|
|
 |
|
Calanoida ( Order ) |
|
|
|
Arietelloidea ( Superfamily ) |
|
|
|
Heterorhabdidae ( Family ) |
|
|
|
Heterorhabdus ( Genus ) |
|
|
| |
Heterorhabdus abyssalis (Giesbrecht, 1889) (F,M) | |
| | | | | | | Syn.: | Heterochäta abyssalis Giesbrecht,1889; 1892 (p.373, 383, 773, Descr.M, figs.M);
no Heterorhabdus abyssalis : Park, 1968 (p.561, figs.F,M); Heptner, 1971 (p.147, figs.F) | | | | Ref.: | | | Giesbrecht & Schmeil, 1898 (p.116, Rem.M, fig.M); Thompson & Scott, 1903 (p.235); Farran, 1905 (p.45); 1908 b (p.65); Wolfenden, 1911 (p.303); Farran, 1926 (p.280); Rose, 1929 (p.35); 1933 a (p.204, fig.M); Jespersen, 1940 (p.54); Lysholm & al., 1945 (p.36); Sewell, 1947 (p.175, fig.44, figs.F,M, Rem.: p.178); Farran, 1948 e (n°16, p.3, fig.M); Brodsky, 1950 (1967) (p.354, figs.M, Rem.F,M); Vervoort, 1957 (p.133, figs.F); Paiva, 1963 (p.62); Tanaka, 1964 a (p.2, figs.F, non M, Rem.); Owre & Foyo, 1967 (p.78, figs.F,M); Mazza, 1967 (p.198, 202, figs.F,M, juv., Rem.); Razouls, 1972 (p.95, Annexe: p.75); Nishida & Ohtsuka, 1996 (p.620); Chihara & Murano, 1997 (p.818, Pl.119,121: F,M); Lapernat, 1999 (p.18, 55, fig.F); Bradford-Grieve & al., 1999 (p.883, 944, figs.F,M); Bradford-Grieve,1999 b (p.78, figs.F,M, Rem., figs.174, 191); Park, 2000 (p.134, figs.F,M, Rem.); Vives & Shmeleva, 2007 (p.299, figs.F,M, Rem.) | 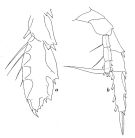 issued from : W. Vervoort in B.A.N.Z. Antarctic Research Expedition, Rep. Ser. B. III., 1957 [Fig.125] Female (from 47°43'S, 76°48'E): a, distal part of exopod of left P3; b, right P5 (posterior aspect).
|
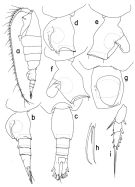 issued from : T. Park in Bull. Scripps Inst. Oceanogr. Univ. California, San Diego, 2000, 31. [p.258, Fig.106]. Female: a, habitus (left side); b, c, urosome (left, dorsal, respectively); d, genital somite (left); e, f, genital somite with operculum open (right, left, respectively); g, genital somite (ventral); h, 4th lobe of left Mx2 (posterior); i, exopod of P1 (anterior). Nota: - Urosome 30%length of body/ Length ratios of 4 urosomal somites and left caudal ramus 38.1 : 18.2 : 13.3 : 7.7 : 22.7 = 100. - Left caudal ramus extending beyond posterior end of right by about 1/10 its length as measured along medial margins. Dorsal appendicular seta of left ramus about 1.5 times length of that of right ramus. Anteriorly, each caudal ramus with 2 pores. - A1 extending beyond posterior end of caudal ramus by its last 2 segments. - Mx2: Posterior subterminal spine of 4th lobe 43% as long as 2nd saberlike spine. - P1 exopod with outer spines of 2nd and 3rd segments relatively small; outer spine of 2nd segment similar in size to 1st outer spine of 3rd segment.
|
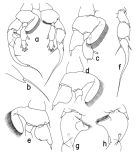 issued from : T. Park in Bull. Scripps Inst. Oceanogr. Univ. California, San Diego, 2000, 31. [p.259, Fig.107]. Male: a, P5 (anterior); b, distal end of exopod of right P5 (lateral); c, d, e, basipod of right P5 (anterior, tilted clockwise, posterior, respectively); f, exopod of left P5 (anterior); g, h, second exopodal segment of right P5 (anterior, posterior, respectively). Nota: - Prosome 73% length of body and 2.6 times length of urosome. - Urosome 28% length of body. - Caudal rami and station as in female. - Left geniculate A1 similar in morphology to that of H. spinifrons. - All other cephalosomal appendages and first 4 pairs of legs as in female. - P5: right basal inner lobe initially extending and tapering mediad for a distance nearly equal to length of segment and then widely recurved with lateral margin deeply folded, furnished with a single band of setules along whole medial margin. In right exopod , 2nd segment (fig. 107 g, h) about 1.5 times length of 1st, with a rounded tubercular process preceding the medial projection. Proximal margin of medial projection sloping at an angle of about 55° with reference to longitudinal axis of segment; distal end more or less truncated. Outer spine of 2nd segment well-developed, about 3/4 length of segment from proximal end. 3rd segment (fig. 107 a) rather strongly curved, about twice the length of the 2nd , with outer spine 37% length of segment from proximal end, terminal spine 63% as long as segment, terminal lobe (fig.107 b) 11% as long as terminal spine. -In left P5 (fig.107 a, f), basis with strongly arched medial margin, of which the distal half fringed with a single band of setules. In left exopod, 2nd segment about 1.5 times length of 1st, without lateral conical process, with outer spine only slightly longer than that of 1st segment. 3rd segment tapering distally into a long spiniform process, with outer spine about 1/2 length of inner spine and a little longer than outer spine of 2nd segment. Together with distal spiniform process, 3rd segment 69% as long as whole exopod.
|
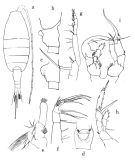 issued from : O. Tanaka in Publs Seto Mar. Biol. Lab., 1964, XII (1). [p.3, Fig.175]. doubt. Female: a, habitus (dorsal); b, last thoracic segment and genital somite (lateral left slide); c, genital somite (lateral), other specimen; d, idem (ventral); e, Mx1; f, Mx2; g, Mxp; h, P5. Nota: The urosome segments and furca are in the proportional lengths as 48 : 13 : 10 : 7 : 22 = 100 (in the first group and 48 : 13 : 10 : 8: 21 = 100 (in the second group). A1 extends beyond the end of furca at least by 2 terminal segments. Male: i, P5. Nota: The urosome segments and furca are in the proportional lengths as 24 : 17 : 16 : 12 : 7 : 24 (right) = 100 in the first group and 22 : 17 : 15 : 13 : 9: 24 in the second group. A1 extends to the distal end of the furca.
|
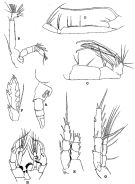 issued from : R.B.S. Sewell in The John Murray Expedition, 1933-34, Scientific Reports, VIII (1), 1947. [p.177, Fig.46]. Female (from Arabian Sea): A, urosome (lateral right side); B, A2; C, Mx2; D, Mxp; E, P1; F, exopod of P3; G, P5. Male: H, P5.
|
 issued from : R.B.S. Sewell in The John Murray Expedition, 1933-34, Scientific Reports, VIII (1), 1947. [p.166, Fig.44, K]. Process on the posterior aspect of the 2nd basal segment of P1. Remarks: The shape of the hook-like or spine-like process shows some variation in different genera, but it is undoubtedly homologous throughout the whole series. The function of this organ is unknown.
|
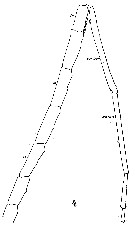 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf. 19 , Fig.4 ]. As Heterochäta abyssalis. Male: 4, distal part of A1.
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf. 20 , Figs.29, 30 ]. As Heterochäta abyssalis. Male: 29, left P5 (anterior view); 30, right P5 (anterior view).
|
 issued from : P.E. Lapernat & C. Razouls in Vie Milieu, 2002, 52 (1). [p.23, Pl. III, fig.1]. Biting edge of Md gnathobase female (from off Malta, Mediterranean Sea): respectibely: right side appendage (on the left) and left side appendage (on the right). The Itoh's index value = 2290.6 (number of teeth: 4) on the right Md, and 3281.5 on the left Md (number of teeth: 3).
|
 issued from : J.M. Bradford in N.Z. Jl mar. freshw. Res., 1971, 5 (1). [p.127, Fig.6, a-c). Female (from 42°24.5'S, 174°01.8'E): a, genital segment (right side). Male: b, lobe 4 of Mx2; c, P5.
|
 Heterorhabdus abyssalis Heterorhabdus abyssalis female: 1 - See key to species groups of Heterorhabdus: ''abyssalis'' Group (p.90, 114). 2 - Genital somite without a conical projection mediodorsally (Fig.106-a, b). 3 - Laterally, genital somite without a tubercular outgrowth posteriorly on dorsal margin (Fig.106-f). 4 - Laterally, genital operculum reaching close posterior end of somite (Fig.106-b). 5 - Laterally, genital flange elongate and low, separate from posterior ventral margin of somite by a shallow notch (Fig.106-d). Posterior subterminal spine of 4th lobe of Mx2 almost 1/2 length of 2nd saberlike spine (Fig.106-h).
