|
|
 |
|
Calanoida ( Order ) |
|
|
|
Clausocalanoidea ( Superfamily ) |
|
|
|
Euchaetidae ( Family ) |
|
|
|
Paraeuchaeta ( Genus ) |
|
|
| |
Paraeuchaeta antarctica (Giesbrecht, 1902) (F,M) | |
| | | | | | | Syn.: | Euchaeta antarctica Giesbrecht, 1902 (p.21, figs.F, non M); Wolfenden, 1908 (p.18, figs.F); 1911 (p.295); Vervoort, 1951 (p.91, Rem.); 1957 (p.74, Rem.); Littlepage, 1964 (p.463, lipid content); Bradford, 1971 b (p.21, figs.F,M); Park, 1978 (p.220, fig.F,M, Rem.); Séret, 1979 (p.89, figs.F,M, juv.); Hirche, 1984 a (p.361, respiration); Hubold, 1985 (p.43, Table 1, predation by fish); Hopkins, 1985 (p.197, Table 1, gut contents); Dearborn & al., 1986 (p.1, predation by benthic star); Ferrari & Dojiri, 1987 (p.458, figs.F,M, Rem.: Sgn, P5, spermatophore); Zmijewska, 1987 (tab.2a); Ward & Robins, 1987 (p.127, Table VI, reproduction); Hopkins & Torres, 1988 (tab.1); Fontaine, 1988 (p.32, figs.F,M, Rem.); Ward & Wood, 1988 (p.45, tab.1); Ferrari & Dearborn, 1989 (p.1315, predator); Ward, 1989 (tab.2); Yen & Nicoll, 1990 (p.222); Øresland, 1990 (p.201, Table 1, predator); Rau & al., 1991 (p.1, isotopic forms vs feeding); Conover & Huntley, 1991 (p.1, Table 2, 3, 5, 7, 10, polar seas comparison); Øresland, 1991 (p.41, diet); Øresland & Ward, 1993 (p.73, diet); Zmijewska, 1993 (p.73, seasonal and vertical distribution, life history); Hosie & Cochran, 1994 (p.21); Donnelly & al., 1994 (p.171, chemical composition); Huntley & Nordhausen, 1995 (p.457, elemental composition, ammonium excretion rates); Ward & al., 1995 (p.195, abundance, biomass, vertical distribution); Albers & al., 1996 (p.347, lipids vs. diet); Zmijewska & al., 1997 (p.127); Fransz & Gonzalez, 1997 (p.395, weight-length, biomass vs. N-S transect); Atkinson & al., 1999 (p.63, Rem.: p.66); Chiba & al., 2001 (p.95, tab.4); Ward & al., 2002 (p.2183, tab.2); Ward & al., 2003 (p.121, tab.4); Tsujimoto & al., 2006 (p.140, Table1); Ward & al., 2012 (p.78, Table A1, B1, abundance, weight);
Pareuchaeta sp. Tanaka, 1960 (p.39, fig. Juv. stage III);
Pareuchaeta antarctica : Farran, 1929 (p.208, 238); Sewell, 1929 (p.154); Hardy & Gunther, 1935 (1936) (p.160, Rem., distribution charts); Baker, 1954 (p.203, 211, fig.5); Vinogradov, 1968 (1970) (p.68); Zvereva, 1976 a (p.70, fig.F); Mauchline, 1998 (tab.20, 30, 33, 45, 58, 64); Veistheim & al., 2005 (p.382, tab.1, 2, fig.1); Ward & al., 2006 (p.83: tab.4); | | | | Ref.: | | | Brady, 1918 (p.21); Sewell, 1948 (p.573, 574); Björnberg & al., 1981 (p.633, 635, figs.F,M); Bradford, 1981 (p.391, 398, figs.F,M, Rem.); Bradford & al., 1983 (p.25, figs.F,M, Rem.); Razouls, 1994 (p.85, figs.F, M); Park, 1994 (p.321); 1995 (p.88, Rem.F,M, figs.F,M); Mazzocchi & al., 1995 (p.176, figs.F,M, Rem.); Bradford-Grieve & al., 1999 (p.880, 926, figs.F,M); Braga & al., 1999 (p.79, 84, tab.1, figs.6, 7, 8, Rem.: Biol. mol.); Michels & Schnack-Schiel, 2005 (p.483, fig.8: Md); Cheng F. & al., 2013 (p.119, molecular biology, GenBank); Xavier & al., 2020 (p.15, fig., Rem.). | 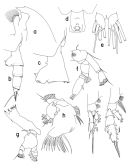 issued from : T. Park in Bull. Scripps Inst. Oceanogr. Univ. California, San Diego, 1995, 29. [p.194, Fig.84]. Female: a, forehead (left side); b, urosome (left); c, d, genital somite (left, ventral, respectively); e, caudal rami (ventral); f, A2; g, Md; h, Mx1 (first inner lobe separated), posterior; i, P1 (anterior); j, P2 (anterior). Nota: Basis of A2 with a single seta; in Md, the basis with a small additional seta, 1st endopodal segment without a seta, 2nd endopodal segment with an appendicular seta; Mx1 with 2 setae on the 2nd inner lobe, 3 setae on the basis, and 5 or 6 setae on the 1st endopodal segment.
|
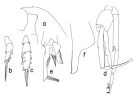 issued from : T. Park in Bull. Scripps Inst. Oceanogr. Univ. California, San Diego, 1995, 29. [p.195, Fig.85]. Male: a, forehead (left side); b, exopod of P1 (anterior); c, exopod of P2 (anterior); d, P5 (anterior); e, distal end of left 5th leg exopod (medial); f, serrated lamella (lateral).
|
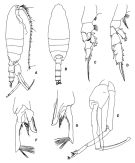 issued from : T. Park in Biology of the Antactic Seas VII. Antarctic Res. Ser. Washington, 1978, 27. [p.222, Fig.78]. As Euchaeta antarctica. Male: A, habitus (lateral); B, idem (dorsal); C, P1; D, P2; E, P5; F, distal end of exopod of left P5 (medial); G, idem (lateral). P1-5: legs (anterior).
|
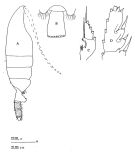 issued from : J.M. Bradford, L. Haakonssen & J.B. Jillett in Mem. N.Z. Oceanogr. Inst., 1983, 90. [p.27, Fig.10]. Female (from 61°56'S, 170°26'E) : A, habitus (lateral right side); B, distal part of last thoracic segment and genital segment (dorsal); C, exopod of P1; D, exopod segment 3 of P2. Nota: P1 exopod: Aa ≤ AB; Bb> BC; Cc ≥ BC. P2 exopod: Aa > AB; Bb = 1/2 BC; Cc ± 2/3 CD. (see code of lengths outer spine in the Genus' figure, or in Paraeuchaeta sp. A).
|
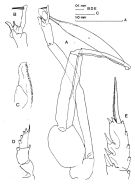 issued from : J.M. Bradford in N.Z. Jl Mar. Freshw. Res., 1981, 15 (4). [p.396, Fig.5]; Male: A, P5 (clasping spermatophore); B, terminal part of left exopod of P5; C, serrated lamella; D, exopod of P1; E, terminal part of exopod of P2. Nota: Long outer edge spine of the 2nd exopodal spine of P2. P5 with short serrated lamella of the 2nd exopodal segment, which scarcely extends beyond the short, hairy tubercle and is one-half the length of the 3rd exopodal segment.
|
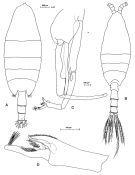 Issued from: M.G. Mazzocchi, G. Zagami, A. Ianora, L. Guglielmo & J. Hure in Atlas of Marine Zooplankton Straits of Magellan. Copepods. L. Guglielmo & A. Ianora (Eds.), 1995. [p.178, Fig.3.31.1]. Female: A, habitus (dorsal). Nota: Proportional lengths of urosomites and furca 40:26:25:9 = 100. 2nd and 3rd urosomal somites covered with short bristles forming characteristic checker board pattern on dorsal and lateral surfaces, with long hairs on ventral surface. Posterior margins of urosomal somites 2-3 bordered by row of triangular flaps. Male: B, habitus (dorsal); C, P5; D, last two segments of left P5. Nota: Proportional lengths of urosomites and furca 14:32:25:21:8 = 100.
|
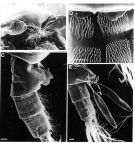 Issued from: M.G. Mazzocchi, G. Zagami, A. Ianora, L. Guglielmo & J. Hure in Atlas of Marine Zooplankton Straits of Magellan. Copepods. L. Guglielmo & A. Ianora (Eds.), 1995. [p.179, Fig.3.31.2]. Female (SEM preparation): B, forehead (ventral); C, urosome (lateral right side); D, detail of spinulation on 2nd and 3rd urosomal somites; E, urosome with spermatophores attached to genital somte. Bars: B, C, E 0.100 mm; D 0.050 mm.
|
 issued from : J. Michels & S.B. Schnack-Schiel in Mar. Biol., 2005, 146. [p.489, Fig.8]. Mandibular gnathobase. a-c: Female (from Weddell and Bellingshausen Seas); d, adult Male, e, male (copepodite stage 5). a: left gnathobase from cranial; b: right gnathobase from from distal; c: right gnathobase from caudal; d: left mandible from lateral; e: left mandible from cranial; f: detailed view of mandibular gnathobase from e. V: ventral tooth, C1-C3: central teeth, D1+D2: dorsal teeth, B: dorsal bristle; R: ridge-like structure; Bg: group of bristles. Ganthobase teeth in panels a-c are partly damaged, third central tooth in panels e and f is damaged. Scale bars 0.020 mm. Nota: The gnathobases of mandibles of the male adults are absent totally, while the male copepodite stage 5 possesses completely developed mandibular gnathobase (Figs. d, e-f). It seems to be probable that the uptake of food is not important for males with reduced mouthparts and the only goal is to locate females and to transfer the spermatophores (see in Boxshall& al., 1997 for Euchaeta rimana. Ohtsuka & Huys (2001) mention the avoidance of cannibalism as a possible explanation for the complete reduction of gnathobases in males of the family Euchaetidae. Males with reduced gnathobases are not danger for the females during mating, and they cannot feed on copepodite stages of their own species. Another explanation could be lower energy needs for the production of spermatophores in males in contrast to the production of eggs in females This species feeds on zooplankton, mainly on other copepod species. The early copepodite stages prefer small species as Oncaea spp. and Microcalanus pysgmaeus, while adulults feed on relatively large species such as Metridia gerlachei (Hopkins, 1985, 1987; Hopkins & Torres, 1989; Oresland, 1991, 1995; Yen, 1991; Oresland & Ward, 1993). Hopkins (1985) found parts of Euphausia superba in the guts of adult females. The smaller number of teeth can be an advantage in piercing prey organisms due to reduced working surface. Since special modifications of the gnathobase morphology can mainly be found in predatory copepod species (Ohtsuka & al., 1997). In this context the large amount of small bristles on the gnathobases can be interpreted as a special adaptation to motile prey; this bristles could be associated with mechanoreceptors. According to the gnathobase morphology it is even imaginable that individuals of this species are able to rip pieces out of zooplankton organisms that are much larger than themselves. It is assumed here that the phytoplankton organisms that have been found in guts of this species have generally been secondary food items that were ingested undirectedly inside the prey organisms while feeding on zooplankton , or/and that phytoplankton organisms that adhere to the bristles of the moothparts of zooplankton organisms become ingested together with such organisms
|
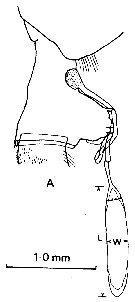 issued from : J.M. Bradford in N. Z. Jl Mar. Freshw. Res., 1981, 15. [p.392, Fig.1, A]. Female (from McMurdo Sound, Ross Sea): A, lateral view of genital segment with non-direct-placement spermatophore attached. Nota: Females of P. anractica are easily distinguished by the shape of their genital segment
|
 issued from : J.M. Bradford in N. Z. Jl Mar. Freshw. Res., 1981, 15. [p.401, Table 5 (part.)]. Female & Male (from Giesbrecht, 1902): Distinguishing features of species of Paraeuchaeta closely related to P. antarctica (* for code see p.393, Fig.2 in P. erebi). Nota: The McMurdo material reveals that Gisebrecht (1902, p.21, Pl.3, figs 4, 6-8) assigned the wrong male to P. anractica. As Zvereva (1976) observed, the male may attach a short, wide spermatophore with a long neck to the anteroventral surface of the female genital segment. The male figured by Giesbrecht (1902) holds a spermatophore of different proportions in its left P5. males were distinguished from other Paraeuchaeta males by the long outer edge spine of the 2nd exopodal spine of P2 (see figG.5 above) and the short serrated lamella of the 2nd exopodal segment of the left P5, which scarcely extends beyond the short, hairy tubercle and is 1/2 the length of the 3rd exopodal segment. All spermatophores on females were of the one type. Rhere is evidence that P. antarctica males can place their spermatophores on other species, as 2 females of P. erebi were found with attached spermatophores of simolar dimensions to those of P. antarctica.. One female had the spermatohore attached ventrally, as is characteristic for P. antarctica; on the other, the spermatophore was attached to the left posterodorsal surface of the genital segment.
|
 issued from : M. Fontaine in Biol. Antarctic Seas XIX, Antact. Res. Ser., 1988, 47. [p.33, Fig.3]. As Euchaeta antarctica. Female (from Ross Sea) genital segment: A-c, right lateral, ventral, and left lateral views, respectively; D, details of ventral view; E, details of ventrolateral view.
|
 issued from : M. Fontaine in Biol. Antarctic Seas XIX, Antact. Res. Ser., 1988, 47. [p.34, Fig.4]. As Euchaeta antarctica. Female: A, A2 (inset shows seta on endopod segment 2); B, Md (manducatory plate); C, left Md (mandibular palp); D, right Md (mandibular palp); E-G, Mx1 (posterior surface); H, basal segment 2 and endopod of Mx1 (anterior surface). Scale bars 0.25 mm each. Nota: Endopod of A2 2/3 as long as exopod; segment 1 with 2 setae at distal inner corner; segment 2 with 14 terminal setae (8 on inner lobe, 6 on outer lobe); exopod 1 with no seta, segment 2 not clearly defined and with 1 very minute seta; segments 3-6 with 1 long plumose seta; exopod 7 with 3 terminal setae and appendicular seta placed about 1/3 the length of the segment. Mx1 inner lobe 1 bearing 13 setae of the following types: 3 plumose setae (covered with short slender hairs) along the posterior edge; 4 setae median to these: 2 curved strong setae with both hairs and long sharp spines and 2 more that are slender and straighter and have very short hairs; 6 very stout clawlike setae (the unguiform setae of Arashkevich's (1969) predatory copepod) with long, sharp spines or teeth, or both; inner lobe 2 with 2 strong curved plumose setae; inner lobe 3 with 1 long, curved, strong plumose seta; 1st outer lobe with 9 plumose setae, the 2 proximal setae smaller than the others; 2nd basal segment with 3 long, curved internal setae: exopod 1-segmented, with 11 plumose setae, the most proximal one and the most distal 3 much smaller than the others; endopod segments partially fused and difficult to distinguish, with a total of 8 setae, segment 1 without seta, segment 2, which is partly fused with it, with 5 setae: 3 long, 2 short, all curved and plumose, segment 3 with 3 long curved plumose setae. mxp without any tiny ''flagellum'' on tips of long endopodal setae.
|
 issued from M. Fontaine in Biol. Antarctic Seas XIX, Antact. Res. Ser., 1988, 47. [p.35, Fig.5]. As Euchaeta antarctica. Female: A, P1; B, P2. Nota: Relative lengths of spines and setae. Exopod of P1: Aa > AB (Aa ± 1.25 AB; Bb > BC (Bb ± 1.25 BC); Cc > BC. Exopod of P2: Aa > AB (Aa ± 1.25 AB); Bb ± 2/3 BC; Cc ± 3/4 CD.. Male: C-D, lateral and medial views, respectively, of distal part of left P5.
|
 issued from M. Fontaine in Biol. Antarctic Seas XIX, Antact. Res. Ser., 1988, 47. [p.37, Fig.7]. As Euchaeta antarctica. Females: Right lateral views of genital segment from different size at different stations: A, 76°30'S, 168°35'E (Body length: 7.9 mm); B, 64°32'S, 104°48'E (Body length: 7.6 mm); C, idem (Body length: 7.2 mm). Scale bars: 0.5 mm each. Nota: This species was first described by Giesbrecht (1902) from specimens collected through the ice near Peter I Island. In the Ross Sea the species is most abundant close to the ice shelf waters in the south east corner of the Ross Sea. A comparison of the occurrence with hydrographic stations (Jacobs & al. 1970) indicates that the numbers drop off significantly at stations made in Circumpolar Deep Water. However, the species is again abundant at stations made in the open ocean. Possibly the distribution, although widespread, is not continuous. Possibly more than one species needs to be considered here. The small differences in the shape of the genital segments of smaller females, for example from stations 1906 and 396 may prove to be more than merely individual variations. The extremely wide size range of P. antarctica has been remarked by Vervoort (1957). In the samples most of the adult females measured more than 9 mm, while small numbers of them were between 7 and 8 mm. A bar graph of measurements of females from station 1906 in the Ross Sea suggests that there may be two size-groups present; in an another station in the Ross Sea, two out of 65 females were smaller than the others, each of these was was extruding an egg, showing that the \"smalls\" are reproductively active. According to Vervoort (1957) a similar size difference has not been observed in males.
|
 issued from : W. Giesbrecht in Copepoden. Res. voyage du S. Y. Belgica. Rapports scientifiques, Zoologie, 1902. [Taf. III, Figs. 1-8]. As Euchaeta antarctica. Female (from S Peter Ist Island): 1-2, last thoracic segment and genital segment (ventral and lateral, respectively); 3, exopod of P1; 5, exopodite 3 of P2. Male: 4, P1; 6, exopodite 3 of P2; 7, P5 with spermatophore (anterior); 8, distal part of left P5.
|
 issued from : T. Park in Biology of the Antarctic Seas VII. Antarctic Res. Ser., 1978, 27 (4). [p.221, Fig.77]. As Euchaeta antarctica. Female: A-B, habitus (dorsal and lateral, respectively); C-D, distal end of metasome and genital segment (right side and ventral, respectively); E, P1 (anterior); F, P2 (anterior).
Rem.: An peculiar outgrowth innediately anterior to the genital orifice of the genital segment.
Nota : Proportional lengths of prosome and urosome70 : 30. Frontal eminence of forehead low, bearing suprafrontal sensilla ; rostrum moderately developed, nearly downward.
Posterolateral corner of metasome produced posteriorly, covering about 1/4 of genital segment, appearing broadly rounded dorsally but more or less angular laterally.
Dorsally, genital segment symmetrical , its sides nearly straight. Genital prominence large, very characteristic, and close to distal end of segment ; peculiar lappetlike outgrowth along anterior edge of genital field (fig.C). Laterally, genital flange appearing like a tooth. Each side of posterior surface of genital prominence prolonged posteriorly into flaplike structure appearing like posterior lobe of genital flange. Laterally, genital operculum visible posterior to genital flange. Ventrally, genital segment symmetrical. Ventral wall of genital segment forming conspicuous pair of folds immediately anterior to genital prominence.
2 nd medial seta of caudal ramus large, much elongated ; appendicular caudal setae not geniculated, thinner and shorter than 2 nd medial seta.
A1 reaching middle of genital segment.
Outer coxal lobe of Mx1 with 9 setae.
Mx2 : None of 6 apical setae bearing long spines in addition to usual short spinules.
Mxp : No spinules found on tip of long endopodal setae.
In P1, 1st and 2 nd exopodal segments fused ; external spine of 1st slender, reaching close to distal end of segment ; external spine of 2 nd segment extending beyond distal end of 3rd.
In P2, external spine of 2 nd exopodal segment reaching base of 1st external spine of 3rd. 2 nd external spine of 3rd segment reaching about 2/3 way to base of 3rd external spine. Marginal lobe bearing 2 nd external spine separated from segment by moderately deep incision.
|
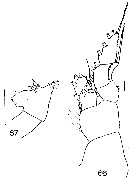 Issued from : J.M. Bradford in N.Z. Oceanogr. Inst., 1971, 206, Part 8, No 59. [p.21, Figs.66, 67]. As Euchaeta antarctica. Female (from Ross Sea): 66, P1; 67, genital segment (left side). Scale bar: 1 mm (67); 100 µm (66).
|
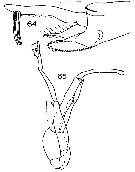 Issued from : J.M. Bradford in N.Z. Oceanogr. Inst., 1971, 206, Part 8, No 59. [p.21, Figs.64, 65]. As Euchaeta antarctica. Male (from Ross Sea): 64, terminal segments of left P5; 65, P5. Scale bar: 1 mm (65); 100 µm (64).
|
 issued from : W. Giesbrecht in Copepoden. Res. voyage du S. Y. Belgica. Rapports scientifiques, Zoologie, 1902. [Taf. III, Figs.1, 2, 3, 5]. As Euchaeta antarctica. Female (from S Peter Ist Island): 1-2, last thoracic segment and genital segment (ventral and lateral, respectively); 3, exopod of P1; 5, exopodite 3 of P2.
|
 Paraeuchaeta antarctica Female: 1 - See key to species Groups and independent species of Paraeuchaeta (p.30). 2 - None of endopodal setae of Mx2 armed with long spinules in addition to usual spinules. 3 - Genital segment with transverse ridge on ventral side anterior to genital prominence (Fig.84-C).
|
 Issued from : C. Séret inThesis 3ème Cycle UPMC, Paris VI, 1979. [Pl. XVII, Figs.101-105]. As Euchaeta antarctica. Female (from off Kerguelen Is.): 101, habitus (dorsal); 102, corner of the last thoracic segment and genital segment (lateral); 103, same (rihgt side): 104, P1; 105, P2.
|
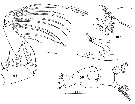 Issued from : C. Séret inThesis 3ème Cycle UPMC, Paris VI, 1979. [Pl. XVIII, Figs.106-108]. As Euchaeta antarctica. Female: 106, right Md; 107, Md (mastcatory edge of gnathobase); 108, right Mxp, with detail of the distal seta.
|
 Issued from : C. Séret inThesis 3ème Cycle UPMC, Paris VI, 1979. [Pl. XVIII, Figs.109, 110]. As Euchaeta antarctica. Male: 109, P5 (D = right leg; G = left leg); 110, detail of distal segment of left leg vearing the spermatophore.
|
 Issued from : C. Razouls in Ann. Inst. océanogr., Paris, 1994, 70 (1). [p.85]. Caractéristiques morphologiques de Paraeuchaeta antarctica femelle et mâle adultes. Terminologie et abbréviations: voir à Calanus propinquus. Nota: Le segment génital femelle permet de caractériser cette espèce; chez le mâle, l'examen soigneux de la P5 s'avère nécessaire.
| | | | | Compl. Ref.: | | | Rudyakov, 1972 (p.886, Table 1: sinking rate); Errhif & al., 1997 (p.422); Razouls & al., 2000 (p.343, tab. 3, 5, Appendix); Bocher & al., 2002 (p.1317); Cabal & al., 2002 (p.869, Table 1, abundance); Alonzo & al., 2003 (p.525); Ward & Shreeve, 2001 (p.50, tab.3, 4); Auel & Hagen, 2005 (p.1272, Table 2); Deibel & Daly, 2007 (p.271, Table 7a, Rem.: Antarctic polynyas); Kattner & al., 2007 (p.1628, Table 1); Schnack-Schiel & al., 2008 (p.1045: Tab.2); Ward & al., 2008 (p.241, Tabls, Appendix II ); Galbraith, 2009 (pers. comm.); Laakmann & al., 2009 (p.679, fig.2, 3, 4, Table 2); Park & Ferrari, 2009 (p.143, Table 3, 7: common deep water species, 8, fig.1, Appendix 1, biogeography, Rem. p. 166); Takahashi & al., 2010 (p.1, Table 4); Swadling & al., 2010 (p.887, Table 2, 3, A1, abundance, indicator species); Yang & al., 2011 a (p.921, Table 2, inter-annual variation 1999-2006); Swadling & al., 2011 (p.118, Table 2, figs. 5, 7, interannual abundance); Guglielmo & al., 2012 (p.1301,Table 3); Laakmann & al., 2012 (p.535, Table 1, fig.2, Rem.: mol. Biol.); Thompson G.A. & al., 2012 (p.127, Table 2, 3); Michels & al., 2012 (p.369, Table 1, occurrence frequency); Yang G. & al., 2013 (p.1701, fig.4, Table 2, feeding); Schründer & al., 2013 (p.1, sodium/ammonium and ph effects in the hemolymph); Ojima & al., 2013 (p.1293, Table 2, 3, abundance); Lee D.B. & al., 2013 (p.1215, Table 1, abundance, composition); Ward & al., 2014 (p.305, Table 7, seasonal and abundance in the ''Discovery'' Investigations in the 1930s); Kouwenberg & al., 2014 (p.290, biogeography, Map 3); Acha & al., 2020 (p.1, Table 3: occurrence % vs ecoregions). | | | | NZ: | 6 | | |
|
Distribution map of Paraeuchaeta antarctica by geographical zones
|
| | | | | | | | |  issued from M. Fontaine in Biol. Antarctic Seas XIX, Antact. Res. Ser., 1988, 47. [p.36, Fig.6]. issued from M. Fontaine in Biol. Antarctic Seas XIX, Antact. Res. Ser., 1988, 47. [p.36, Fig.6].
Occurrence of P. antarctica.
Open circles show stations where no adults were found. Solid line indicates the Antarctic Convergence.
Nota: P. antarctica appears to be ubiquitous south of the Antarctic Convergence. |
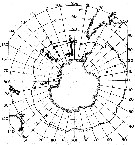 issued from : T. Park in Biology of the Antarctic Seas VII. Antarctic Res. Ser., 1978, 27 (4). [p.223, Fig.79]. issued from : T. Park in Biology of the Antarctic Seas VII. Antarctic Res. Ser., 1978, 27 (4). [p.223, Fig.79].
Occurrence of Euchaeta ( = Paraeuchaeta ) antarctica.
Closed circles: stations where the species was found; A.C: Antarctic Convergence. |
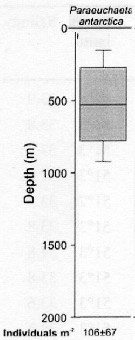 issued from : S. Laakmann, M. Stumpp & H. Auel in Polar Biol., 2009, 32. [p.682, Fig.2]. issued from : S. Laakmann, M. Stumpp & H. Auel in Polar Biol., 2009, 32. [p.682, Fig.2].
Vertical distribution (stages C3 to C6 from Antarctic Polar Front: Atlantic sector).
Error bars encompass the 5th to the 95th percentile. Abundance data are given as mean ± SD.
Nota: The species had the shallowest distribution range between 270 and 740 m. It overlapped in their ranges with P. rasa and P. biloba. |
 issued from : S. Laakmann, M. Stumpp & H. Auel in Polar Biol., 2009, 32. [p.682, Fig.2]. issued from : S. Laakmann, M. Stumpp & H. Auel in Polar Biol., 2009, 32. [p.682, Fig.2].
Vertical distribution, abundance and stage composition ( C1- C6) from Antarctic Polar Front: Atlantic sector).
a: All stations along the transect: stations 342 (52°18'S, 53°54'W) to 347 (51°30'S, 23°50'W) and 350 (51°32'S, 7°58''W) and 351 (51°33'S, 2°05'W).
Nota: The ontogenic vertical partitioning of the species can be explained by different dietary preferences. Young stages find smaller pret in higher concentrations at shallower depths. Mandibular gnathobases do not differ morphologically between different copepodite stages (see Michels & Schnack-Qchiel, 2005) indicating that even young stages are already predatory, feeding on small copepods (see Yen, 1991; Øresland & Ward, 1993). |
 Issued from : M.I. Zmijewska in Oceanologia, 1993, 35. [Fig. 3.5]. Issued from : M.I. Zmijewska in Oceanologia, 1993, 35. [Fig. 3.5].
Diel changes in abundance distribution (ind./ m2) of Euchaeta antarctica (= Paraeuchaeta antarctica) adult female and male (from 64°50'S, 61°50'W, Croker Passage, Antarctic Peninsula) during 3 austral seasons.
N = night; D = day. |
 Issued from : M.I. Zmijewska in Oceanologia, 1993, 35. [Fig. 3.5]. Issued from : M.I. Zmijewska in Oceanologia, 1993, 35. [Fig. 3.5].
Diel changes in abundance distribution (ind./ m2) of Euchaeta antarctica (= Paraeuchaeta antarctica) adult female and male (from 64°50'S, 61°50'W, Croker Passage, Antarctic Peninsula) during 3 austral seasons.
N = night; D = day. |
 Issued from : M.I. Zmijewska in Oceanologia, 1993, 35. [Fig. 3.5]. Issued from : M.I. Zmijewska in Oceanologia, 1993, 35. [Fig. 3.5].
Diel changes in abundance distribution (ind./ m2) of Euchaeta antarctica (= Paraeuchaeta antarctica) adult female and male (from 64°50'S, 61°50'W, Croker Passage, Antarctic Peninsula) during 3 austral seasons.
N = night; D = day. |
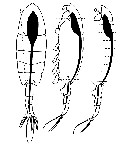 Issued from : V. Øresland in Mar. Ecol. Prog. Ser., 1991, 78. [p.42, Fig.2]. Issued from : V. Øresland in Mar. Ecol. Prog. Ser., 1991, 78. [p.42, Fig.2].
Euchaeta antarctica (= Paraeuchaeta antarctica). Dissection details. Dashed lines: cuts using microscalpel; circle: position of tungsten needle during cutting.
Nota: The copepods were dissected under the steromicroscope using an insect pin (sharpened and formed under stereomicroscope to the shape of a 3 mm long microscalpel) and needles made of tungsten wire (0.1 to 0.3 mm in diameter) sharpened in melted NaNo2.
The gut, with the attached anterior end of the prosome and the urosome, was dissected out in one piece and the gut was then transferred to a few drops of polyvinyl-lactophenol on a microscope slide (fig.2).
The anterior end of the prosome and the urosome were removed, and the gut cut into 5 to 10 small pieces after which the gut contents could easily be removed. The part of the gut found in the urosome was not analysed.
Analysis of gut contents was inferred from identification of prey mandibles or other identifiable prey parts observed through an inverted microscope. |
 Issued from : V. Øresland in Mar. Ecol. Prog. Ser., 1991, 78. [p.45, Table 4]. Issued from : V. Øresland in Mar. Ecol. Prog. Ser., 1991, 78. [p.45, Table 4].
Euchaeta antarctica (= Paraeuchaeta antarctica) from two stations in Weddell Sea. Percentage , in 4 different categories, of prey items found singly or together with 1, 2, 3 or more other prey (not necessarily of the same category) at stages V and VI.Nota: The gut contents included a high proportion of large copepods and metridia sp.; this indicates that they may be much more important as food than small copepods. |
 issued from A. de C. Baker in 'Discovery' Rep., 1954, 27. [p.215, Fig.5]. issued from A. de C. Baker in 'Discovery' Rep., 1954, 27. [p.215, Fig.5].
Occurrence of Euchaeta antarctica (= Paraeuchaeta antarctica) in all longitudes around Antarctic zone of the Southern Ocean.
The percentage frequency of occurrence in samples taken within every 20° of longitude.
Nota: During the 'Discovery' investigations some thousands of plankton samples have been taken from stations spread over the whole of the Southern Ocean at all seasons of the year, the majority south of the Antarctic Convergence. An arbitrary selection of samples has been made from hauls between the surface and a depth of 250 m, which means that they have been taken from within the limits of the Antarctic surface water.
The data suggest that the Southern Ocean is an uninterrupted circumpolar belt with more or less uniform conditions prevailing in east and west directions, the range of planktonic species of the Antarctic surface water may be expected to extend as far as these uniform conditions persist, i.e to be circumpolar. |
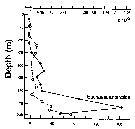 Issued from : P. Ward, A. Atkinson, A.W.A. Murray, A.G. Wood, R. Williams & S. Poulet in Polar Biol., 1995, 15. [p.200, Fig.3]. As Euchaeta antractica. Issued from : P. Ward, A. Atkinson, A.W.A. Murray, A.G. Wood, R. Williams & S. Poulet in Polar Biol., 1995, 15. [p.200, Fig.3]. As Euchaeta antractica.
Abundance and biomass (g dry mass/m3) profiles for the shelf station (54°48'S, 38°15'W) in January 1990.
Values on the horizontal axes at the base represent abundance and the one above biomass. Solid line = midday haul; hatched line = midnight haul. |
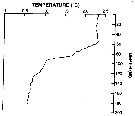 Issued from : P. Ward, A. Atkinson, A.W.A. Murray, A.G. Wood, R. Williams & S. Poulet in Polar Biol., 1995, 15. [p.198, Fig.1, A (modified C.R.)]. Issued from : P. Ward, A. Atkinson, A.W.A. Murray, A.G. Wood, R. Williams & S. Poulet in Polar Biol., 1995, 15. [p.198, Fig.1, A (modified C.R.)].
Temperature profile for the shelf station at Bird Island, South Georgia (54°48'S, 38°15'W) in January 1990. |
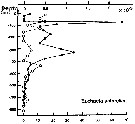 Issued from : P. Ward, A. Atkinson, A.W.A. Murray, A.G. Wood, R. Williams & S. Poulet in Polar Biol., 1995, 15. [p.203, Fig.4, B]. As Euchaeta antarctica. Issued from : P. Ward, A. Atkinson, A.W.A. Murray, A.G. Wood, R. Williams & S. Poulet in Polar Biol., 1995, 15. [p.203, Fig.4, B]. As Euchaeta antarctica.
Abundance (10/m3) and biomass (g dry mass/m3) profiles at the oceanic station from the shelf break in water 4000 m deep off Bird Island, South Georgia (53°04'S, 39°51'W) in January 1990.
Values on the horizontal axes at the base of each profile represent abundance and the one above biomass. Solid line = midday haul; hatched line = midnight haul. |
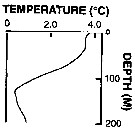 Issued from : P. Ward, A. Atkinson, A.W.A. Murray, A.G. Wood, R. Williams & S. Poulet in Polar Biol., 1995, 15. [p.198, Fig.1, B (modified C.R.)]. Issued from : P. Ward, A. Atkinson, A.W.A. Murray, A.G. Wood, R. Williams & S. Poulet in Polar Biol., 1995, 15. [p.198, Fig.1, B (modified C.R.)].
Profile temperature-depth at the oceanic stations from the shelf break in water 4000 m deep off Bird Island, South Georgia, in January 1990. |
 Issued from : A.C. Hardy & E.R. Gunther in Discovery Reports, 1935 (1936), 11. [p.162, Fig.75]. Issued from : A.C. Hardy & E.R. Gunther in Discovery Reports, 1935 (1936), 11. [p.162, Fig.75].
Charts showing the distribution of Pareuchaeta (= Paraeuchaeta) antarctica in the upper layers of waters at stations in the 1926-7 surveys around South Georgia.
The squares represent the average numbers per 50 m vertical haul from 250 m (or less at shallow-water stations) to the surface with N 100 H nets.
See remarks in Hardy & Gunther (p.142, Figs.66, 67) for Rhincalanus gigas concerning the effects of the mesh apertures of nets. |
 Issued from : C. Séret in Thesis 3ème Cycle, UPMC, Paris 6. 1979, Annexe. [p.34]. Issued from : C. Séret in Thesis 3ème Cycle, UPMC, Paris 6. 1979, Annexe. [p.34].
Geographical occurrences of Euchaeta antarctica (= Paraeuchaeta anterctica) in the Indian Ocean and Antarctic zone. [after publications from: Brady, 1883, 1918; Thompson, 1900; Wolfenden, 1908, 1911; With , 1915; Rosendorn, 1917; Farran, 1929; Sewell, 1929, 1947; Brady & Gunther, 1935; Steuer, 1929, 1392, 1933; Ommaney, 1936; Vervoort, 1957; Tanaka, 1960; Brodsky, 1964; Seno, 1966; Andrews, 1966; Grice & Hulsemann, 1967; Seno, 1966; Frost & Fleminger, 1968; Voronina, 1970; Zverva, 1972].
C. Séret notes the occurrences at stations 56°S, 70°E; 51°S, 65°E and 46°S, 64°E. |
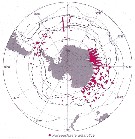 Issued from : J.H.M. Kouwenberg, C. Razouls & N. Desreumaux in Biogeographic Atlas of the Southern Ocean, Scient. Comm. Antarct. Res., Cambridge, 2014, 6.6. [p.291, Map 6]. Issued from : J.H.M. Kouwenberg, C. Razouls & N. Desreumaux in Biogeographic Atlas of the Southern Ocean, Scient. Comm. Antarct. Res., Cambridge, 2014, 6.6. [p.291, Map 6].
Distribution of Paraeuchaeta antarctica. |
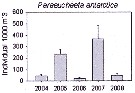 Issued from : K.M. Swadling, F. Penot, C. Vallet & al. in Polar Sci., 2011, 5. [p.125, Fig.5]. Issued from : K.M. Swadling, F. Penot, C. Vallet & al. in Polar Sci., 2011, 5. [p.125, Fig.5].
Mean abundance (ind. per 1000 m3) and standard errors of Paraeuchaeta antarctica collected each summer (January), 2004-2008 in the region covered the area from 139°E to 145°E from Terre Adélie to the Mertz Glacier Tongue.
Nota: The entire region sampled during 2004 to 2008 surveys was south of the continental slope and was under the influence of the westward flowing Antarctic Coastal Current. Macrozooplankton were collected by oblique tows of a Bongo net (500 µm mesh aperture) |
 Issued from : K.M. Swadling, So. Kawaguchi & G.W. Hosie in Deep-Sea Research II, 2010, 57. [p.896, Fig.6]. Issued from : K.M. Swadling, So. Kawaguchi & G.W. Hosie in Deep-Sea Research II, 2010, 57. [p.896, Fig.6].
Distribution of indicator species Paraeuchaeta antarctica from the BROKE-West survey (southwest Indian Ocean) during January-February 2006.
Sampling with a RMT1 net (mesh aperture: 315 µm), oblque tow from the surface to 200 m.
The survey area was located predominantly within the seasonal ice zone, and in the month prior to the survey there was considerable ice coverage over the western section but none over the east.
See map showing sampling sites in Calanus propinquus. |
| | | | Loc: | | | Antarct. (Amundsen Sea, Gerlache & Bransfield Straits, S PeterIst Island, Croker Passage, Peninsula, South Georgia, Scotia Sea, Weddell Sea, Knapp-Norvegia, Halley Bay, McMurdo Sound, SW & SE Atlant., Prydz Bay, Indian, Lützow-Holm Bay, Dumont d'Urville Sea, SW & SE Pacif.), Cape Evans (under ice), Croker Passage, sub-Antarct. (Drake Passage, N South Georgia, SW Atlant., Indian, SW & SE Pacif., Strait of Magellan, SW Atlant., S Indian, S Tasman Sea, S Chile | | | | N: | 97 | | | | Lg.: | | | (3) F: 9,9-8,4; M: 7,3-6,6; (9) F: 6,8; (25) F: 9,32-7,47; M: 7,07-6,39; (32) F: 8-7,6; (33) F: 8; (36) F: 6,51-7,53; M: 5,14-5,53; (102) F: 10,4-8,2; M: 6,8-7,8; (103) F: 9,8-8,2; M: 7,6-6,3; (876) F: 7,4-9,6; M: 5,5-7; {F: 6,51-10,40; M: 5,14-7,80}
The mean female size is 8.321 mm (n = 16; SD = 1.1602), and the mean male size is 6.586 mm (n = 12; SD = 0.8512). The size ratio (male : female) is 0.769 (n = 6; SD = 0.0231) or ± 77 %. | | | | Rem.: | Epi-meso-bathypélagic.
Sampling depth (Antarct., sub-Antarct.) : 0-1000-4000 m. After Park (1978, p.223) the species was originally described by Giesbrecht (1902) from specimens collected between 69°51'S and 71°15'S in the Bellingshaussen Sea.
For Vervoort (1951, p.92) this species is present in all the seas round the Antarctic continent South of the Antactic circle. Also to the North convergence (45°-47°S, 76°-87°E, in Vervoort, 1957); it seems likely that the species is occasionally swept to the North in the Antarctic intermediate current.
Park (1995, p.89) mentions this species in the Tasman Sea at 50°S.
The species moves rapidly towards the surface at sunset, and is capable of covering considerable vertical distances in a short time; during daytime it is completely absent from the surface. In living condition it have an opaque white body with a faint greenish tinge; A1, A2 and mouth parts are deep red (with colour often preserved after fixation).
According to Ikeda & Hirakawa, using a breeding index similar to that of Mauchline (1994), Ward & Robins (1987) have suggested two generations per year of P. antarctica in the South Georgia. | | | Last update : 17/06/2021 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed February 06, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |