|
|
 |
|
Calanoida ( Order ) |
|
|
|
Eucalanoidea ( Superfamily ) |
|
|
|
Eucalanidae ( Family ) |
|
|
|
Subeucalanus ( Genus ) |
|
|
| |
Subeucalanus subtenuis (Giesbrecht, 1888) (F,M) | |
| | | | | | | Syn.: | ? Eucalanus setiger Brady, 1883;
Eucalanus subtenuis Giesbrecht, 1888; 1892 (p.132, 150, 772, figs.F,M); Giesbrecht & Schmeil, 1898 (p.21, Rem. F,M); Thompson & Scott, 1903 (p.233, 242); Cleve, 1904 a (p.190); Esterly, 1905 (p.135, figs.F); Carl, 1907 (p.16); A. Scott, 1909 (p.21, Rem.); Wolfenden, 1911 (p.204); Sewell, 1912 (p.353, 358); 1914 a (p.200); Sars, 1925 (p.21); Farran, 1929 (p.207, 218); Mori, 1929 (p.170, figs.F); Tanaka, 1935 (p.147, figs.F,M, juv.); Mori, 1937 (1964) (p.25, Rem.); Wilson, 1942 a (p.184); Lysholm & al., 1945 (p.8); Vervoort, 1946 (p.106, Rem.); Sewell, 1947 (p.48); C.B. Wilson, 1950 (p.211); Sewell, 1951 (p.370, fig. juv.M, Rem.: parasites); Carvalho, 1952 a (p.141, figs.M); Tanaka, 1956 (p.270); Chiba al., 1957 (p.306); Fukase, 1957 (p.17, figs.F,M, Rem.); Heinrich, 1961 b (p.93); Fagetti, 1962 (p.10); Cervigon, 1962 (p.181, tables: abundance distribution); Grice, 1962 (p.181, figs.F,M, Rem.); Brodsky, 1962 c (p.109, figs.F,M); Paiva, 1963 (p.19, figs.F); Vervoort, 1963 b (p.91, Rem.); Gaudy, 1963 (p.20, Rem.); De Decker & Mombeck, 1964 (p.12); Anraku & Azeta, 1965 (p.13, Table 2, fish predator); Chen & Zhang, 1965 (p.35, figs.F,M); Furuhashi, 1966 a (p.295, vertical distribution in Kuroshio region, Table 10); Neto & Paiva, 1966 (p.19, Table III); Mazza, 1967 (p.83, figs.F,M, juv., fig.64); Fleminger, 1967 a (tabl.1); Vinogradov, 1968 (1970) (p.78); Vidal, 1968 (p.21, figs.F,M); Marques, 1968 a (p.396); Samyshev, 1970 (p.32, nutrition); Itoh, 1970 a (p.4: tab.1); Park, 1970 (p.474); Deevey, 1971 (p.224); Timonin, 1971 (p.281, trophic group); Gueredrat, 1971 (p.300, fig.2, 10, Table 1, 2); Bainbridge, 1972 (p.61, Appendix Table I: vertical distribution vs day/night); Binet & al., 1972 (p.68); Kos, 1972 (Vol.1, figs.F,M, Rem.); Heinrich, 1973 (p.95); Fleminger, 1973 (p.969, 978, 979, 982, 999, figs.F, fig.20); Björnberg, 1973 (p.297, 386); Marques, 1974 (p.12); Fleminger, 1975 (p.395); Arashkevich, 1975 (p.351, digestion time); Timonin, 1976 (p.79, vertical distribution); Geletin, 1976 (p.83, 91, Rem.); Greenwood, 1976 (p.16); Deevey & Brooks, 1977 (tab.2); Tranter, 1977 (p.596, 597, fig.1); Carter, 1977 (1978) (p.35); Timonin & Voronina, 1977 (p.285, fig.3); Dessier, 1979 (p.204); Judkins, 1980 (p.475, Rem.: p. 479); Björnberg & al., 1981 (p.621, figs.M); Arashkevich & al., 1982 (p.477, Table 2, diet); Marques, 1982 (p.753); Vives, 1982 (p.290); Dessier, 1983 (p.89, Tableau 1, 2, Rem., %); Van der Spoel & Heyman, 1983 (p.40, fig.52); De Decker, 1984 (p.315); Guangshan & Honglin, 1984 (p.118, tab.); Stephen, 1984 (p.161, 169, Distribution vs thermocline & geographic); Baars & Oosterhuis, 1985 (p.71, Table 4: gut passage time); Brenning, 1985 a (p.23, Table 2); Longhurst, 1985 (tab.2); Madhupratap & Haridas, 1986 (p.105, tab.1); Chen Y.-Q., 1986 (p.205, Table 1: abundance %, fig.2, Table 2: vertical distribution, Rem.: p.207); Jimenez-Perez & Lara-Lara, 1988; Herman, 1989 (p.247); Othman & al., 1990 (p.565, Rem.); Hirakawa & al., 1990 (tab.3); Yoo, 1991 (tab.1); Samba Diouf, 1991 (p.104); He & al., 1992 (p.250); Kim & al., 1993 (p.269); Hirakawa & al., 1995 (tab.2); Shih & Young, 1995 (p.69); Kotani & al., 1996 (tab.2); Park & Choi, 1997 (Appendix); Go & al., 1997 (tab.1); Suarez-Morales & Gasca, 1997 (p.1525); Chihara & Murano, 1997 (p.789, Pl.100,101: F,M); Padmavati & al., 1998 (p.349); Hwang & al., 1998 (tab.II); Wong & al, 1998 (tab.2); Noda & al., 1998 (p.55, Table 3, occurrence); Mauchline, 1998 (tab.23, 30); Smith S. & al., 1998 (p.2369, Table 6, moonsoon effects); Lavaniegos & Gonzalez-Navarro, 1999 (p.239, Appx.1); Dolganova & al., 1999 (p.13, tab.1); Fernandez-Alamo & al., 2000 (p.1139, Appendix); Hidalgo & Escribano, 2001 (p.159, tab.2); Hidalgo & Escribano, 2001 (p.158, fig.5); Wang & Zuo, 2004 (p.1, Table 2, dominance, origin); Rezai & al., 2004 (p.489, tab.2); Shimode & al., 2005 (p.113 + poster); Ashjian & al., 2005 (p.1380: tab.2); Ferrari & Dahms, 2007 (p.71, Rem.); Morales-Ramirez & Suarez-Morales, 2008 (p.519); Franco-Herrera & Castro, 2008 (p.361, seasonal variations in grazing); Medellin-Mora & Navas S., 2010 (p.265, Tab. 2); Johan & al., 2012 (p.647, Table 1, fig.2, salinity range); Johan & al., 2012 (2013) (p.1, Table 1); Naz & al., 2012 (p.61, Table 4, relative abundance); Jang M.-C & al., 2012 (p.37, abundance and seasonal distribution); Lidvanov & al., 2013 (p.290, Table 2, % composition);
? Eucalanus subtilis : Rosales & Sepulveda, 1992 (p.38, 46: ? lapsus calami);
Eucalanus subtenuis japonica : Fukase, 1957;
Euchaeta subtenuis : Huntley & Lopez, 1992 (p.201, Table B1, growth rate, temperature-dependent production [lapsus calami]); | | | | Ref.: | | | Geletin, 1976 (p.91); Bradford-Grieve, 1994 (p.87, 88); Lapernat, 1999 (p.16, 55); Bradford-Grieve & al., 1999 (p.878, 912, figs.F,M); Prusova, 2003 (p.62, figs.M); Conway & al., 2003 (p.169, figs.F,M, Rem.); Goetze, 2003 (p.2322 & suiv.); Mulyadi, 2004 (p.119, figs.F, Rem.); Vives & Shmeleva, 2007 (p.883, figs.F,M, Rem.); Goetze & Ohman, 2010 (p.2110, Table 1, A1, Fig.2, molecular study, biogeography) | 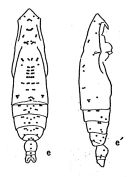 issued from : A. Fleminger in Fishery Bull. natn. Ocean. Atmos. Adm., 1973, 71 (4). [p.985, Fig.9]. As Eucalanus subtenuis. Female: e, habitus (dorsal), e', idem (lateral right side).
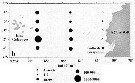
Dorsal and lateral pattern of integumental organs (black point = sites occuring at 100% frequency, o = 80-99% frequency, x = 10-79% frequency, triangle are sites which are also visible in lateral view but which are assigned to dorsal sets);
|
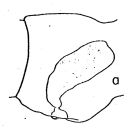 issued from : A. Fleminger in Fishery Bull. natn. Ocean. Atmos. Adm., 1973, 71 (4). [p.968, Fig.1, a (p.969)]. As Eucalanus subtenuis. Female (27°08'S, 72°02'W): a, genital segment (lateral right side).
|
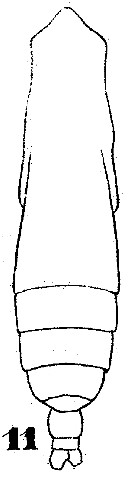 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf.35, Fig.11]; As Eucalanus subtenuis.
|
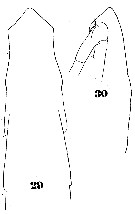 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf.35, Fig.29-30]; As Eucalanus subtenuis. Female: 29, antrior part of body (dorsal); 30, idem (lateral).
|
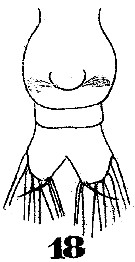 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf.35, Fig.18]; As Eucalanus subtenuis. Female: 18, urosome (ventral).
|
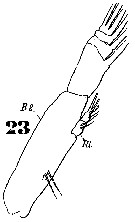 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf.11, Fig.23]; As Eucalanus subtenuis. Female: 23, Md.
|
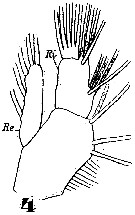 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf.11, Fig.4]; As Eucalanus subtenuis. Female: 4, Mx1 (posterior).
|
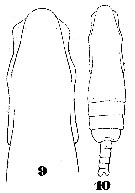 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf.35, Fig.9-10]; As Eucalanus subtenuis. Male: 9, anterior part of ephalothorax (dorsal); 10, habitus (dorsal).
|
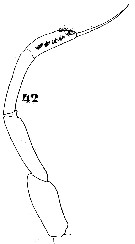 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf.11, Fig.42]; As Eucalanus subtenuis. Male: 42, P5.
|
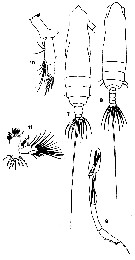 issued from : T. Mori in The pelagic Copepoda from the neighbouring waters of Japan, 1937 (2nd edit., 1964). [Pl.8, Figs.7-11]. As Eucalanus mucronatus. Female: 7, habitus (dorsal); 10, Md; 11, Mx1. Male: 8, habitus (dorsal); 9, left P5.
|
 issued from : T. Mori in Zool. Mag. Tokyo, 1929, 41 (486-487). [Pl. III, Figs.25-28]. Female (from Cosen Strait, Korea-Japan): 25, Md (mandibular palp); 26, Mx2; 27, forehead; 28, A2.
|
 issued from T. Mori in Zool. Mag. Tokyo, 1929, 41 (486-487). [Pl. IV, Figs.3-9]. As Eucalanus subtenuis. Female: 3, urosome (dorsal); 5, P1; 6, P4. Male: 4, A2; 7, P4; 8, urosome (dorsal); 9, P5.
|
 issued from : S. Fukase in J. Oceanogr. Soc. Japan, 1957, 13 (1). [p.17, Fig.1, a-c, e-g]. As Eucalanus subtenuis. Female (from warm waters of Japan): a, forehead (dorsal); b, same (lateral); c, habitus (dorsal); e, A2; f, Mx1; g, Mx2. Nota: Head and 1st thoracic segment fused. Abdomen 6.47 times in the length of prosome. Head triangular in dorsal view, lateral margins slightly concave but sometimes nearly straight; angle of the frontal margin of the head ranges from 81.6° to 94.5°, apical portion rounded. Abdomen 3-segmented, caudal rami fused with the 4th and 5th segments; proportional lengths of the urosomal segments and caudal rami 50: 18: 32 = 100. A2: 1st basal segment has a plumose seta and the 2nd basal segment has 2 setae; exopod 7-segmented, 1st and 2nd fused and 12 setae present; endopod 1.7 times as long as the exopod and consists of 2 segments, the 1st is as long as the 2nd and about 3 times longer than the width, and has 2 setae; the 2nd has 8 setae on the inner lateral margin and 1 accessory seta on the posterior surface of the base of the distal lateral seta; the distal margin of the 2nd segment has 6 terminal setae and 1 accessory seta. Mx1: 1st inner lobe with 8 long and strong spines, 5 short and stout spines, and 1 very short spine; 2nd inner lobe absent; 3rd inner lobe with 4 setae; 1st outer lobe with 9 long plumose setae and 2nd outer lobe a single short seta; 2nd basal segment with 4 setae on the inner distal margin, but some specimens have 5; exopod with 5 setae; endopod 3-segmented, the 1st and 2nd segments with each 4 setaze, the terminal segment with 5 setae. Mx2 : uniramous ; 1single plumose seta on the middle of the iouter margin of the 1st basal segment ; there are 5 distinct and 1 small lobes, the 1st lobe with 5 long and 1 short setae, 2nd, 3th and 4th lobes with each 3 setae, 5th with 3 long and 1 short setae, 6th lone with 1 seta ; endopod 3-segmented, the 1st and 2nd segments with each 1 seta, the 3rd segment with 3 setae. P5 absent.
|
 issued from : S. Fukase in J. Oceanogr. Soc. Japan, 1957, 13 (1). [p.18, Fig.2a-d]. As Eucalanus subtenuis. Female: a, Md; b, Mxp; c, P1; d, P2. Nota: Md: 1st basal segment short and 2nd basal segment has 3 setae along the internal margin; exopod with 6 setae; endopod 2-segmented, the 1st segment with 2 setae and the 2nd with 4 setae (3 long and 1 short); the apical portion of the endopod does not reach the end of the 2nd basal segment which is divided into two parts, 1:3 by the endopod; the cutting edge has 1 spine and 8 teeth. Mxp : 1st basal segment with 9 setae, 2nd basal segment with 5 setae ; endopod 5-segmented, the 1st and 2nd with each 4 setae but some specimens have each 3 setae, the 3rd segment with 3 setae, the 4th with 3 long and 1 short exteral setae, the 5th with 3 long and 1 short external setae.
|
 issued from : S. Fukase in J. Oceanogr. Soc. Japan, 1957, 13 (1). [p.18]. As Eucalanus subtenuis. Female: Proportional lengths of A1 segments. Nota: A1 23-segmented (segments 1 and 2, 8 and 9 dused, respectively), reaches back beyond the end of the caudal rami by the last 6 segments at least.
|
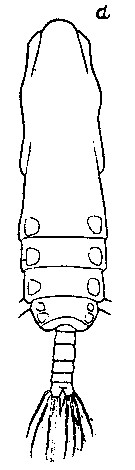 issued from : S. Fukase in J. Oceanogr. Soc. Japan, 1957, 13 (1). [p.17, Fig.1d]. As Eucalanus subtenuis. Male: d, habitus (dorsal). Nota: Head and 1st thoracic segment fused. In dorsal view the frontal margin of the head and the lateral corners of the 5th thoracic segment evenly rounded.. Abdomen 5-segmented, contained 4.83 times the length of the prosome ; proportional lengths of the urosomal segments plus caudal rami 19 : 25 : 18 : 13 : 25 (Ur5+caudal rami) = 100.
|
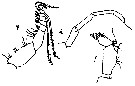 issued from : S. Fukase in J. Oceanogr. Soc. Japan, 1957, 13 (1). [p.18, Fig.2e-h]. As Eucalanus subtenuis. Male: e, A2; f, Md (mandibular palp); g, Mxp; h, P5. Nota: A2: the 1st basal segment differs from that of the female and free from inner distal seta; 2nd basal segment with 2 setae; exopod as in female; endopod 2-segmented, the 1st with only 1 seta, 2nd with 6 terminal and 8 marginal setae (as in female), of which the proximal one is very short ( while the other 2 setae which are found in the female are absent). Md: number of setae is ame as in female. Mx1 and Mx2 are similar to those of the female. Mxp: 1st and 2nd segments of the exopod with each 3 setae; the external setae of the 4th and 5th segments are longer and stronger than those of the female; one of the 3 setae of the terminal segment is short. Right P5 absent; left leg 4-segmented ( 1st and 2nd basal segments and the 1st and 2nd exopods); the terminal segment has hairs and a spibulose spine which is shorter that the terminal segment and slightly curved; the proportional lengths of the segments and the spine 21: 26: 22: 18: 13 = 100.
|
 issued from : S. Fukase in J. Oceanogr. Soc. Japan, 1957, 13 (1). [p.19]. As Eucalanus subtenuis. Male: Proportional lengths of the A1 segments. Nota: A1 23-segmented (stronger than that of the female), 1st and 2nd segments, 8th and 9th fused; reaches beyond the end of the caudal rami by the last 6 segments.
|
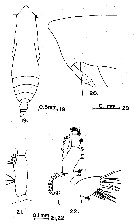 issued from : G.D. Grice in Fish. Bull. Fish and Wildl. Ser., 1962, 61. [p.179, Pl.2, Figs.19-22]. As Eucalanus subtenuis var. japonicus. Female (from equatorial Pacific): 19, habitus (dorsal); 20, forehead (lateral); 21, basal segments and endopod of Md; 22, Mx1. mandibular palpus with 3 setae Distal segment of P5 longer than the terminal spine.
|
 issued from : G.D. Grice in Fish. Bull. Fish and Wildl. Ser., 1962, 61. [p.182, Pl.3, Figs.1-4]. As Eucalanus subtenuis var. japonicus. Male: 1-2, habitus (dorsal and lateral, respectively);3, mandibular palpus; 4, P5.
|
 issued from : P.E. Lapernat & C. Razouls in Vie Milieu, 2002, 52 (1). [p.23, Pl. III, fig.1]. Masticatory edge of Md gnathobase female (from off Malta, Mediterranean Sea). Nota: The Itoh's index value = 966.9 (number of teeth: 8).
|
 issued from : Mulyadi in Published by Res. Center Biol., Indonesia Inst. Sci. Bogor, 2004. [p.119, Fig.68]. Female (from 01°30'N, 124°00'E): a, habitus (dorsal); b, cephalon (lateral); c, posterior part of thoracic segments and urosome (lateral right side); d, P1 (exopod incomplete); e, P2.
|
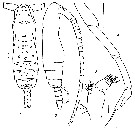 issued from : I. Prusova in Vestnik zoologii, 2003, 37 (2). [p.63, Fig.1]. Male (from Arabian Sea): 1, habitus (dorsal); 2, idem (lateral right side); 3, Md (mandibular palp); 4, P5. Scale bar: 0.1 mm. Nota: The setation on the Md palp male is different that for S. mucronatus Md palp male. Nota: Males of S. subtenuis (Giesbrecht, 1888) and S. mucronatus (Giesbrecht, 1888) were stained according to the Fleminger's method and identified based on the patterns of integumental pores of the cephalothorax and abdomen.
|
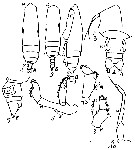 Issued from : M.C. Kos in Field guide for plankton. Zool Institute USSR Acad., Vol. I, 1972. After Brodsky, 1962. As Eucalanus subtenuis. Female; 1-2, habitus (dorsal and lateral, respectively); 3, forehead (lateral); 4, urosome (dorsal); 5, same (lateral); 6, A2; 7, Md (palp); 8, Mx2. Male: 9, habitus (dorsal); 10, P5 (left)
| | | | | Compl. Ref.: | | | Madhupratap & Haridas, 1990 (p.305, fig.6: vertical distribution night/day; fig.7: cluster); Suarez-Morales & Gasca, 1998 a (p.109); Suarez-Morales & al., 2000 (p.751, tab.1); Lapernat & Razouls, 2001 (p.123, tab.1); Hwang & al., 2003 (p.193, tab.2); Orusova, 2003 (p.61, figs.M); Hsiao & al., 2004 (p.326, tab.1); Lan & al., 2004 (p.332, tab.1); Lo & al.*, 2004 (p.218, tab.1, fig.6); Lo & al., 2004 (p.89, tab.1); Kazmi, 2004 (p.229); Prusova & Smith, 2005 (p.76); Zuo & al., 2006 (p.159, tab.1, 3, abundance, fig.8: stations group); Hwang & al., 2006 (p.943, tabl. I); Sterza & Fernades, 2006 (p.95, Table 1, occurrence); Dias & Araujo, 2006 (p.48, Rem., chart); Lavaniegos & Jiménez-Pérez, 2006 (p.157, tab.2, 4, Rem.); Escribano, 2006 (p.20, Table 1); Hwang & al., 2007 (p.24); Dur & al., 2007 (p.197, Table IV); Wishner & al., 2008 (p.163, Table 2, fig.8, oxycline); Lopez Ibarra, 2008 (p.1, Table 1, figs.11, 16: abundance, Table 3: N, C isotopes, figs.17, 18, 19, 20, 22, 24, 25, 26, Annexe 2, Table 4: index trophic); Lan Y.C. & al., 2008 (p.61, Table 1, % vs stations); Tseng L.-C. & al., 2008 (p.153, Table 2, fig.5, occurrence vs geographic distribution, indicator species); Tseng & al., 2008 (p.402, Table 2); Zhang W & al., 2009 (p.261, table 2); Lan Y.-C. & al., 2009 (p.1, Table 2, % vs hydrogaphic conditions); Escribano & al., 2009 (p.1083, Table 1, figs.6, 7, 10); C.E. Morales & al., 2010 (p.158, Table 1); Hernandez-Trujillo & al., 2010 (p.913, Table 2); Schnack-Schiel & al., 2010 (p.2064, Table 2: E Atlantic subtropical/tropical); Hidalgo & al., 2010 (p.2089, fig.4, Table 2, cluster analysis); Xu & Gao, 2011 (p.514, figs.3, 4, Table 2: optimal salinity); Hsiao S.H. & al., 2011 (p.475, Appendix I); Kâ & Hwang, 2011 (p.155, Table 3: occurrence %); Guo & al., 2011 (p.567, fig.6, abundance vs spatial distribution); Magris & al., 2011 (p.260, abundance, interannual variability); Cass, 2011 (p.1, oxygen consumption, excretion, composition, metabolism, lipids); Tseng L.-C. & al., 2011 (p.47, Table 2, occurrences vs mesh sizes); Tutasi & al., 2011 (p.791, Table 1, 3, fig.8b, abundance distribution vs La Niña event, Rem. p.797, fig.11); Lavaniegos & al., 2012 (p. 11, Table 1, fig.3, seasonal abundance, Appendix); Teuber & al., 2013 (p.28, Table 1, respiration rates); Palomares-Garcia & al., 2013 (p.1009, Table I, abundance vs environmental factors); in CalCOFI regional list (MDO, Nov. 2013; M. Ohman, comm. pers.); Tachibana & al., 2013 (p.545, Table 1, seasonal change 2006-2008); Tseng & al., 2013 (p.507, seasonal abundance); Tseng & al., 2013 a (p.1, Table 3, 4, abundance); Hirai & al., 2013 (p.1, Table I, molecular marker); Williams R., 2013 (p.71, figs., Table 3.2, 3.4, 3.6, 3.7, Appendix A, C; C & N composition, trophic level); Hwang & al., 2014 (p.43, Appendix A: seasonal abundance); Bonecker & a., 2014 (p.445, Table II: frequency, horizontal & vertical distributions); Lopez-Ibarra & al., 2014 (p.453, fig.6, biogeographical affinity); Nakajima & al., 2015 (p.19, Table 3: abundance); Jackson & Smith, 2016 (p.305, figs. 3, 4, 5, vertical abundance vs day/night cycle); Marques-Rojas & Zoppi de Roa, 2017 (p.495, Table 1); Palomares-Garcia & al., 2018 (p.178, fig.3: relative frequency, Table 1). | | | | NZ: | 18 | | |
|
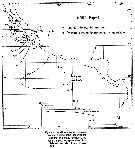
Distribution map of Subeucalanus subtenuis by geographical zones
|
| | | | | | | | | | | |  issued from : A. Fleminger in Fishery Bull., 1973, 71 (4). [p.1000, Fig.30]. As Eucalanus subtenuis. issued from : A. Fleminger in Fishery Bull., 1973, 71 (4). [p.1000, Fig.30]. As Eucalanus subtenuis.
Geographical distribution (localities shown based on records verified by analysis of integumental organs). |
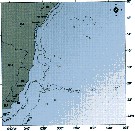 issued from : C. de O. Dias & A.V. Araujo in Atlas Zoopl. reg. central da Zona Econ. exclus. brasileira, S.L. Costa Bonecker (Edit), 2006, Série Livros 21. [p.48]. issued from : C. de O. Dias & A.V. Araujo in Atlas Zoopl. reg. central da Zona Econ. exclus. brasileira, S.L. Costa Bonecker (Edit), 2006, Série Livros 21. [p.48].
Chart of occurrence in Brazilian waters (sampling between 22°-23° S).
Nota: sampling only 2 specimens. |
 Issued from : E. Goetze & M.D. Ohman in Deep-Sea Res. II, 2010, 57. [p.2114, Fig.2]. Issued from : E. Goetze & M.D. Ohman in Deep-Sea Res. II, 2010, 57. [p.2114, Fig.2].
Biogeographic distribution of Subeucalanus subtenuis, a circumglobal species in subtropical and tropical waters.
Solid circles mark sampling locations of specimens whose species identify was verified by DNA sequencing of 165 rRNA.
The species distribution from Lang (1965) is outlined wuth hatched lines.
Records from morphological studies by Lang (1965) and Fleminger (1973) are marked as in the legend.
Note: The 'positive' records plotted from Lang (1965) are a subset of her original material. |
 Issued from : P. Tutasi, S. Palma & M. Caceres in Scienc. Mar., 2011, 75 (4). [p.798, Fig.8b] Issued from : P. Tutasi, S. Palma & M. Caceres in Scienc. Mar., 2011, 75 (4). [p.798, Fig.8b]
Geographic distribution of Subeucalanus subtenuis in September and October 2001, associated with the weak La Niña event of 2001. |
 Issued from : M. Madhupratap & P. Haridas in J. Plankton Res., 12 (2). [p.313, Fig.6]. Issued from : M. Madhupratap & P. Haridas in J. Plankton Res., 12 (2). [p.313, Fig.6].
Vertical distribution of calanoid copepod (mean +1 SE), abundance No/100 m3. 59- Subeucalanus subtenuis.
Night: shaded, day: unshaded.
Samples collected from 6 stations located off Cochin (India), SE Arabian Sea, November 1983, with a Multiple Closing Plankton Net (mesh aperture 300 µm), in vertical hauls at 4 depth intervalls (0-200, 200-400, 400-600, 600-1000 m). |
 Issued from : Y.-Q. Chen in CalCOFI Rep., 1986, XXVII. [Fig.2, p.210]. As Eucalanus subtenuis (= Subeucalanus subtenuis): Vertical abundance of females, males and late copepodites. Issued from : Y.-Q. Chen in CalCOFI Rep., 1986, XXVII. [Fig.2, p.210]. As Eucalanus subtenuis (= Subeucalanus subtenuis): Vertical abundance of females, males and late copepodites.
Nota: Samples collected in May-June 1974 with an opening-closing bongo nets (mesh apertures of both nets 0.330 mm and 0.220 mm) from Baja California to Galapagos Is.
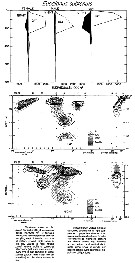
This species was the most abundant copepods (33.5% of the total catch in combined day and night samples. It was more numerous in day samples-56.5% of the total daytime catch- and in agreement with Timonin & Voronina's (1977) results. The distribution of this species was marked by large concentrations. Greatest numbers extended from 25 to 175 m, and adults were concentrated at 50-100 m at stations 17 and 19 (see chart Figs.1, 12, 13). |
 Issued from : Y.-Q. Chen in CalCOFI Rep., 1986, XXVII. [Fig.1, p.207]. Issued from : Y.-Q. Chen in CalCOFI Rep., 1986, XXVII. [Fig.1, p.207].
Chart of the KRILL Expedition. May and June1974 on RV Alexander Agassiz of Scripps Institution of Oceanography. |
 Issued from : Y.-Q. Chen in CalCOFI Rep., 1986, XXVII. [Fig.13, p.221]. Issued from : Y.-Q. Chen in CalCOFI Rep., 1986, XXVII. [Fig.13, p.221].
Vertical hydrographic conditions during the KRILL Expedition (May and June1974).
The hydrographic conditions were originally described by Brinton (1979);
between stations 10 and 11, flow appeared easterly, as indicated by the slope of the Sigma t and the surface temperatures of 23.8° and 24.9°C (Figs.12, 13).
At station 12, near 21°N off the middle of the mouth of the Gulf, subsurface influence of the California Current was evident from the higher oxygen content (Fig. 13b) and from the presence of northern euphausid species, although surface temperatures were < 24°C (Fig.13a).
Stations 13 and 14 were south of 21°N, lying in waters considerably warmer at all depths than those to the north, and showed fully developed characteristics of the East tropical Pacific, evidently associated with northerly or easterly flow toward the Gulf.
Along the Krill Expedition's cruise track, the weak thermocline at 50-100 m off baja California gave way to the strong east tropical Pacific thermocline between 25 and 60 m off the Gulf (Figs. 12 and 13a).
The distribution of oxygen (Fig.23b) also showed strenhjgthening of east tropical Pacific characteristics along the soyutherly tyrack, as oxygen values in the minimum layer progressively decreasely south of 25°N, where the lowest measurable value (< 0.05 ml/l-1) was first encountered. This low value was uniformly distributed in a thick, oxygen-deficient layer between 11°N and 21°N (Fig.13b).Extremely low oxygen concentrations (< 0.05 ml/l-1) were also present at some depths at stations 11 and 22.
Salinity (Fig.13c) was < 34 p.1000 above 100 m depth off Baja California, indicating water of northern origin.
The core of the flow appeared in the 33.7 p.1000 southerly tongue at 50-100 m.
The axis of the deep countercurrent of the Calfornia Current (Reid & al., 1958) was evident at 200-400 m, where water having salinity in excess of 34.5 p.1000 extended northward.
To the south, East Tropical pacific salinities were high (34.6-35.0 p.1000), except for a localized subsurface patch off the mid-Gulf (station 12), where salinity was lower (34.5 p.1000) and associated with an increase in oxygen above 300 m and with plankton species indicative of California Current influence.
South of the station 17, the characteristic east tropical Pacific oxygen minimum was highly developed beneath 70 m, indicating little dilution by other water masses (Fig.13b). High temperature (> 28°C) and low salinity (< 34 p.1000) were found in the 50 m-thick mixed layer (Fig.13 a, c).
Stations 18 and 19 were occupied between 6°N and 8°N, typically the zone of the North Equatorial Countercurrent. Tsuchiya (1974) showed easterly flow across 97°E between 4°N and 6°N during April and May1967, and Love (1971) reported easterly flow between 4°N and 8°N during July 1967. Wyrtki & Kendall (1967) provided evidence that eastward geographic flow in the countercurrent is maximal from March through June, overlapping the time of the present study. Brinton (1979) reported finding considerable faunistic evidence of the countercurrent's influence in this zone.
At station 20 (2°41'N), extremely low surface salinity (< 33.3 p.1000) indicated westerly flow in the mixed layer. However, the presence here of 13°C water (Jones, 1973) and a secondary oxygen maximum at 100-200 m indicated a contribution from the easterly Equatorial Undercurrent, which mixed with the Equatorial Tropical Pacific water beneath and lateral to the undercurrent's core.
At equatorial station 21 (0°19'N), stronger evidence of the undercurrent's activity was seen between 100- and 225-m depth in the 13°C water, the secondary oxygen maximum in excess of 2.5 ml/l-1, and the secondary salinity maximum.
Station 22 (south of the Galapagos Is.) was also in water of high surface salinity, again indicating little or no contribution of westerly water by the South Equatorial Current. Salinity was homogeneous between 60 and 275 m, but an oxygen maximum near 150 m intercepting the generally oxygen-deficient water beneath the mixed layer indicated penetration by water from a direction other than east. Faunistically, this locality was mixed, but without the easterly intrusions found at the equator (Brinton, 1979). |
 Issued from : Y.-Q. Chen in CalCOFI Rep., 1986, XXVII. [Fig.12, p.220]. Issued from : Y.-Q. Chen in CalCOFI Rep., 1986, XXVII. [Fig.12, p.220].
Vertical distribution of thermosteric anomaly Sigma t of the KRILL Expedition (May and June1974). Direction of zonal components of flow is inferred from slopes of isopleths; inferences within about 2° of the equator are least reliable. From Brinton, 1979.
The slope of sigma t surface indicated an easterly component of flow at all observed depths between stations 16 and 17; however, south of station 17 there was a westerly component centered near 200 m depth. |
 Issued from : G.A. Lopez Ibarra in Tesis, Inst. Politec. Nac., CICIMAR, 2008. [p.48, Fig. 22 and p.51, Fig.24]. Issued from : G.A. Lopez Ibarra in Tesis, Inst. Politec. Nac., CICIMAR, 2008. [p.48, Fig. 22 and p.51, Fig.24].
Spatial distribution of the isotopic values from the dominant species for localities (see map for the different zones: a to f). A: 13C; B: 15N.
Nota: Sampling in Julio to December 2003, with a Bongo net (333 µm mesh aperture) from obliquely drawing from 200 m depth to surface.
Cf. Map of the 6 zones sampled, legend and remarks by the same author in Subeucalanus subcrassus. |
 Issued from : G.A. Lopez Ibarra in Tesis, Inst. Politec. Nac., CICIMAR, 2008. [p.37, Table 3]. Issued from : G.A. Lopez Ibarra in Tesis, Inst. Politec. Nac., CICIMAR, 2008. [p.37, Table 3].
Summary of abundance and steady isotopics 15N and 13C in Subeucalanus subcrassus.
O = main food; T = tropical biogeographical affinity; zones (a-f) = Cf. fig.1 in the same author.
Compare with Subeucalanus subcrassus and other species. |
| | | | Loc: | | | South Africa (E & W), off N St. Helena Is., Angola (Baia Farta), Congo, G. of Guinea, Ivorian shelf, Dakar, Cape Verde Is., off Morocco-Mauritania, Azores, Brazil (off Rio de Janeiro, Vitoria Bay, Cabo de Sao Tomé, Mucuri estuary), Caribbean Sea, Caribbean Colombia, Bahia de Mochima (Venezuela), Cariaco Basin, Salamanca Gulf, Yucatan, Cuba, Florida, Sargasso Sea, off Bermuda: Station "S" (32°10'N, 64°30'W), New York, Medit. (W Basin, off Malta), Red Sea, Arabian Sea, Maldive Is., Natal, Rodrigues Is., Seychelles, Karachi coast, Sri Lanka, N Indian, Bay of Bengal, Ambon Bay, Burma (coast), W Australia, Perai River estuary (Penang), Straits of Malacca, Indonesia-Malaysia, Sarawak: Bintulu coast, Tioman Is., N Celebes (Manado Bay), Philippines, Viet-Nam, China Seas (Yellow Sea, East China Sea, South China Sea), Taiwan Strait, Taiwan (S, E, SW, NW, N, NE, Mienhua Canyon), Okinawa, S Korea, Korea Strait, Japan Sea, Tsushima Straits, Nagazaki, Kuchinoerabu Is., Japan, Tokyo Bay, off SE Japan, Kuroshio zone, Pacif. (W equatorial), Indonesia (S Celebes Sea), Australia (Moreton Bay, G. of Carpentaria), California, W Baja California (Bahia Magdalena), Gulf of California (.., Bahia de los Angeles), Central America, New Caledonia, Pacif. (equatorial), G. of Tehuantepec, La Paz, W Mexico , off W Guatemala , Tehuantepc Bowl - Cosra Rica Dome, W Costa Rica , Galapagos S, Ecuador, off Peruvian coast, NE Easter Is., Chile (N, off Valparaiso in Rosales & Sepulveda, 1992, Concepcion) | | | | N: | 186 | | | | Lg.: | | | (14) F: 3,4-3,04; M: 2,95; (28) F: 2,2-1,8; (35) F: 3,4; (47) F: 3,1-2,65; M: 2,75; (73) F: 3,53-3,29; (91) F: ± 2,5; M: ±2,4; (125) F: 3,22-2,4; M: 3,06-2,63; (142) F: 2,7; (150) F: 3,53-2,85; M: 2,82-2,65; (187) F: 3,1-3; M: 2,85; (207) F: 2,56-2,52; (290) F: 3-3,15; M: 2,6-2,7; (332) F: 2,77-2,14; (335) F: 3,41-2,14; (340) F: 2,65; (388) M: 2,72-2,6; (792) F: 3,7-2,9; M: 3,08-2,6; (896) M: 2,44-2,83; (991) F: 2,61-3,15; M: 2,7-2,75; (1122) F: 1,94; (1125) F: 3,2; {F: 1,80-3,70; M: 2,60-3,08} | | | | Rem.: | epipelagic-mesopelagic; (off Malta: 2000 m). Sargasso Sea: 0-1000 m.
For Vervoort (1963 b, p.92) the male in the Atlantide collection, is extraordinarily like the male of Eucalanus crassus (= Subeucalanus crassus), but differs of the latter in the more elongated basipodite of Md, small structural details of P5 and the strongly developed asymmetry of the caudal rami, one of the setae on the left side being lengthened and thickened. The differences in the stucture of P5, which are very small, can best be appreciated from a comparison of Giesbrecht's (1892 Pl.11, fig.42, representing P5 in the male of E. subtenuis, with fig.4d in Vervoort, representing P5 in the male of E. crassus; the number of setae on the basipodite of Md is 3; the same number has been found in several females of E. subtenuis that Vervoort had dissected. The Atlantide specimens are in complete agreement with Eucalanus subtenuis var. japonica Fukase (1957). In the Giesbrecht's specimen, the basipodite of Md carries 2 setae whilst the structure of P5 in the male is slightly different from that studied by Vervoort. Giesbrecht mentions both Atlantic and Pacific localities and it is not at all clear from which locality the figured specimen originares.
For Fukase (1957, p.20-21) Eucalanus (= Subeucalanus) subtenuis obtained from the warm waters of Japan, is distinct from Giesbrecht's subtenuis in the proportional length of the genital segment, the number of setae on the 2nd basal segment of Md, the proportional length of the terminal segment and the spine on the P5 of the male. So this way concludes that is best at present to recognized the japanese species as a variety.
For Itoh (1970 a, fig.2, from co-ordonates) the Itoh's index value of the mandibular gnathobase = 210.
Timonin (1971, p.282) considers the trophic interrelations in the equatorial and tropical Indian Ocean, and divides the plankters into 6 trophic groups from the litterature and the results of studies of mouth-parts structure and intestine content. This species is a coarse-filter feeder.
See in DVP Conway & al., 2003 (version 1)
See in vertical distribution day and night in the Costa Rica Dome by Jackson & Smith (2016, p.310, fig.3 and p.311, fig.4) | | | Last update : 14/06/2021 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed January 16, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |

