|
 Heterorhabdus abyssalis Heterorhabdus abyssalis male: 1 - See key to species groups of Heterorhabdus: ''abyssalis'' Group (p.90, 114). 2 - Basis of left P5 without a well-developed inner lobe (Fig.107-a). 3 - Basal inner lobe of right P5 armed with normal bristles (Fig.107-a, c). 4 - 3rd exopodal segment of right P5 with a short terminal lobe, less than 1/3 length of terminal spine (Fig.107-a, b). 5 - Basal lobe of right P5 arising from anteromedial side of segment (Fig.107-a). 6 - 2nd exopodal segment of left P5 with outer spine not borne on a conical process (Fig.107-a, f). 7 - 3rd exopodal segment of right P5 with terminal spine longer than 1/2 length of segment (Fig.107-a). 8 - Anteriorly and when tilted clockwise, basal lobe of right P5 recurved by folding (Fig.107-d, e). 9 - Anteriorly, basal lobe of right P5 strongly folded (Fig.107-d).
| | | | | Compl. Ref.: | | | Cleve, 1904 a (p.191); Pearson, 1906 (p.26); Wilson, 1942 a (p.189); Sewell, 1948 (p.329, 503, 519, 521, 530, 532, 539, 547, 558, 567); C.B. Wilson, 1950 (p.239, Rem.); V.N. Greze, 1963 a (tabl.2); Grice, 1963 a (p.496); Shmeleva, 1964 a (p.1068); De Decker & Mombeck, 1964 (p.12); Grice & Hulsemann, 1965 (p.224); Furuhashi, 1966 a (p.295, vertical distribution in Oyashio/Kuroshio transitional area, Table 8); Mazza, 1966 (p.71); 1967 (p.367); Grice & Hulsemann, 1967 (p.18); Park, 1970 (p.477); Gamulin, 1971 (p.382, tab.3); Björnberg, 1973 (p.345, 387); Harding, 1974 (p.141, tab. 3, gut contents); Vives & al., 1975 (p.49, tab.II, III); Deevey & Brooks, 1977 (p.256, tab.2, Station "S"); Carter, 1977 (1978) (p.36); Vives, 1982 (p.293); Kovalev & Shmeleva, 1982 (p.84); Scotto di Carlo & Ianora, 1983 (p.150); Scotto di Carlo & al., 1984 (1042); Lozano Soldevilla & al., 1988 (p.59); Wiebe & al., 1988 (tab.7); Scotto di Carlo & al., 1991 (p.270); Hattori, 1991 (tab.1, Appendix); Shih & Young, 1995 (p.70); Hure & Krsinic, 1998 (p.66, 102); Razouls & al., 2000 (p.343, Appendix); d'Elbée, 2001(tabl. 1); Lapernat & Razouls, 2001 (p.123, tab.1); Rebstock, 2001 (tab.2); Holmes, 2001 (p.15); Rebstock, 2002 (p.71, Table 3, 5, 6, Fig.2, climatic variability); Beaugrand & al., 2002 (p.179, figs.5, 6); Vukanic, 2003 (139, tab.1); Hsiao & al., 2004 (p.326, tab.1); Lo & al., 2004 (p.89, tab.1); Dur & al., 2007 (p.197, Table IV); Fernandes, 2008 (p.465, Tabl.2); Wishner & al., 2008 (p.163, Table 2, fig.8, oxycline); Galbraith, 2009 (pers. comm.); Park & Ferrari, 2009 (p.143, Table 8, fig.1, biogeography); Licandro & Icardi, 2009 (p.17, Table 4); Schnack-Schiel & al., 2010 (p.2064, Table 2: E Atlantic subtropical/tropical); Mazzocchi & Di Capua, 2010 (p.425); Medellin-Mora & Navas S., 2010 (p.265, Tab. 2); Hsiao S.H. & al., 2011 (p.475, Appendix I); Benedetti & al., 2016 (p.159, Table I, fig.1, functional characters); El Arraj & al., 2017 (p.272, table 2); Belmonte, 2018 (p.273, Table I: Italian zones) | | | | NZ: | 18 | | |
|
Distribution map of Heterorhabdus abyssalis by geographical zones
|
| | | | | | | | | | | | | | | | | | 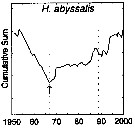 issued from : G.A. Rebstock in Global change Biology, 2002, 8. [p.77, Fig.2 d]. issued from : G.A. Rebstock in Global change Biology, 2002, 8. [p.77, Fig.2 d].
Climatic regime shifts and decadal-scale variability in calanoid copepod populations off southern California (31°-35°N, 117°-122°W, from years 1950 to 2000.
