|
|
 |
|
Calanoida ( Order ) |
|
|
|
Eucalanoidea ( Superfamily ) |
|
|
|
Eucalanidae ( Family ) |
|
|
|
Subeucalanus ( Genus ) |
|
|
| |
Subeucalanus pileatus (Giesbrecht, 1888) (F,M) | |
| | | | | | | Syn.: | Eucalanus pileatus Giesbrecht,1888; 1892 (p.132, 151, 772, figs.F,M); Giesbrecht & Schmeil, 1898 (p.21, Rem.); Thompson & Scott, 1903 (p.233, 242); Cleve, 1904 a (p.189); A. Scott, 1909 (p.21); Wolfenden, 1911 (p.204); Sewell, 1912 (p.353, 357); 1914 a (p.202); 1929 (p.51); Tanaka, 1935 (p.148, figs.F,M); Sewell, 1947 (p.47); C.B. Wilson, 1950 (p.211); Sewell, 1951 (p.369, fig.F, Rem.: parasites); Tanaka, 1956 (p.270); Grice, 1962 (p.181, figs.F, Rem.); Grice & Hart, 1962 (p.287, table 3); Vervoort, 1963 b (p.92, figs.F,M, Rem.); Ahlstrom & Thrailkill, 1963 (p.57, Table 5, abundance); De Decker, 1964 (p.15, 22, 28); Neto & Paiva, 1966 (p.19, Table III, annual cycle); Dawson, 1966 (p.176); Mazza, 1967 (p.80, figs.F,M, juv., p.321, fig.64); Fleminger, 1967 a (tabl.1); Matthews, 1968 a (p.35, Rem.); Itoh, 1970 a (p.4: tab.1); Park, 1970 (p.474); Deevey, 1971 (p.224); Timonin, 1971 (p.281, trophic group); Bainbridge, 1972 (p.61, Appendix Table I: vertical distribution vs day/night, Table II: %, Table IV); Binet & al., 1972 (p.71); Marques, 1973 (p.236); Björnberg, 1973 (p.299, 386); Fleminger, 1973 (p.969, 978, 979, 983, 999, figs.F); 1975 (p.395); Greenwood, 1976 (p.12, figs.F,M); Tranter, 1977 (p.596); Carter, 1977 (1978) (p.35); Paffenhöfer & Knowles, 1978 a (p.143, feeding); Binet, 1979 (p.400, 401, fig.2); Dessier, 1979 (p.64, 201, 204); Paffenhöfer & Knowles, 1979 (p.35, fecal pellets); Koehl & Strickler, 1981 (p.1062, food capture vs current); Zheng & al., 1982 (p.19, figs.F,M); Arashkevich & al., 1982 (p.477, Table 2, diet); Schnack, 1982 (p.145, fig.Md); Paffenhöfer & al., 1982 (p.193, feeding); Gardner & Paffenhöfer, 1982 (p.725, nitrogen ingestion/eccretion); Vives, 1982 (p.290); Zheng & al., 1982 (p.193, figs.F,M); Dessier, 1983 (p.89, Tableau 1, Rem., %); Price & al., 1983 (p.116, feeding); 1984 (p.35, feeding); Guangshan & Honglin, 1984 (p.118, tab.); De Decker, 1984 (p.315, 348: chart); Paffenhöfer & Gardner, 1984 b (p.505, nitrogen release; Stephen, 1984 (p.161, 169, Distribution vs thermocline & geographic); Paffenhöfer, 1984 c (p.75, feeding); Strickler, 1984 (p.187, feeding behavior); Kimmerer & al., 1985 (p.428); Brenning, 1985 a (p.23, Table 2); Sazhina, 1985 (p.34, figs.N); Longhurst, 1985 (tab.2); Paffenhöfer & Van Sant, 1985 (p.55, feeding): Price & Paffenhöfer, 1985 (p.115, Table 1, feeding); Brinton & al., 1986 (p.228, Table 1); Valentin & al., 1986 (p.117, Table V); Madhupratap & Haridas, 1986 (p.105, tab.1); Ambler, 1986 a (p.957, fig.7, selectivity ); Dagg & Walser, 1986 (p.1066, Chl.a concentration vs. fecal pellets); Diouf & Diallo, 1987 (p.260); Ayukai, 1987 a (p.584: Rem., feeding); Huntley, 1988 (p.83, Table 1, feeding history); Lozano Soldevilla & al., 1988 (p.57); Jimenez-Perez & Lara-Lara, 1988; Dessier, 1988 (tabl.1); Tester & Turner, 1989 (p.231, feeding rate); Turner & Tester, 1989 (p.21, grazing); Schnack, 1989 (p.137, tab.1, fig.6: Md); Paffenhöfer & Lewis, 1989 (p.129, feeding behavior of nauplii); 1990 (p.933, feeding behavior); Hirakawa & al., 1990 (tab.3); Suarez & al., 1990 (tab.2); Suarez & Gasca, 1991 (tab.2); Suarez, 1992 (App.1); Samba Diouf, 1991 (p.104); Bundy & Paffenhöfer, 1993 (p.3, figs.F); Paffenhöfer, 1994 (p.617, feeding, fecal pellts); Lopes, 1994 (tab.1); Shih & Young, 1995 (p.69); Paffenhöfer & al., 1996 (p.1699, motion behavior); Padmavati & Goswami, 1996 a (p.85, fig.2, 3, vertical distribution); Ramaiah & Nair, 1997 (tab.1); Noda & al., 1998 (p.55, Table 3, occurrence); Achuthankutty & al., 1998 (p.1, Table 2, seasonal abundance vs monsoon); Lopes & al., 1998 (p.195); Mauchline, 1998 (tab.21, 24, 25, 47, 56, 63); Smith S. & al., 1998 (p.2369, Table 6, moonsoon effects); Neumann-Leitao & al., 1999 (p.153, tab.2); Fernandez-Alamo & al., 2000 (p.1139, Appendix); Paffenhöfer & Loyd, 2000 (p.171, fig.F); Lavaniegos & Gonzalez-Navarro, 1999 (p.239, Appx.1); Lopes & al., 1999 (p.215, tab.1); Madhupratap & al., 2001 (p. 1345, vertical distribution vs. O2, figs.4, 5: clusters, p.1353); Beaugrand & al., 2002 (p.179, figs.5, 6); Rezai & al., 2004 (p.489, tab.2); Krumme & Liang, 2004 (p.407, tab.1); Frangoulis & al., 2005 (p.254, Table I: C/N/P fecal pellet composition); Smith & Madhupratap, 2005 (p.214, tab.4); Miller C.B., 2005 (p.132, feeding); Morales-Ramirez & Suarez-Morales, 2008 (p.519); Pagano, 2009 (p.115); Ferrari & Dahms, 2007 (p.34, Rem. N, p.63: Rem.); Fazeli & al., 2010 (p.153, Table 1); Maiphae & Sa-ardrit, 2011 (p.641, Table 2); Patonai & al., 2011 (p.1146, fecal pellet vs sinking velocities); Naz & al., 2012 (p.61, Table 4, relative abundance); Köster & Paffenhöfer, 2013 (p.323, fecal pellets vs O2 consumption); Chiba S. & al., 2015 (p.968, Table 1: length vs climate);
E. monachus : Dakin & Colefax,1940 (p.96, figs.F,M);
E. saravatae Oliveira, 1945;
? E. pileatus-subcrassus : Deevey, 1960 (p.16);
E. subcrassus-pileatus : Björnberg, 1963 (p.20, Rem);
? E. subcrassus/ pileatus : Roe, 1972 (p.277, tabl.1, 2); 1972 a (p.326, Rem.) | | | | Ref.: | | | Bradford-Grieve, 1994 (p.88, 91, figs.F,M); Bradford-Grieve & al., 1999 (p.878, 912, figs.F,M); Conway & al., 2003 (p.167, figs.F,M, Rem.); Goetze, 2003 (p.2322 & suiv.); Vives & Shmeleva, 2007 (p.880, figs.F,M, Rem.); Goetze & Ohman, 2010 (p.2110, Table 1, 5, A1, Fig.8, 9, 10, biogeography); Blanco-Bercial & al., 2011 (p.103, Table 1, fig.8, 9, 10, Table A1, molecular study, phylogeny, biogeography); | 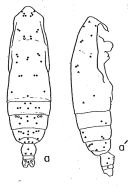 issued from : A. Fleminger in Fishery Bull. natn. Ocean. Atmos. Adm., 1973, 71 (4). [p.987, Fig.11]. As Eucalanus pileatus. Female: b, habitus (dorsal), b', idem (lateral right side).
Dorsal and lateral pattern of integumental organs (black point = sites occuring at 100% frequency, o = 80-99% frequency, x = 10-79% frequency, triangle are sites which are also visible in lateral view but which are assigned to dorsal sets);
|
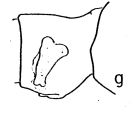 issued from : A. Fleminger in Fishery Bull. natn. Ocean. Atmos. Adm., 1973, 71 (4). [p.968, Fig.1, g (p.969)]. As Eucalanus pileatus. Female (from 08°30'N, 13°17'W): g, genital segment (lateral right side).
|
 issued from : A. Fleminger in Fishery Bull. natn. Ocean. Atmos. Adm., 1973, 71 (4). [p.968, Fig.1, f (p.969)]. As Eucalanus pileatus. Female (from 08°30'N, 13°17'W)): f, forehead (lateral right side).
|
 issued from : Q.-c. Chen & S.-z. Zhang & C.-s. Zhu in Studia Marina Sinica, 1974, 9. [Pl.1, Figs.8-9]. As Eucalanus pileatus. Female (from China Seas): 8, habitus (dorsal); 9, forehead.
|
 issued from : Q.-c. Chen & S.-z. Zhang & C.-s. Zhu in Studia Marina Sinica, 1974, 9. [Pl.2, Figs.10-14]. As Eucalanus pileatus. Female: 10, urosome (dorsal); 11, A2. Male: 13, habitus (dorsal); 14, P5.
|
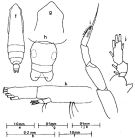 issued from : J.G. Greenwood in Proc. R. Soc. Qd, 1976, 87. [p.13, Fig.4, f-k]. As Eucalanus pileatus. Female: f, habitus (dorsal); g, forehead (dorsal); h, urosome (dorsal). Nota: Genital segment reaches maximum width in middle of segment; lateral tooth on endopodal segment 2 of P2 to P4 present but smaller than in S. subcrassusMale: i, P5; j, endopod of P4; k, Md (mandibular palp).
|
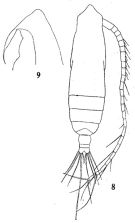
 issued from : W. Vervoort in Atlantide Rep., 1963, 7. [p.99, Fig.4, a-c]; As Eucalanus pileatus. Female (off Liberia, Monrovia): a, habitus (dorsal and lateral, respectively) (x45); c, posterior part cephalothorax and urosome (dorsal (x88)). Nota: The present material presents a considerable degree of variability in the development of the anterior part of the cephalic somite, which is composed of the fused head and the 1st pedigerous somite.. Two extreme cases have been figured; all transitional stages in respect to the development of the head are present in the material. The line of the back shows a distinct though shallow depression opposite the oral field. There is no trace of fusion between head and 1st thoracic somite. Rostrum fairly thick, sausage-shaped, with 2 fine filaments. Urosome composed of 3 parts: the genital complex (fused urosomal somites 1 and 2), the free 3rd abdominal somite and the anal complex (fused abdominal somites 4 and 5 and the caudal rami); the vaious parts have the fommowing proportional lengths: 45:17:38 = 100. The caudal rami are scarcely asymmetrical; the 2nd seta on the left side is lengthened (it reaches the length of the body). A1 23-segmented (1st and 2nd segments, 24th and 25th segments fused)
|
 issued from : W. Vervoort in Atlantide Rep., 1963, 7. [p.97, Fig.3, a-f]; As Eucalanus pileatus. Female: a-b, A1 (x135); c, Md (mandibular palp) (x135); d, Md (cutting edge) (x240); e, A2 (x135); f, Mx1 x135). Nota: A2 endopod 2-segmented; exopod 7-segmented (segments 1 and 2 fused). Mandibular palp long and slender; the fused basis and coxa are styliform and carry 3 fine setae; the endopodite is small and 2-segmented; it articulates with the basipodite in the upper part ofthat segment, its apex just reaches the base of the exopodite; on the endopodite there are 5 free exopodal segments. Mx1: praecoxal arthrite with 9 strong, setiform spines and 4 smaller spines; epipodite with 9 setae; only one of the endites is developed (apparently that ofthe basis), it carries 4 setae; the basal exite is indicated, but no seta is founded on this lobe (in such cases where a seta on the basal exite has been observed itappears to be very small, so is possible overlooked it).; there are 5 setae on the 4th segment, this segment is distinctly separate from the basis; the exopodite is small and has 5 setae in all; the segmentation of the endopodite is indistinct, but apparently 3-segmented, with 4, 4 and 5 setae on the vaious segments.
|
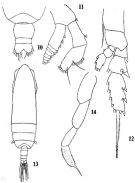
 issued from : W. Vervoort in Atlantide Rep., 1963, 7. [p.95, Fig.2]; As Eucalanus pileatus. Female: a-d, P1 to P4; e, Mx2; f, Mxp. All appendages x 135. Nota: Mx2 with praecoxa and coxa indistinctly separated and each have 2 endites, with 6, 3,
and 3 setae respectively (from the base outwards
|
 issued from : Z. Zheng, S. Li, S.J. Li & B. Chen in Marine planktonic copepods in Chinese waers. Shanghai Sc. Techn. Press, 1982. [p20, Fig.8]. As Eucalanus pileatus. Female: a, habitus (dorsal); b, forehead (ventral); d, idem (lateral); c, urosome (ventral); e, A2; f, mandibular palp of Md; g, Mx1; h, P1; i, P2. Male: j, habitus (dorsal); k, P5. Scales in mm.
|
 issued from : G.D. Grice in Fish. Bull. Fish and Wildl. Ser., 1962, 61. [p.182, Pl.3, Figs.5-12]. As Eucalanus pileatus. Female (from equatorial Pacific): 5-6, habitus (dorsal and lateral, respectively); 7-8, forehead (dorsal and ventral, respectively); 9, same (lateral); 10, urosome (dorsal); 11, posterior part of thorax and urosome (lateral, right side); 12, Mx1 (part.).
|
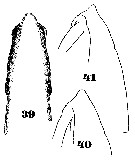 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf. 35, Figs.39, 40, 41]. As Eucalanus pileatus. Female: 39, head (dorsal); 40-41, forehead (lateral).
|
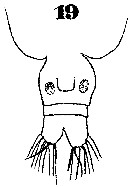 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf. 35, Fig.19]. As Eucalanus pileatus. Female: 19, urosome (ventral).
|
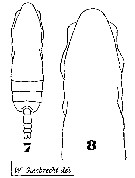 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf. 35, Figs.7, 8]. As Eucalanus pileatus. Female: 7, habitus (dorsal); 8, head (dorsal).
|
 issued from : S.B. Schnack in Crustacean Issue, 1989, 6. åp.143, Fig.6: 4]. 4, As Eucalanus pimeatus (from off NW Africa, upwelling region): Cutting edge of Md.
| | | | | Compl. Ref.: | | | Madhupratap & Haridas, 1990 (p.305, fig.6: vertical distribution night/day; fig.7: cluster); Suarez-Morales & Gasca, 1997 (p.1525); Hsieh & Chiu, 1998 (tab.2); Suarez-Morales, 1998 (p.345, Table 1); Suarez-Morales & Gasca, 1998 a (p.109); Mauchline, 1998 (tab.62); Suarez-Morales & al., 2000 (p.751, tab.1); Lopez-Salgado & al., 2000 (tab.1); Hwang & al., 2003 (p.193, tab.2); Chang & Fang, 2004 (p.456, tab.1); Gallienne & al., 2004 (p.5, tab.3); Lo & al., 2004 (p.89, tab.1); Kazmi, 2004 (p.229); Dias & Bonecker, 2005 (p.100 + poster); Waggett, 2005 (p.17, Table 3.3, behaviour); Hwang & al., 2006 (p.943, tabl. I); Sterza & Fernades, 2006 (p.95, Table 1, occurrence); Dias Araujo, 2006 (p.47, Rem., chart); Lavaniegos & Jiménez-Pérez, 2006 (p.156, tab.2, 3, Rem.; Dur & al., 2007 (p.197, Table IV); McKinnon & al., 2008 (p.844: Tab.I, p.848: Tab. IV); Neumann-Leitao & al., 2008 (p.799: Tab.II, fig.6); Waggett & Buskey, 2008 (p.111, fig.2, Table 1); Muelbert & al., 2008 (p.1662, Table 1); Magalhaes & al., 2009 (p.187, Table 1, %); Miyashita & al., 2009 (p.815, Tabl.II); Hernandez-Trujillo & al., 2010 (p.913, Table 2); Cornils & al., 2010 (p.2076, Table 3); Schnack-Schiel & al., 2010 (p.2064, Table 2: E Atlantic subtropical/tropical); Dias & al., 2010 (p.230, Table 1); Medellin-Mora & Navas S., 2010 (p.265, Tab. 2); W.-B. Chang & al., 2010 (p.735, Table 2, abundance); Magalhaes & al., 2011 (p.1520, seasonal abundance); Magris & al., 2011 (p.260, abundance, interannual variability); Cass, 2011 (p.1, oxygen consumption, excretion, composition, metabolism, lipids); Tutasi & al., 2011 (p.791, Table 1, 3, fig.7, abundance distribution vs La Niña event, Rem. p.796, fig.11); Almeida LR. & al., 2012 (p.13, Table 1, abundance); Miyashita & al., 2012 (p.1557, Table 2: occurrence); in CalCOFI regional list (MDO, Nov. 2013; M. Ohman, comm. pers.); Tachibana & al., 2013 (p.545, Table 1, seasonal change 2006-2008); Peijnenburg & Goetze, 2013 (p.2765, genetic data); Lidvanov & al., 2013 (p.290, Table 2, % composition); Bonecker & a., 2014 (p.445, Table II: frequency, horizontal & vertical distributions); Dias & al., 2015 (p.483, Table 2, abundance, biomass, production, Table 4: % vs. ceason); Hernandez-Trujillo & Esqueda Escarcega, 2016 (p.1, egg production); Araujo & al., 2016 (p.1, Table 3, abundance, %) ; Marques-Rojas & Zoppi de Roa, 2017 (p.495, Table 1); Jerez-Guerrero & al., 2017 (p.1046, Table 1: temporal occurrence); Atique & al., 2017 (p.1, Table 1); Dias & al., 2018 (p.1, Table 2: vertical distribution, abundance vs. season); Palomares-Garcia & al., 2018 (p.178, Table 1: occurrence); Acha & al., 2020 (p.1, Table 3: occurrence % vs ecoregions, Table 5: indicator ecoregions). | | | | NZ: | 15 | | |
|
Distribution map of Subeucalanus pileatus by geographical zones
|
| | | | | | | | | | | |  issued from : C. de O. Dias & A.V. Araujo in Atlas Zoopl. reg. central da Zona Econ. exclus. brasileira, S.L. Costa Bonecker (Edit), 2006, Série Livros 21. [p.47]. issued from : C. de O. Dias & A.V. Araujo in Atlas Zoopl. reg. central da Zona Econ. exclus. brasileira, S.L. Costa Bonecker (Edit), 2006, Série Livros 21. [p.47].
Chart of occurrence in Brazilian waters (sampling between 22°-23° S).
Nota: sampling 27 specimens. |
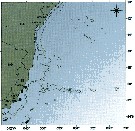
 Isseud from : E. Goetze & M.D. Ohman in Deep-Sea Res. II, 2010 (57). [p.2120, Fig.8]. Isseud from : E. Goetze & M.D. Ohman in Deep-Sea Res. II, 2010 (57). [p.2120, Fig.8].
Biogeographic distribution of Subeucalanus pileatus, a circumglobal species in eutrophic or broadly neritic waters.
This nominal species contains two distinct genetic lineages.
Solid and open circles mark sampling locations of specimens whose species identity was verified to be S. pileatus (Atlantic) or S. pileatus (Pacific/Indian), respectively, by DNA sequencing of 165 rRNA. |
 Isseud from : E. Goetze & M.D. Ohman in Deep-Sea Res. II, 2010 (57). [p.2121, Fig.10]. Isseud from : E. Goetze & M.D. Ohman in Deep-Sea Res. II, 2010 (57). [p.2121, Fig.10].
Frequencies of 165 rRNA haplotypes in Subeucalanus pileatus population samples from the Indian, Pacific and Atlantic Oceans. |
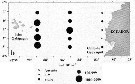 Issued from : P. Tutasi, S. Palma & M. Caceres in Scienc. Mar., 2011, 75 (4). [p.798, Fig.7 b] Issued from : P. Tutasi, S. Palma & M. Caceres in Scienc. Mar., 2011, 75 (4). [p.798, Fig.7 b]
Geographic distribution of Subeucalanus pileatus in September and October 2001, associated with the weak La Niña event of 2001. |
 Issued from : K. Patonai, H. El-Shaffey & G.-A. Paffenhöfer in J. Plankton Res., 2011, 33 (7). [p.1148, Table I (modified)]. Issued from : K. Patonai, H. El-Shaffey & G.-A. Paffenhöfer in J. Plankton Res., 2011, 33 (7). [p.1148, Table I (modified)].
Summary of results for sinking rates and estimated volumes for E. pileatus fecal pellets.
Specimens were collected outer shelf off Georgia, USA (31°20"N, 80°16'W) and placed in jars for experimentation with an average food level of 143 g C per l., and harvested after 15-22 h. |
 Issued from : A. Magalhaes, D.S.B. Nobre, R.S.C. Bessa, L.C.C. Pereira & R.M. da Costa in J. Coastal Res., 2011, SI 64. [p.1522, Fig.2]. Issued from : A. Magalhaes, D.S.B. Nobre, R.S.C. Bessa, L.C.C. Pereira & R.M. da Costa in J. Coastal Res., 2011, SI 64. [p.1522, Fig.2].
Seasonal variation in the abundance, biomass and productivity of Subeucalanus pileatus in the Taperaçu estuary (00°55'06.8''S, 46°44'00''W) in July (rainy season) and October (dry season), 2004, during spring tides (flood and ebb periods), at 3-hour intervals over 24-hour period. with a plankton net (300 µm mesh aperture). |
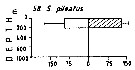 Issued from : M. Madhupratap & P. Haridas in J. Plankton Res., 12 (2). [p.313, Fig.6]. Issued from : M. Madhupratap & P. Haridas in J. Plankton Res., 12 (2). [p.313, Fig.6].
Vertical distribution of calanoid copepod (mean +1 SE), abundance No/100 m3. 58- Subeucalanus pileatus.
Night: shaded, day: unshaded.
Samples collected from 6 stations located off Cochin (India), SE Arabian Sea, November 1983, with a Multiple Closing Plankton Net (mesh aperture 300 µm), in vertical hauls at 4 depth intervalls (0-200, 200-400, 400-600, 600-1000 m). |
 Issued from : S. Hernandez-Trujillo & G.Ma. Esqueda Escarcega in CICIMAR Oceanides, 2016, 31 (1). [p.3, Table 1 & 2]. Issued from : S. Hernandez-Trujillo & G.Ma. Esqueda Escarcega in CICIMAR Oceanides, 2016, 31 (1). [p.3, Table 1 & 2].
Table 1: Egg production rate (TPH: eggs female-1 day -1 mean during Marca Roja-VII cruise in Abril 2015. n = number of experiments with 5 replicates. Superficial water temperature, salinity and chlorophyll a at 10 m depth. Min: minimum number of eggs; Max: number maximum of eggs.
Table 2: Mean length of prosome (mm), quantity of carbone (µgC), K: enviromental and physiological factor according to Froese (2006), Rennie & Verdon (2008), Blackwell & al. (2000), Trudel & al;, (2005), Hernandez-Trujillo & al. (2008, 2013). |
| | | | Loc: | | | South Africa (E), Namibia, Angola (Baia Farta), off S St. Helena Is., off Ascension Is., Congo, G. of Guinea, off Lagos, Ivorian shelf, Casamance, Dakar, Morocco-Mauritania, Canary Is., Argentina, Uruguay (continental shelf), Brazil (S, Paranagua Bay, off Rio de Janeiro, Campos Basin, Cabo Frio, Vitoria Bay, off Macaé, Guarairas Lagoon, Mucuri estuary, Camamu, off Natal, Curuça estuary, Taperaçu estuary, Caeté Estuary, off Amazon), Barbados Is., Caribbean Colombia, Bahia de Mochima (Venezuela), Caribbean Sea, Yucatan, G. of Mexico, Aransas Ship Channel, Louisiana (off Grand Isle), Mississipi (off Southwest Pass), Florida, off SE Wassaw Seabuoy (31°19'N, 80°15'W), off Savannah shelf, Georgia, off Bermuda, ? Delaware Bay (outside), Medit. (W Basin), Red Sea, Gulf of Oman, Arabian Sea, Karachi coast, Laccadive Is., Maldive Is., Rodrigues Is. - Seychelles, Natal, Bombay, Goa, Sri Lanka, Bay of Bengal, Nicobar Is., S Burma, Australia (W, North West Cape), Straits of Malacca, Indonesia-Malaysia, SW Celebes, Philippines, China Seas (East China Sea, South China Sea), Taiwan (S, SW, Kaohsiung Harbor, N: Mienhua Canyon), Kuchinoerabu Is., Japan, Tokyo Bay, Pacif. (W equatorial), Australia (Moreton Bay, Shark Bay), New Caledonia, Pacif. (equatorial), Clipperton Is., California, W Baja California, Bahia Magdalena, Gulf of California, La Paz, G. of Tehuantepec, off Guerrero coast, W Mexico, off W Guatemala, W Costa Rica, W Panama, Bahia Cupica (Colombia), Ecuador, Galapagos, off Peruvian coast, off Chile (53° S) | | | | N: | 171 | | | | Lg.: | | | (14) F: 2,5-1,8; M: 2,25-1,8; (47) F: 2,25-1,96; M: 2; (101) F: 1,94; 1,8; (125) F: 2,41-2,11; M: 2,16; (207) F: 2,09-1,83; (237) F: 2,0-2,25; M: 1,7-2,03; (336) F: 2,3-1,8; M: 2,2-1,9; (338) F: 1,85-2,25; M: 1,75-2,15; (786) F: 2,22-2,19; (1023) F: 1,97-2,20; M: 1,85; (1125) F: 2,5; {F: 1,80-2,50; M: 1,70-2,25}
Chiba S. & al., 2015 (p.971, Table 1: Total length female (June-July) = 2.2 mm [optimal SST (°C) = 15.2].
Prosome length: (1254 )F: 2.17 ± 0.19
| | | | Rem.: | Sometimes confused with S. monachus (Vervoort, 1963 b, p.100), Mazza (1967, p.81) and Roe (1972 a, p.326) underline the difficulty to distinguish this species from E. subcrassus. For Grice (1962, p.181) this species closely resembles S. subcrassus and the two may be prove to be conspecific. The females may be distinguished as follows (Pacific specimens): 1- the size; 2- shape of forehead (in dorsal view anterior end of forehead more produced in S. pileatus than in S. subcrassus; 3- shape of genital segment (S. pileatus greatest diameter at a point approximately 1/2 the length of the segment, S. subcrassus, greatest diameter in lower third of segment).
For Itoh (1970 a, fig.2, from co-ordonates) the Itoh's index value of the mandibular gnathobase = 205, and for Schnack (1989) 430.
Timonin (1971, p.282) considers the trophic interrelations in the equatorial and tropical Indian Ocean, and divides the plankters into 6 trophic groups from the litterature and the results of studies of mouth-parts structure and intestine content. This species is a coarse-filter feeder.
After Ayukai (1987 a, p.584-585), Paffenhöfer & al. (1982), Paffenhöfer & Van Sant (1985) observed that Eucalanus pileatus (= Subeucalanus p.) ingested Thalassiosira weissflogii in a active mode and noted that the filtering rate of this species on beads decreased at a rate similar to that on T. weissflogii when beads and T. weissflogii were offered in mixtures.. Also, reported that the presence of beads (18 µm diameter) did not affect the ingestion rate. Therefore it is presumed that bead interference does not occur when copepods perceive and ingest cells individually. bead interfernce, in turn, may be indirect evide,nce that copepods ingest cells in a passive mode. beads may be accumulated along with cells near the mouthpart of copepods and 'post-capture rejection' of beads seems to be required to ingest cells selectivity. As suggested by Huntley & al. (1983), the time constraint due to post-capture rejection is the possible cause of bead interference.
See in DVP Conway & al., 2003 (version 1) | | | Last update : 11/07/2022 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2025. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed December 02, 2025] © copyright 2005-2025 Sorbonne University, CNRS
|
|
 |
 |
























