued from : G. Belmonte & A.C. Pati in J. Plankton Res., 2007, 29 (1). [p.141, Fig.1]. Issued from : G. Belmonte & A.C. Pati in J. Plankton Res., 2007, 29 (1). [p.141, Fig.1].
Types and number of eggs produced during the investigation period by reared females of Paracartia latisetosa from Acquatina brackish water lake (Italian coast of the Adriatic Sea); From after 20 January 2004 to 4 May 2004 (excluded) sampling was not carried out. From 23 November 2004 to 28 December 2004, the population of P. latisetosa was absent from the water column.
Nota: During the whole investigation period, 329 females were reared, 229 of them (69.6%) produced eggs. A total of 1037 eggs were obtained. The average egg production was 3.15 ± 3.11 eggs by female and by day (4.52 ± 2.77 eggs fecund by female and by day).
Egg production varied considerably during the year (from 0.45 ± 1.06 eggs/female/day in May 2004 to 6.5 ± 2.12 eggs/female/day in August 2004).
Three morphological types of eggs were produced (in Table1). Both smooth and brush eggs which did not hatch were observed to be discoloured and to disintegrate in a short time; these were presumed to be unfertilized or non viable (abortive). The majority of both smooth and brush eggs hatched during the first week of incubation; so they were considered to be subitaneous eggs. UInstead, hatching of spiny eggs occurred after an incubation of at least 54 days, thus these eggs were considered to be diapausal.
Diapause eggs were produced only in Autumn (September-early January), before the species disappears from the water column. In both the sampling periods, the production of diapause eggs occurred under a short-day regime, that is when the photoperiod varied from about 12L : 12D (September) to 9L : 15D (early January). The peak of diapause egg production occurred in October.
The conversion in the production of eggs types (i.e. from subitaneous to diapause eggs) was never complete, it did not affect all the females of the same generation, and both egg types were present even in the same clutch. |
 Issued from : G. Belmonte & A.C. Pati in J. Plankton Res., 2007, 29 (1). [p.142, Fig.2]. Issued from : G. Belmonte & A.C. Pati in J. Plankton Res., 2007, 29 (1). [p.142, Fig.2].
Typologies of females reared depending on produced eggs. From after 20 January 2004 to 4 May 2004 (excluded) sampling was not carried out. From 23 November 2004 to December 2004, the population of P. latisetosa |
 Issued from : G. Belmonte & A.C. Pati in J. Plankton Res., 2007, 29 (1). [p.145, Fig.5]. Issued from : G. Belmonte & A.C. Pati in J. Plankton Res., 2007, 29 (1). [p.145, Fig.5].
Percentage of diapause eggs hatched during different 15-day periods of incubation. |
 Issued from : G. Belmonte & A.C. Pati in J. Plankton Res., 2007, 29 (1). [p.145, Fig.6]. Issued from : G. Belmonte & A.C. Pati in J. Plankton Res., 2007, 29 (1). [p.145, Fig.6].
Linear regression analysis between dormancy, days of diapausal eggs and photoperiod for the day of their production.
Sanpling dates are reported between brackets.
Regression analysis shows that the diapause duration is positively correlated with photoperiod length at the time of collection of the adults. |
 Issued from : M.G. Mazzocchi, L. Dubroca, C. Garcia-Comas, I. Di Capua & M. Ribera d'Alcalà in Progr. Oceanogr., 2012, 97-100. [p.143, Fig.10]. Issued from : M.G. Mazzocchi, L. Dubroca, C. Garcia-Comas, I. Di Capua & M. Ribera d'Alcalà in Progr. Oceanogr., 2012, 97-100. [p.143, Fig.10].
Long-term variability of the abundance (Ind. m3) of rare copepod species that disappeared in the second period of the time-series at Station MC (Gulf of Naples), by vertical tows in the upper 50 m. |
| | | | Loc: | | | Atlant.: Mauritania (in Rose, 1933 a), Medit. (Ras Kebdana, Bay of Algiers, SE Spanish : Laguna Mar Menor, Baleares; Thau lagoon, Marseille (very rare), Toulon Harbour, Genova Harbour, W Sardinia, Tyrrhenian Sea, G. of Naples, Brindisi laggon, Lake Faro (Sicily), Milazzo, Tunisia, El Kala, Bay of Tunis: lagoon, G. of Gabès, Sfax, G. of Taranto, Adriatic Sea (Venice, Lago Acquatina, Po delta, G. of Trieste), Salento coasts, Malta, G. of Saronikos, Aegean Sea, NW Lebanon Basin, Iskenderun Bay, Lebanon, Alexandria, Bardawill Lagoon, Black Sea, Sevastopol Bay, see in Remarks), Azov Sea, Port Said Harbour, Suez Canal, Lake Timsah, Madagascar: Tulear (in Dussart, 1982) | | | | N: | 78 | | | | Lg.: | | | (46) F: 0,93-0,89; M: 0,91-0,87; (164) F: 0,91-0,819; M: 0,845-0,806; (168) F: 0,951-0,781; 0,923-0,766; 0,951-0,766; 1,109-1,01; M: 0,908-0,795; 0,888-0,752; 0,908-0,781; 1,055-1,01; (325) F: 1,23-0,82; M: 1,2-0,81; (449) F: 0,93-0,89; M: 0,91-0,87; (791) F: 1-0,9; M: 1-0,9; {F: 0,766-1,230; M: 0,750-1,200} | | | | Rem.: | Coast, littoral lagoons. Euyhaline
Species is apparently strongly oligohaline.
Certain authors (Rose, 1925 e, p. 305) identify as different the two species verrucosa and latisetosa and do not follow the synonymy established by Giesbrecht & Schmeil (1898).
For Gurney (1927, p.156), this species is a purely Mediterranean and Black Sea littoral form wich is distinctly euryhaline, and may occur in water which is almost fresh, as for instance, in Garaa Ackeul (NE Tunisia). It is abundant in Lake Menzaleh and also in the channel leading to Port Said.
After Gubanova & al. (2013, p.4) the species was bundant on the coastal areas of the Black Sea but now disappeared completely from the Black Sea in relation with strong eutrophisation and pollution in the coastal areas. It can be supposed that A. tonsa replaced the native P. latisetosa, because these species occupied the same ecological niche, but the alien A. tonsa was apparently more resistant to eutrophisation. and pollution than P. latisetosa. | | | Last update : 17/09/2020 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed February 01, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |