|
|
 |
|
Calanoida ( Order ) |
|
|
|
Clausocalanoidea ( Superfamily ) |
|
|
|
Euchaetidae ( Family ) |
|
|
|
Euchaeta ( Genus ) |
|
|
| |
Euchaeta rimana Bradford, 1974 (F,M) | |
| | | | | | | Syn.: | Euchaeta prestandreae : Brady, 1883 (p.60, figs.F,M);
Euchaeta marina : Wolfenden, 1905 (p.1007, figs.); A. Scott, 1909 (p.67, figs.F,M); Mori, 1937 (1964) (p.44, figs.F,M); Dakin & Colefax, 1940 (p.99, figs.F,M); Sewell,1947 (p.113, figs.F); Brodsky, 1962 c (p.120, figs.F,M); Chen & Zhang, 1965 (p.53, figs.F,M); ? Lubny-Gertzyk, 1968 (p.275); Greenwood, 1977 (p.57, figs.F); ? Takenaka & al., 2012 (p.1669, fig.2, 3, Table 1, bioluminescence assay); ? Lopez Ibarra (2008, Table 1, 2);
Euchaeta marina (Prestandrea, 1833) : often confused with Euchaeata rimana until it was separated by Bradford (1974) mainly in the Indo-Pacific. | | | | Ref.: | | | Bradford, 1974 (p.165, figs.F,M, fig.7); Bradford & al., 1983 (p.20, figs.F,M, Rem.); Kimmerer & al., 1985 (p.428); Renon, 1987 (tab.2); Madhupratap & Haridas, 1990 (p.312, 318, Rem.); Yen & Nicoll, 1990 (p.222); Park, 1995 (p.16, Rem.F,M, figs.F,M); Boxshall & al., 1997 (p.387, figs.F,M); Chihara & Murano, 1997 (p.798, Pl.104: F,M); Braga & al., 1999 (p.84, 86, 89, Rem.: Biol. mol., Table 1 , figs.6, 7, 8); Conway & al., 2003 (p.186, figs.F,M, Rem.); Phukham, 2008 (p.132, figs.M); Al-Yamani & al., 2011 (p.40, figs.F,M); Jeong & al., 2011 (p.117, 127, figs.F,M, Rem.: genital aperture); Mercier & al., 2013 (p.760, neural ultrastructure); Soh & al., 2013 (p.32, figs.F,M)
| 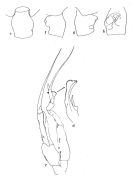 issued from : J.M. Bradford in Pacific Science, 1974, 28 (2). [Fig.4, p.165; Fig.6., p.166]. Female: genital segment: e, dorsal view; f, left side; g, right side; h, ventral view. Male: c, P5; d, serrate lamella of left P5 exopod 2.
|
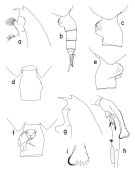 issued from : T. Park in Bull. Scripps Inst. Oceanogr. Univ. California, San Diego, 1995, 29. [p.114, Fig.4]. Female: a, forehead (left side); b, urosome (left side); c, genital somite (left side); d, idem (dorsal); e, idem (right side); f, idem (ventral view). Nota: In both dorsal and ventral view, he left side of the genital somite is smoothly curved. Laterally, the left genital flange is divided into 2 similar lobes and rxtends distally farther than the right genital flange and its posterior margin is nearly perpendicular to the ventral wall of the somite. The right genital flange in lateral view is relatively narrow and produced ventrally into a large rounded lobe, which has a small semicircular ridge on the top. In ventral view thereis almost no gap medially between both genital flanges. Male: g, forehead (left side); h, exopod of left 5th leg (anterior, tilted clockwise); i, distal end of serrated lamella (anterior).
|
 issued from : J.M. Bradford, L. Haakonssen & J.B. Jillett in Mem. N.Z. Oceanogr. Inst., 1983, 90. [p.22, Fig.7]. Female: A, habitus (lateral right side); B-E, genital segment (dorsal, right lateral, left lateral, ventral, respectively); F, exopod of P1; G, exopod segment 3 of P2. - P1 exopod: Bb ± 2/3 BC; Cc ≥ BC. (see code of lengths outer spine in the Genus' figure of Paraeuchaeta, or in Paraeuchaeta sp. A). Male: H, habitus (lateral left side); I, P5; J, terminal part of left P5 exopod.
|
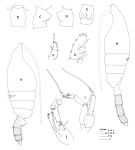
 issued from: Q.-c Chen & S.-z. Zhang in Studia Marina Sinica, 1965, 7. [Pl.14, 1-8]. As Euchaeta marina. Female (from E China Sea): 1, habitus (dorsal); 2, forehead (lateral); 3, genital segment (lateral right side); 4, idem (ventral); 5, outer marginal spines of 3rd exopodal segment of left P2 (posterior). Male: habitus (dorsal); 7, P5 (anterior); 8, 2nd exopodal segment of left P5 (anterior).
|
 issued from : A. Scott in Siboga-Expedition, 1909, XIX a. [Plate XIX, Figs.9-20]. As Euchaeta marina. Female (from Indonesia-Malaysia): 9, head (dorsal); 10, forehead (lateral); 11, last thoracic segment and abdomen (dorsal); 12, last thoracic and genital segments (left side); 13, idem (right side); 14, mxp (end hair); 15, P1 (exopodite only); 16, P2 ( outer margin of exopodite); 17, part of terminal spine of exopodite of P3. Male: P1 (exopodite only); 19, P2 (outer margin of exopodite); 20, P5 (part of left leg).
|
 issued from : G.A. Boxshall, Yen J. & Strickler J.R. in Bull. mar. Sci., 1997, 61 (2). [p.388, Fig.1]. Female and Male (from Kailua Kona, Hawaii Island): A, proximal 15 segments (I to XVIII) of A1 adult female; B, proximal 14 segments (I to XVIII) of A1 of adult male.
|
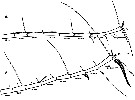 issued from : G.A. Boxshall, Yen J. & Strickler J.R. in Bull. mar. Sci., 1997, 61 (2). [p.389, Fig.2]. Female and Male: A, distal part of A1 of adult female; B, idem of adult male.
|
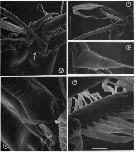 issued from : G.A. Boxshall, Yen J. & Strickler J.R. in Bull. mar. Sci., 1997, 61 (2). [p.390, Fig.3].Female: A, Apex of A1, showing apical segments (XXVII-XXVIII) bearing 5 elements, partly defined from preceding segment (XXVI) by integumental fold (arrowed). B, Same, showing detail of whip seta and aesthetasc. C, Spine on segment 11 (XIV). D, basal articulation of long, anteriorly-directed seta on segment 23 (XXVI), showing typical grooved surface of long setae and process (arrowed) on setal base. E, detail of posterior seta on segment 21 'XXIV), showing flattened pinnules of plumose seta. Scale bars A = 0.020 mm, B = 0.043 mm, C = 0.0075 mm, D = 0.010 mm, E = 0.005 mm.
|
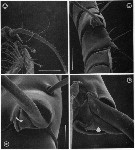 issued from : G.A. Boxshall, Yen J. & Strickler J.R. in Bull. mar. Sci., 1997, 61 (2). [p.391, Fig.4]. Female: A, ventral view of proximal part of A1, showing 3-dimensional array of long setae. B, detail of segments 7 (IX) and 8 (X-XI), showing ventrally and dorsally directed long setae. C, basal articulation of long seta on segment 7 (IX), with arrow indicating triangular integumental pad within socket. D, basal articulation of long seta on segment 8(X-XI), with arrow indicating integumental pad within socket. Scale bars A = 0.430 mm, B = 0.060 mm, C = 0.020 mm, D = 0.015 mm.
|
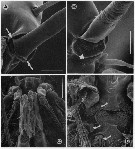 issued from : G.A. Boxshall, Yen J. & Strickler J.R. in Bull. mar. Sci., 1997, 61 (2). [p.392, Fig.5]. Female: A, basal articulation of long seta on segment 13 (XVI) of A1, with arrows indicating pivots of hinge line around which rotation of seta occurs. B, basal articulation of long, anteriorly-directed seta on segment 23 (XXVI) of A1 with arrow indicating swelling distal to base that prevents over-rotation of seta. Male: C, venral view of oral region showing orientation of Mxp; D, same, with Mxp removed, showing reduced labrum and mouth opening, with arrows indicating reduced mandibular gnathobase, reduced praecoxal arthrite of Mx1 and entire Mx2 Nota: The basal articulation between setae and antennule segments permit the setae to rotate from their resting position, to lie virtually flat against the A1, with their tips directed toward the apex of the limb. Rotation of the setae so that they lie flat against the limb will increase streamlining and hehp to reduce drag. This rotation occurs when the A1 are rapidly flexed back, parallel with the body, during the initial phase of an escape response in female. The two strongly curved, long setae on segments 7 (IX) and 8 (X-XI) have modified articulations which also allow the setae to rotate distad, but because of the curve these two setae are unable to be oriented parallel to the long axis of the limb. The basal articulations of the various setation elements of the compound apical segment also restrict the range of possible movements. As the seta rotates, the process on the setal base (arrowed in figure 3,D) comes into contact with the integumental pad and prevents over-rotation ot the seta, i.e., allowing it to rotate to lie parallel with the long axis of the limb but no farther.
|
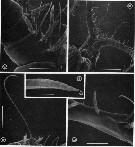 issued from : G.A. Boxshall, Yen J. & Strickler J.R. in Bull. mar. Sci., 1997, 61 (2). [p.394, Fig.6]. Male: A, ventral view of proximal part of A1, showing double aesthetascs on 2nd segment. B, ventral view of head, showing proximal parts of A1. C, long seta on segment 3 (V) of A1. D, tip of long seta, showing grooved surface and apical pore (arrowed). E, triple segment 8(X-XII) of A1 showing anteriorly-directed aesthete. Scale bars A, E = 0.060 mm, B = 0.300 mm, C = 0.100 mm, D = 0.0075 mm.
|
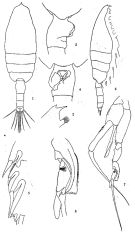
 issued from : T. Mori in The pelagic Copepoda from the neighbouring waters of Japan, 1937 (2nd edit., 1964). [Pl.19, Figs.1-8]. As Euchaeta marina. Female: 2, P2; 3, forehead (lateral); 4, habitus (dorsal); 5, last thoracic and genital segments (lateral); 6, genital segment (ventral). Nota: Body hairy. A1 extend beyond the end of the 2nd urosomal segment. Among the 3 marginal spines on the 3rd segment of exopodite of P2, the middle spine is the longest. Genital segment asymmetical, the right side with a swelling, and is shorter than the following 3 segments together. Male: 1, habitus (dorsal); 7, P5 (anterior); 8, distal portion exopodite of left P5. Nota: Urosome 5-segmented. Genital segment symmetrical.
|
 issued from : J.G. Greenwood in Proc. R. Soc. Qd, 1977, 88. [p.56, Fig.3, a-e]. As Euchaeta marina. Female (from 27°20'S, 153°15'E): a-b, forehead (dorsal and lateral, respectively); c-d, urosome (lateral and dorsal, respectively); e, exopod of P2.
|
 Issued from : G.S. Brady in Rep. Scient. Results Voy. Challenger, Zool., 1883, 8 (23). [Pl.XIX]. As Euchaeta prestandreae. Female: 1-2, habitus (dorsal and lateral, respectively); 3, A2; 4, Md; 5, Mx1; 6, Mx2; 7, Mxp; 8, P1; 9, P2; 10, P3; 11, terminal spines of one the swimming legs
|
 Issued from : G.S. Brady in Rep. Scient. Results Voy. Challenger, Zool., 1883, 8 (23). [Pl.XVIII, Figs.7-13, 15]. As Euchaeta prestandreae. Male: 7, habitus (lateral); 8, A1; 9, Md; 10, Mx1; 11, Mx2; 12, Mxp; 13, P5 with attached spermatophore (a); 15, urosome.
|
 issued from : Y. Al-Yamani, V. Skryabin, A. Gubanova, S. khvorov & I. Prusova in Marine Zooplankton Practical Guide for the Northwestern Arabian Gulf, 2, 2011. [p.41, Fig.159, a-b]. Male (from Kuwait): a, habitus (dorsal); b, anterior part of cephalosome (dorsal).
|
 issued from : N. Phukham in Species diversity of calanoid copepods in Thai waters, Andaman Sea (Master of Science, Univ. Bangkok). 2008. [p.214, Fig.88]. Male (from W Malay Peninsula): a, habitus (dorsal); b, forehead (lateral); c, last thoracic segment and urosome (dorsal); d, P5; e-f, serrated lamella. Body length after drawing: M = 2.795 mm (caudal rami included).
|
 issued from : M.-K. Jeong, H.-L. Suh & H.Y. Soh in Ocean Sci., 2011, 46 (2). [p.127, Fig.7]. Female (E Korea): A-B, habitus (dorsal and lateral, respectively); C, genital double-somite; D, A1. Male: E-F, habitus (dorsal and lateral, respectively); G, P5; H, 2nd exopodal segment of left P5; I, A1. Scale bars in micrometers.
|
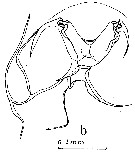 issued from : J.M. Bradford in Pacific Science, 1974, 28 (2). [p.166, Fig.5, b]. Female: c, genital field and right border of genital segment. Nota: Genital field in ventral view with genital pads not meeting in midline, gap between them about 1/2 width of right pad.
|
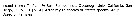 Euchaeta rimana Euchaeta rimana Female: 1 - See Key to marina species Group. 2 - Laterally, genital somite less than 1.5 times as long as deep (Fig.4-b). 3 - In P2 exopod, 2nd outer spine of 3rd segment longer than outer spine of 2nd segment. 4 - Ventrally, genital field with narrow gap between genital flanges (Fig.4-f).
|
 issued from : J.M. Bradford in Pacific Science, 1974, 28 (2). [p.164, Fig.3]. Comparison between exopods of P1 and P2 female of Euchaeta marina (a, b), Euchaeta rimana (c, d) and Euchaeta marinella (e, f). Nota: Exopod of P1: Bb ± 2/3 BC, Cc ± 4/3 BC. Exopod of P2: Aa ≤ AB, Bb ± 1/2 BC, Cc ≤ CD. In E. rimana P1 exopod: Bb ±2/3 BC, Cc ≥ BC. In E. marinella exopod of P1: Bb ≥ 1/2 BC, Cc ≥BC; exopod of P2: Aa ± 3/4 AB, Bb ≤ 1/2 BC, Cc ≤ 1/2 CD.
|
 issued from : J.M. Bradford in Pacific Science, 1974, 28 (2). [p.167, Table 2] Female and Male: comparison between Euchaeta rimana and allied forms.
|
 Issued from : H.Y. Soh, S.Y. Moon & J.H. Wi in Invertebrate Fauna of Korea (eds) Incheon: NIBR, 2013, 21 (28). [p.33, Fig.16]. Female (from Korean waters): A-B, habitus (dorsal and lateral, respectively); C, A1. Scale bars: A-B = 500 µm; C = 400 µm.
|
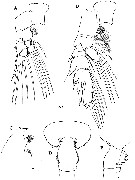 Issued from : H.Y. Soh, S.Y. Moon & J.H. Wi in Invertebrate Fauna of Korea (eds) Incheon: NIBR, 2013, 21 (28). [p.34, Fig.17]. Female: A, P1; B, P2; C, forehead (lateral); D, last thoracic segment and genital segment (dorsal); E, same (lateral). Scale bars: 200 µm.
|
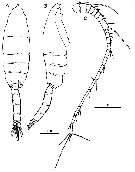 Issued from : H.Y. Soh, S.Y. Moon & J.H. Wi in Invertebrate Fauna of Korea (eds) Incheon: NIBR, 2013, 21 (28). [p.35, Fig.18]. Male: A-B, habitus (dorsal and lateral, respectively); C, A1. Scale bars: A, B = 500 mm; C = 200 µm.
|
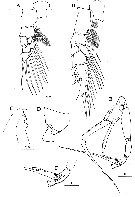 Issued from : H.Y. Soh, S.Y. Moon & J.H. Wi in Invertebrate Fauna of Korea (eds) Incheon: NIBR, 2013, 21 (28). [p.36, Fig.19]. Male: A, P1; B, P2; C, forehead (lateral); D, last thoracic segment and 1st urosomal segment (lateral); E, P5; F, distal segments of left P5. Scale bars: 200 µm.
| | | | | Compl. Ref.: | | | Hayward, 1980 (p.295, Table 2, vertical distribution, feeding); Arashkevich & al., 1982 (p.477, Table 2 cont; as rimani, diet); Dessier, 1983 (p.89, Tableau 1, 2, Rem. p.99, %); Brinton & al., 1986 (p.228, Table 1); Chen Y.-Q., 1986 (p.205, Table 1: abundance %, Table 2: vertical distribution, Rem.: p.209); Madhupratap & Haridas, 1986 (p.105, tab.1); Ambler & Miller, 1987 (tab.2, 3, 4, 5); Dessier & Donguy, 1987 (p.14393, abundance vs. annuals 1979-1984); Dessier, 1988 (tabl.1); Yen J., 1988 (p.395, behaviour, prey-predator, male-female interactions); Hirakawa & al., 1990 (tab.3); Heinrich, 1990 (p.17); Schulz, 1990 (p.188); Mahdupratap & Haridas, 1990 (p.305, fig.5, vertical distribution, fig.7: cluster, Rem.: p.318); Yen & al., 1991 (p.362, behaviour, energy dissipation); 1992 (p.495, tab.1, mechanoreception); Madhupratap & al., 1996 (p.866); Yen & Strickler, 1996 (p.191); Ramaiah & Nair, 1997 (tab.1); Fields & Yen, 1997 a (p.79, feeding current vs. prey); Padmavati & al., 1998 (p.349); Achuthankutty & al., 1998 (p.1, Table 2, seasonal abundance vs monsoon); Hsieh & Chiu, 1998 (tab.2); Mauchline, 1998 (tab.63, 64, 65); Suarez-Morales & Gasca, 1998 a (p.109); Smith S. & al., 1998 (p.2369, Table 6, moonsoon effects); Lenz & al., 2000 (p.338); Madhupratap & al., 2001 (p. 1345, vertical distribution vs. O2, figs.4, 5: clusters; Rebstock, 2001 (tab.2); Lo & al., 2001 (1139, tab.I); Fields & Yen, 2002 (p.747); Rebstock, 2002 (p.71, Table 3, 4, climatic variability); Hwang & al., 2003 (p.193, tab.2); Hsiao & al., 2004 (p.326, tab.1); Hsieh & al., 2004 (p.397, tab.1); Lo & al.*, 2004 (p.218, fig.6); Lo & al., 2004 (p.468, tab.2); Lan & al., 2004 (p.332, tab.1); Gallienne & al., 2004 (p.5, tab.3); Lo & al., 2004 (p.89, tab.1); Kazmi, 2004 (p.229); Prusova & Smith, 2005 (p.77); Miller C.B., 2005 (p.363, fig.16.16Lavaniegos & Jiménez-Pérez, 2006 (p.144,tab.2, 3, Rem.); Zuo & al., 2006 (p.162: tab.1, 3, abundance, fig.8: statiopns group); Hwang & al., 2006 (p.943, tabl. I); Fang & al., 2006 (p.224, metals concentration); Mackas & al., 2006 (L22S07, Table 2); Rakhesh & al., 2006 (p.93, Table 2, spatial distribution); Hwang & al., 2007 (p.24); Dur & al., 2007 (p.197, Table IV); Jitlang & al., 2008 (p.65, Table 1); Lan Y.C. & al., 2008 (p.61, Table 1, % vs stations); Tseng L.-C. & al., 2008 (p.153, Table 2, occurrence vs geographic distribution); Ayon & al., 2008 (p.238, Table 4: Peruvian samples); Morales-Ramirez & Suarez-Morales, 2008 (p.520); Ohtsuka & al., 2008 (p.115, Table 5); Wishner & al., 2008 (p.163, Table 2, fig.8, zonation-oxycline); C.-Y. Lee & al., 2009 (p.151, Tab.2); Zhang W & al., 2009 (p.261); Cornils & al., 2010 (p.2076, Table 3); Xu & Gao, 2011 (p.514, figs.3, 4, Table 2: optimal salinity); Hsiao S.H. & al., 2011 (p.475, Appendix I); Kâ & Hwang, 2011 (p.155, Table 3: occurrence %); Hsiao & al., 2011 (p.317, Table 2, fig.6, indicator of seasonal change); Tseng L.-C. & al., 2011 (p.47, Table 2, occurrences vs mesh sizes); Tutasi & al., 2011 (p.791, Table 2, abundance distribution vs La Niña event); Naz & al., 2012 (p.61, Table 4, relative abundance); Jang M.-C & al., 2012 (p.37, abundance and seasonal distribution); Tseng & al., 2012 (p.621, Table 3: abundance); Tseng & al., 2013 (p.507, seasonal abundance); Hirai & al., 2013 (p.1, Table I, molecular marker); Oh H-J. & al., 2013 (p.192, Table 1, occurrence); Hwang & al., 2014 (p.43, Appendix A: seasonal abundance); Palomares-Garcia & al., 2018 (p.178, Table 1: occurrence). | | | | NZ: | 9 | | |
|
Distribution map of Euchaeta rimana by geographical zones
|
| | | | | | | | |  issued from : J.M. Bradford in Pacific Science, 1974, 28 (2). [Fig.7, p.167]. issued from : J.M. Bradford in Pacific Science, 1974, 28 (2). [Fig.7, p.167].
Distribution of Euchaeta marina, E. rimana and E. marinella based on specimens seen by the author. |
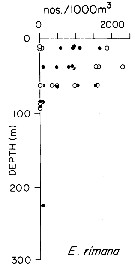 issued from : T.L. Hayward in Mar. Biol., 1980, 58. [p.299, Fig.2]. issued from : T.L. Hayward in Mar. Biol., 1980, 58. [p.299, Fig.2].
Plots of day (open circle) and night (filled circle) depth distributions of Euchaeta rimana in the North Pacific central gyre (main sampling location: 25°N, 155°W with drogue), September 1968. |
 issued from : T.L. Hayward in Mar. Biol., 1980, 58. [p.302, Fig.4]. issued from : T.L. Hayward in Mar. Biol., 1980, 58. [p.302, Fig.4].
Day and night feeding indices (relative degree of gut fullness of copepods collected under differing environmental conditions) for females Euchaeta rimana from N Pacific central gyre (main sampling location: 28°N, 155°W), June 1973.
Numbers in abscisse : stations. |
 Issued from : A. Dessier in Oceanol. Acta, 1983, 6 (1). [p.100, Fig.8 B]. Issued from : A. Dessier in Oceanol. Acta, 1983, 6 (1). [p.100, Fig.8 B].
Geographic distribution of Euchaeta rimana along the transect Noumea (New Caledonia) to Panama.
black square: more than 18 organisms; black point inside square: 1 to 18 organisms.
Nota: Sampling by net (mesh aperture = 330 µ) between depth 5 and 10 m, 34 hours after sunset. From November 1977 to September 1981 (90 % of samples obtained since 1979). |
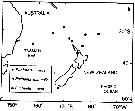 Issued from : J.M. Bradford, L. Haakonssen & J.B. Jillett in N. Z. Oceanogr. Inst. Memoir 90, 1983. [p.128, Top left, 129]. Issued from : J.M. Bradford, L. Haakonssen & J.B. Jillett in N. Z. Oceanogr. Inst. Memoir 90, 1983. [p.128, Top left, 129].
Distribution of Euchaeta indica, E. marinella and E. rimana in the south-west Pacific. |
 Issued from : S.-H. Hsiao, S. Kâ, T.-H. Fang & J.-S. Hwang inHydrobiologia, 2011, 666. [p.326, Fig.6]. Issued from : S.-H. Hsiao, S. Kâ, T.-H. Fang & J.-S. Hwang inHydrobiologia, 2011, 666. [p.326, Fig.6].
Variations in the most abundant copepod species (mean ± SE) along the transect in the boundary waters between the northern part Taiwan Strait and the East China Sea i March (black bar) and October (grey bar) 2005 (Mann-Whitney U test, sig. ns: P >0.05.
See drawings of Hydrological conditions and superficial marine currents in Calanus sinicus. |
 Issued from : M. Madhupratap & P. Haridas in J. Plankton Res., 12 (2). [p.312, Fig.5]. Issued from : M. Madhupratap & P. Haridas in J. Plankton Res., 12 (2). [p.312, Fig.5].
Vertical distribution of calanoid copepod (mean +1 SE), abundance No/100 m3. 43- Euchaeta rimana.
Night: shaded, day: unshaded.
Samples collected from 6 stations located off Cochin (India), SE Arabian Sea, November 1983, with a Multiple Closing Plankton Net (mesh aperture 300 µm), in vertical hauls at 4 depth intervalls (0-200, 200-400, 400-600, 600-1000 m). |
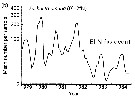 Issued from : C.B. Miller in Biological Oceanography, Blackwell Publishing, 2005. [p.363, Fig. 16.16 (b)]. After Dessier & Donguy, 1987. Issued from : C.B. Miller in Biological Oceanography, Blackwell Publishing, 2005. [p.363, Fig. 16.16 (b)]. After Dessier & Donguy, 1987.
Time series of abundance estimates for Euchaeta rimana species sampled along the Pacific equator from passenger liner on a regular schedule.
Miller underlines that serial abundance for this species from the equator to 2°N shows strong seasonality and dramatic reduction during the 1982-1983 El Niño (with a a sharp reduction of nitrate). A long list of other species also nearly vanished in that event. At least the stocks of hebivorous species rebounded immediately in 1984; E. rimana, a predator, had another reduced seasonal peak in that year. Dessier & Donguy propose that the seasonality is driven by variations in equatorial upwelling strength and production. They note that peaks of herbivorous species (as Clausocalanus spp.) alternate with peaks of carnivores (as the Lokta-Voltera model: p.70 in Miller, 2005), implying a lag along the food chain in response to the seasonal variation. Seasonal advective shifts of the strong, trans-equatorial abundance gradients are also possible explanation. |
 Issued from : G.A. Lopez Ibarra in Tesis, Inst. Politec. Nac., CICIMAR, 2008. [p.37, Table 3]. Probably Euchaeta rimana. Issued from : G.A. Lopez Ibarra in Tesis, Inst. Politec. Nac., CICIMAR, 2008. [p.37, Table 3]. Probably Euchaeta rimana.
Summary of abundance and steady isotopics 15N and 13C in Subeucalanus subcrassus.
C = main feeding: carnivore; T = tropical biogeographical affinity; zones (a-f) = Cf. fig.1 in the same author.
Compare with Subeucalanus subcrassus and other species (Tabla 3).
Note the similarity with Euchaeta marina (? = E. rimana). |
| | | | Loc: | | | G. of Aden, Arabian Sea, Arabian Gulf (Kuwait), Karachi coast, Bombay, Mascarene Basin, Madagascar, Eodrigues Is - Seychelles, Indian, Goa, Bay of Bengal, W Malay Peninsula (Andaman Sea), Indonesia-Malaysia, SW Celebes, China Seas (Bohai Sea, Yellow Sea, East China Sea, South China Sea), Taiwan Strait, Taiwan (S, W, SW, Tapong Bay, Bali, NW, N, NE, Kuroshio Current), S & E Korea, Geumo Is., Korea Strait, Japan Sea, Japan, S. Hokkaido, off SE Japan, California, W Baja California, Gulf of California, La Paz, W Mexico, W Costa Rica, Hawaii, off Hawaii NE, Australia (Shark Bay, New South Wales), New Caledonia, New Zealand, America (central), Clipperton Is., Galapagos-Ecuador, Pacif. (SE tropical), Pacific (central gyre), off Peruvian coast, off Chile | | | | N: | 127 ? | | | | Lg.: | | | (3) F: 3,8-3,4; M: 3,96-3,24; (9) F: 4,3-3,4; M: 4,1-3,6; (53) F: 4,3-2,8; M: 4,1-3,11; (104) F: 3,5; M: 2,8; (150) F: 3,07-3,05; M: 3,62-3,12; (290) F: 3,45-3,75; M: 3,35-3,6; (991) F: 2,8-4,3; M: 3,11-4,1; (1085) F: 2,8-4,3; M: 3,11-4,10; (1106) F: 3,15-3,39; M: 3,10-3,19; (1125) F: 3,2; (1174) F: 3,15-3,39; M: 3,10-3,19; {F: 2,80-4,30; M: 2,80-4,10}
The mean female size is 3.479 mm (n = 16; SD = 0.4953), and the mean male size is 3.473 mm (n = 15; SD = 0.4310). The size ratio (male : female) is 1.002 (n = 7; SD = 0.0331), or ± 1. | | | | Rem.: | For Park (1995, p.14) this species belongs to ‘’marina’’ Group.
Madhupratap & Haridas (1986) do not mention E. marina occurrence in the Indian Ocean; this species must correspond to numerous citations of E. marina in this ocean, just like probably for the Pacific before 1974. Chen Y.-Q. underlines the confusion in the NE Pacific tropical between the two species E. marina and E. rimana, the former in the Atlantic ocean and the last in the Indo-Pacific oceans; from the surface to 600 m but generally concentrated above 100 m (may-June 1974 in the zones 20-25).
After Park (1995, p.16) the female of this species can be distinguished from that of E. marina only by the structure of the genital somite and for the male the structure of the serrated lamella of the left P5.
After Braga & al. (1999, p.89) the investigation of the estimed time of divergence for the sibling species E. rimana and E. marina using a molecular clock approach suggested that these sister taxa diverged 2.6 to 3.4 million years ago. This sibling species pair is found on either side of the Panamanian Isthmus, which severed the connection between the Caribbean and the Eastern Pacific 2.9 to 3.5 million years ago (Bermingham & Lessios, 1993).
Park (1995, p.16) found this species throughout the Pacific and Indian oceans between 35°N and 25°S.
Y.-Q. Chen (1986) during the Krill Expedition from 23°N to 3°S in the Pacific East tropical (May-June 1974), this species shows a mean percentage of the total copepods: 2.41 % (day: 0.1, night: 4.9), occupied 9th position among copepods of the stations (but sampling with a bongo net from mesh apertures 333 µm and 220 µm). It was found in lower numbers in day samples than in night samples. Its vertical distribution extended from the surface to 600 m, but in general it was concentrated above 100 m. The maximum number was 35,600 males, 13,800 females, and 62,400 juveniles per 1000 m3. The species occurred in low numbers (130-800 individuals/1000 m3 below 200 m. (see in Subeucalanus subtenuis Chart Fig.1, hydographic conditions Figs.12, 13).
See in DVP Conway & al., 2003 (version 1) | | | Last update : 09/12/2020 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed February 05, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |


