Cumulative sums of nonseasonal anomalies from the long-term means of copepod abundance .
A negative slope indicates a period of below-average anomalies; a positive slope indicates a period of above-average anomalies. Abrupt changes in slope indicate step changes. Step change ismarked with arrow (downward -pointing for decrease).
The October 1966 cruise (prior to the increase in sampling depth), March 1976 cruise (prior to the 1976-77 climatic regime shift), and October 1988 cruise (prior to the hypothesized 1989 climatic regime shift) are marked with vertical lines. |
| | | | Loc: | | | sub-Antarct. (S Indian), E South Africa, Namibia, Cape Verde Is., off Mauritania-NW Cape Verde Is., off Morocco-Mauritania, Canary Islands, off Madeira, Portugal, Bay of Biscay, Azores, Caribbean Sea, Caribbean Colombia, G. of Mexico, Florida, off Bermuda: Station ‘’ S’’ (32°10’N, 64°30’W), Sargasso Sea, off E Cape Cod, off Nova Scotia, S Iceland, Faroe Is., off Ireland (S & W), North Sea, Ibero-moroccan Bay, Medit. (Alboran Sea, NW Basin, Banyuls, Marseille, Ligurian Sea, Tyrrhenian Sea, Strait of Messina, off Malta, Adriatic Sea, Ionian Sea, Aegean Sea), Arabian Sea, Maldive Is., Natal, Madagascar (Nosy Bé), Indian (NW & S), Bay of Bengal, Philippines, China Seas (East China Sea, South China Sea), Taiwan (SW, E, N: Mienhua Canyon), Japan (Izu, Nansei Is., off Sanriku), E Pacif. (N-S), off British Columbia, California, Guaymas Basin, SE Easter Is., Chile, New Zealand, N Tasman Sea.
Type locality: 14°N, 132°W;
For Park (2000, p.135) number identifications are doubtful. Mainly in the eastern Pacific between 35°S and 33°N and of the Americas westward to 118°W.
Type locality: 14°N, 132°W | | | | N: | 66 | | | | Lg.: | | | (11) F: 3,1-3,08; M: 2,85; (22) F: 2,4; M: 2,75; (24) F: 2,65; (25) F: 2,38; (38) F: 2,6-2,2; M: 2,6-2; (46) M: 2,75; (49’) F: 2,5-2,4; (58) F: 2,4; (73) M: 2,31; (121) F: 2,09; M: 2,34; F: 3,73-3,23; M: 3,08-2,92; (207) F: 2,74-2,5; M: 2,64-2,36; (340) F: 2,5; 2,4; (808) F: 3,11-2,9; (824) F: 3,36-2,88; M: 3,16-2,72; (909) F: 2,7-3,55; M: 2,6-3,4; {F: 2,09-3,73; M: 2,00-3,40} | | | | Rem.: | After Pearson (1906, p.26): vertical range down to 2.800 m. Sampling depth (sub-Antarct.) : 250-500 m. Overall Depth Range in Sargasso Sea: 0-2000 m (Deevey & Brooks, 1977, Station "S"); In vertical tow 4000-3000 m (Harding, 1974).
""Abyssalis" Group.
Epi- to bathypelagic.
Sewell (1947, p.175 & suiv.) emphasizes the difficulties to discriminate between several species: H. abyssalis and H. norvegicus. The form norvegicus appears to be confined to the Arctic and North Atlantic Oceans, the form abyssalis appears to be an Atlantic and Indian Oceans.
For Park (2000, p.135) this species is very close in habitus and details of appendages to H. austrinus, hence certain identifications seem contestable and a poorly assured geographical distribution. After Park, descriptions by Giesbrecht (1889, 1892, 1898) were based solely on the male (2.75 mm long). Subsequently, the species was reported by Sewell (1947), Vervoort (1957), Tanaka (1968), and Bradford (1971a, p.127), but none of the material of these authors is identical to H. abyssalis as described by Giesbrecht (1892). The Park's specimen is in agreement of the male P5 with the one of Giesbrecht and found in its type locality. H. abyssalis is very close in habitus and details of the appendages to H. austrinus but can be distinguished from it in the female by the genital somite, of which the posterior edge of the left genital flange meets the ventral wall of the somite in a characteristic notch, and in the male by the P5 of which the right 3rd exopodal segment has a long terminal spine and the outer spine of the left 2nd exopodal segment is not borne on a conical process.
See remark concerning the variation of genital somite shape in Bradford (1971 a, p.124 in H. spinosus). | | | Last update : 24/10/2022 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed February 06, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |













