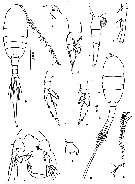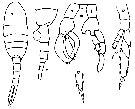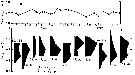|
|
 |
|
Calanoida ( Order ) |
|
|
|
Lucicutiidae ( Family ) |
|
|
|
Lucicutia ( Genus ) |
|
|
| |
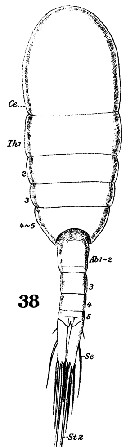
Lucicutia flavicornis (Claus, 1863) (F,M) | |
| | | | | | | Syn.: | Leuckartia flavicornis Claus, 1863 (p.183, figs.F,M); Giesbrecht, 1892 (p.358, 366, 773, figs.F,M); T. Scott, 1894 b (p.44); Oliveira, 1945 (p.191);
no L. favicornis (F) : Brady, 1883 (p.50, figs.F); | | | | Ref.: | | | Giesbrecht & Schmeil, 1898 (p.111, Rem. F,M)); Thompson & Scott, 1903 (p.235); Esterly, 1905 (p.180, figs.F, Rem.F,M) ; Wolfenden, 1905 a (p.23, 24); Farran, 1908 b (p.64, Rem.); A. Scott, 1909 (p.125, Rem.); Wolfenden, 1911 (p.323); Sewell, 1912 (p.353, 366); 1914 a (p.228); Pesta, 1920 (p.530); Lysholm & Nordgaard, 1921 (p.25); Esterly, 1924 (p.98, figs.F); Sars, 1925 (p.207); Farran, 1926 (p.274, 276, figs.F, Rem.); 1929 (p.209, 262); Rose, 1929 (p.33); Sewell, 1932 (p.294); Rose, 1933 a (p.192, figs.F,M); Farran, 1936 a (p.111); Mori, 1937 (1964) (p.72, figs.F,M); Dakin & Colefax, 1940 (p.92, figs.M); Lysholm & al., 1945 (p.34); Sewell, 1947 (p.174, Rem.); Davis, 1949 (p.55, Rem.F,M); Brodsky, 1950 (1967) (p.327, figs.F,M, Rem.); Marques, 1953 (p.117); Chiba & al., 1957 (p.310); 1957 a (p.11); Marques, 1959 (p.216); Tanaka, 1960 (p.52); Grice, 1962 (p.220, figs.F,M, Rem.); Brodsky, 1962 c (p.132, figs.F,M); Tanaka, 1963 (p.39, figs.F, Rem.F,M); Kasturirangan, 1963 (p.42, figs.F,M); Paiva, 1963 (p.57, figs.F,M); Vilela, 1965 (p.9); Chen & Zhang, 1965 (p.83, figs.F,M); Vervoort, 1965 (p.111, Rem.); Hülsemann, 1966 (p.711, figs.F,M, Rem.); Saraswathy, 1966 (1967) (p.80); Owre & Foyo, 1967 (p.76, figs.F,M); Park, 1968 (p.560, figs.F,M, Rem.: anomaly); Vidal, 1968 (p.38, figs.F,M); Vilela, 1968 (p.25, figs.F,M); Ramirez, 1969 (p.78, figs.F, Rem.); Corral Estrada, 1970 (p.185); Minoda, 1971 (p.36); Kos, 1972 (Vol. I, figs. F, M, Rem.); Bradford, 1972 (p.46, figs.F,M, Rem.); Razouls, 1972 (p.94, Annexe: p.72); Marques, 1973 (p.243); 1974 (p.17, fig.M); Arcos, 1975 (p.21, figs.F); Dawson & Knatz, 1980 (p.5, figs.F,M); Björnberg & al., 1981 (p.648, figs.F,M); Ali-Khan & Ali-Khan, 1982 (p.266, figs.M); Marques, 1982 (p.762); Gardner & Szabo, 1982 (p.358, figs.F,M); Zheng & al., 1982 (p.54, figs.F,M); Zheng Zhong & al., 1984 (1989) (p.248, figs.F,M); Roe, 1984 (p.358); Kimmerer & al., 1985 (p.428); He & al., 1992 (p.250); Chihara & Murano, 1997 (p.829, tab.5, Pl.124: F,M); Bradford-Grieve & al., 1999 (p.883, 945, figs.F,M); Bradford-Grieve,1999 b (p.97, figs.F,M, Rem., figs.176, 191); Conway & al., 2003 (p.89, figs.F,M, Rem.); Boxshall & Halsey, 2004 (p.133: F; p.134: M); Avancini & al., 2006 (p.99, Pl. 68, figs.F,M, Rem.); Vives & Shmeleva, 2007 (p.331, figs.F,M, Rem.); Phukham, 2008 (p.53, figs. F,M); Blanco-Bercial & al., 2011 (p.103, Table 1, Biol. mol, phylogeny) |  issued from : J.M. Bradford-Grieve in The Marine Fauna of New Zealand: Pelagic Calanoid Copepoda. National Institute of Water and Atmospheric Research (NIWA). NIWA Biodiversity Memoir, 111, 1999. [p.93, Fig.59]. Female (from 31°19.5'S, 165°19'E): A, habitus (dorsal); B, urosome (left lateral side); C, A1; D, A2; E, Md; F, Mx1; G, Mx2; H, Mxp; I, P1; J, inner distal seta on basipod 2 of P1; K, P2; L, P3; M, P4; N, P5; O, P5 (exopod segment 3 from another specimen); P, P5 (exopod segment 3 from specimen from another station). Nota : Head without lateral protrusions. Genital boss large (0.56 times the length of genital segment), placed centrally on the segment ; genital segment symmetrical in dorsal view. Anal segment as long as urosome segment 3. Caudal rami divergent or parallel and not touching, slightly more than 5 times as long as wide, innermost terminal seta small and slender. A1 extends to middle of caudal rami. Basipod 2 of P1 with a low cylindrical process. Endopod 3-segmented. Endopod of P5 3-segmented ; inner spine on exopd segment 2 long and straight except for a slight bend at tip, reaching beyond base of 1st inner seta on exopod segment 3 ; terminal spine on exopod segment 3 less than half the length of its segment, outer margin of exopod segment 3 with several teeth.
|
 issued from : J.M. Bradford-Grieve in The Marine Fauna of New Zealand: Pelagic Calanoid Copepoda. National Institute of Water and Atmospheric Research (NIWA). NIWA Biodiversity Memoir, 111, 1999. [p.94, Fig.60]. Male: A, habitus (dorsal); B, left A1; C, A2; D, Md (mandibular palp); E, Mx1; F, Mx2; G, Mxp; H, P1; I, P2; J, P3; K, P4; L, P5 (L = left leg; R = right leg). Nota : Head without lateral protrusions. Anal segment almost as long as urosome segment 4. Caudal rami are slightly more than 5 times longer than wide, innermost terminal seta small and slender. A1 extends as far as middle of caudal rami. Endopod of P1 3-segmented. Inner margin of Bbasipod 1 of right P5 with a conspicuous rounded protrusion, basipod 2 with a triangular inner border bearing hairs distally. Basipod 1 of left P5 with a ridge on the inner margin, basipod 2 with inner distal corner protruding and ending in one point and with 3-5 extra teeth and sometimes a proximal spinule.
|
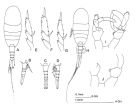 issued from : J.M. Bradford-Grieve in The Marine Fauna of New Zealand: Pelagic Calanoid Copepoda. National Institute of Water and Atmospheric Research (NIWA). NIWA Biodiversity Memoir, 111, 1999. [p.99, Fig.64]. As Lucicutia cf. flavicornis. Female: A, habitus (dorsal); B, urosome (right lateral side); C, idem (dorsal, another specimen); D, idem (left lateral side, another specimen); E, F, G, exopod of P5 of three different specimens. Male: H, habitus (dorsal); I, P5; J, P5 (another specimen). These specimens differ from L. flavicornis s. str.; this form has not been described as new because there is a great deal of variability among the Southwest Pacific specimens and related specimens from other localities. Cf: Lucicutia sp.
|
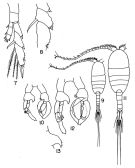 Issued from : T.S. Park in Fishery Bull. Fish Wildl. Serv. U.S., 1968, 66 (3). [p.558, Pl.10, Figs.7-11]. Female: 7, P5; 8, abnormal exopod of left P5. Male: 9, habitus (dorsal); 10, P5 (posterior); 11, habitus (dorsal) of the abnormal P5; 12, P5 (posterior); 13, inner projection of basis of left P5. Nota: The P5 differs of the normality in the followuing aspects: The projection on the internal margin of the basis in the left leg is tapered distally, with 3 to 4 acute teeth along the internal edge. The first exopodal segment of the right leg has proximally a triangular process along the internal margin. The second exopodal segment of the same leg is pronouncedly curved.
|
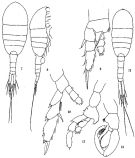 issued from: Q.-c Chen & S.-z. Zhang in Studia Marina Sinica, 1965, 7. [Pl.32, 7-13]. Female (from E China Sea): 7, habitus (dorsal); 8, idem (lateral right side); 9, right P1 (anterior); 10, left P5 (posterior). Male: 11, habitus (dorsal); 12, left P5 (posterior); 13, right P5 (posterior).
|
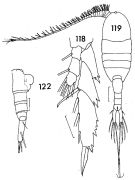 issued from : F.C. Ramirez in Contr. Inst. Biol. mar., Buenos Aires, 1969, 98. [p.78, Lam. XV, figs.118, 119, 122 ]. Female (from off Mar del Plata): 118, P5; 119, habitus (dorsal); 122, urosome (lateral right side). Scale bars in mm: 0.05 (118); 0.2 (119); 0.1 (122).
|
 issued from : R.B.S. Sewell in The John Murray Expedition, 1933-34, Scientific Reports, VIII (1), 1947. [p.166, Fig.44, C]. Process on the posterior aspect of the 2nd basal segment of P1. Remarks: The shape of the hook-like or spine-like process shows some variation in different genera, but it is undoubtedly homologous throughout the whole series. The function of this organ is unknown.
|
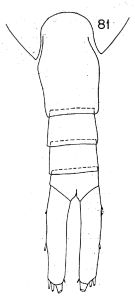 Issued from : K. Hülsemann in Bull. Mar. Sc., 1966, 16 (4). [p.720, Fig.81]. Female: 81, urosome (dorsal).
|
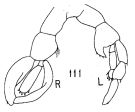 Issued from : K. Hülsemann in Bull. Mar. Sc., 1966, 16 (4). [p.726, Fig.111]. Male: 111, P5.
|
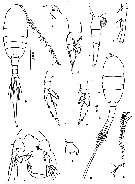 issued from : Z. Zheng, S. Li, S.J. Li & B. Chen in Marine planktonic copepods in Chinese waters. Shanghai Sc. Techn. Press, 1982 [p.55, Fig.31]. Female: a, habitus (dorsal); b, forehead (lateral); c, urosome (lateral); d, P1; e, P4; f, P5. Male: g, habitus (dorsal); h, left A1; i, geniculate segments of right A1; J, P5; k, basipodal segment 2 of P5 (another specimen). Scale bars in mm.
|
 issued from : T. Mori in The pelagic Copepoda from the neighbouring waters of Japan, 1937 (1964). [Pl.37, Figs.1-6]. Female: 2, habitus (dorsal); 3, P5. Nota: Anal segment shorter than the preceding one. Caudal rami symmetrical; ramus about 6 times as long as its width., 2nd terminal setae is thick, and twice as long as the urosome. Male: 1, habitus (dorsal); 4, P5 (anterior); 5, right A1; 6, left A1.
|
 issued from : C.O. Esterly in Univ. Calif. Publs Zool., 1924, 26 (5). [p.98, Fig. I). Female (from San Francisco Bay): 1, forehead (lateral); 2, habitus (dorsal); 3, outline of dorsal part of cephalothorax (lateral); 4, A2; 5, Mxp; 6, urosome (lateral); 7, posterior margin of last thoracic segment and genital segment; 8, basal part of A1; 9, P5; 10, P2; 11, P1; 12, urosome (ventral). Nota: Relative lengths of abdominal segments and caudal rami: 17:5:4:2:24. Caudal rami 5 times as long as width at base, 12 times length of anal segment, nearly as long as combined lengths of first three segments of abdomen
|
 issued from : C.O. Esterly in Univ. Calif. Publs. Zool., 1905, 2 (4). [p.180, Fig.36]. Female (from San Diego Region): a, habitus (lateral); b, P5 (ri = endopodite); c, outer margin of exopod of P3. Nota: Anal segment shorter than the preceding. 2nd terminal bristle of caudal ramithick, twice as long as abdomen.A1 reach beyond middle of the caudal rami, segment 19 as long as tenth to twelfth, inclusive. 2nd basal of Mx1 with 4 bristles.
|
 issued from : J.M. Bradford in Mem. N. Z. Oceonogr. Inst., 1972, 54. [p.47, Fig.12, (4-6]. Female (from Kaikoura, New Zealand): 4, habitus (dorsal); 5, P5. Male: 6, P5. Scale bars: 1 mm (4); 0.1 mm (5, 6).
|
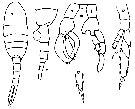 issued from : S. Ali-Khan & J. Ali-Khan in Crustaceana, 1982, 43 (3). [p;306, Figs.9-13]. Female (from 24°09'N, 64°27'E): 11, last thoracic segment and urosome (lateral, left side); 12, P5; 13, terminal portion of the other P5. Male: 9, habitus (dorsal); 10, P5 (R = right leg, L = left leg).
|
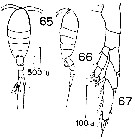 issued from : D.F.R. Arcos in Gayana, Zool., 1975, 32. [Lam.VIII, Figs.65-67]. Female (from Bahia de Concepcion, Chile): 65-66, habitus (dorsal and lateral, respectively); 67, P5.
|
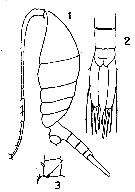 issued from : G.P. Farran in Biscayan Plankton collected during a Cruise of H.M.S. 'Research', 1900.- Part XIV. The Copepoda. (Linn. Journ. Zoology, XXXVI, 1926). [p.304, Pl.9, Figs.1-3]. Female: 1, habitus (lateral); 2, last urosomal segments and caudal rami (dorsal); 3, 2nd basipod of P1.
|
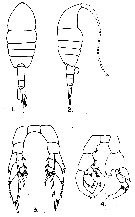 issued from : G.D. Grice in Fish. Bull. Fish and Wildl. Ser., 1962, 61. [p.221, Pl.24, Figs.1-4]. Female (from equatorial Pacific): 1-2, habitus (dorsal and lateral, respectively); 3, P5. Nota: anal segment short. Male: 4, P5 Nota: Protrusion of the 2nd basipodal segment of the left P5 and long terminal segment of the right P5. Remarks: In at least one collection (station:00°11'S, 119°58'W, two size groups were noted (females: 1.46-1.53 mm and 1.87-1.90mm; males: 1.39-1.53 mm and 1.77 mm).
|
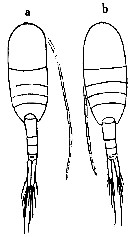 R. Stephen, 2007 : Data sheets of NIO, Kochi, India (on line). Female (Indian Ocean): a, specimen with slender abdomen; b, specimen with short abdomen. Nota: Prosome contained about 1.5 times in the length of the cephalothorax. Prosomal segments and caudal rami in the proportional length 31 : 13: 9 : 9 : 38 = 100. Caudal rami 5.5 times as long as wide at the proximal. A1 extends a little beyond the middle of the caudal rami. The terminal spine of the exopod of P5 is slightly longer than half the length of the 3rd segment of the exopod (7 : 12). According to Giesbrecht the length of the caudal rami varies from 1/3 to 1/4 the combined length of the urosomal segments and rami. Male: Abdomen contained 1.7 times in the length of the cephalothorax. In the left A1 the combined length of the segments 19-31 is equal in length to that of the segments 22-23. In P5 the 2nd basal segment of the left has denticles on the inner marginal process which varies in number ranging from 4 to 8.
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf. 19 , Fig.3 ]. As Leuckartia flavicornis . Female: A1 (segments 1 to 18; ventral view).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf. 19 , Fig.3 ]. As Leuckartia flavicornis . Female: 3, A1 (segments 16 to 25; ventral view).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf. 19 , Figs.10, 19 ]. As Leuckartia flavicornis. Female: 10, Md (mandibular palp; posterior view); 19, Md (edge of gnathobase).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf. 19 , Fig.9 ]. As Leuckartia flavicornis. Female: 9, Mx1 (posterior view).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf. 19 , Fig.8 ]. As Leuckartia flavicornis. Female: 8, Mxp (posterior view).
|
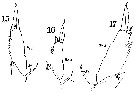 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf. 19 , Figs.15, 16, 17 ]. As Leuckartia flavicornis. Female: 15, exopod of P5; 16 and 17, same. Si = inner spine; Re 3 = exopodite 3.
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf. 19 , Figs.21, 22 ]. As Leuckartia flavicornis. Female: 21, P5 (anterior view); 22, exopod of P3.
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf. 19 , Fig.2]. As Leuckartia flavicornis. Male: 2, A1 (ventral view).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf. 19 , Fig.11 ]. As Leuckartia flavicornis. Male: 11, Mx2 (posterior view). L = lobe; Ri = endopod..
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf. 19 , Figs.20, 23 ]. As Leuckartia flavicornis. Male: 20, P2 (anterior view); 23, P1 (anterior view).. B1 = basipodite 1 (= coxa); B2 = basipodite 2 (= basis); Ri = endopod; Re = exopod; Se = outer spine.
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf. 19 , Figs.29, 38]. As Leuckartia flavicornis. Male: 29, right P5 (basipod); 38, P5 (posterior view).. Pd = right leg; Ps = left leg; B1 = basipodite 1 (= coxa); B2 = basipodite 2 (= basis); Ri = endopod; Re = exopod.
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf. 38, Fig.38]. As Leuckartia flavicornis. Female: 38, habitus (dorsal).
|
 issued from : N. Phukham in Species diversity of calanoid copepods in Thai waters, Andaman Sea (Master of Science, Univ. Bangkok). 2008. [p.138, Fig.12]. Female (from W Malay Peninsula) : a-b, habitus (dorsal and lateral, respectively); c-d, urosome (dorsal and lateral, respectively); e, P5. Male: f, habitus (dorsal); g, urosome (dorsal); h, right P5; i, left P5. Body length after the drawings: F = 1.35 mm; M = 1.103 mm.
|
 Lucicutia flavicornis Lucicutia flavicornis female: 1 - Characters following not combined : Prosome about 3 times longer than urosome. Cephalosome with slightly projecting and rounded anterior corners and well developed lateral spinous projections; anal somite about as long as wide; caudal rami 11.7 times longer than wide and bowed outwards at base, leaving elliptical space between rami proximally. 2 - P1 with 3-segmented endopod. 3 - Cephalosome without lateral spinous projections. 4 - Genital double-somite symmetrical (dorsal view). 5 - Anal somite much shorter than caudal ramus. 6 - P5 with 3-segmented endopod. 7 - Characters not combined between distal 3 to 4 segments of A1 reaching beyond tip of caudal ramus and body length less than 3mm. 8 - Cephalosome without projections. 9 - Terminal setal element of P5 about half length of 3rd exopodal segment; body length less than 2 mm. 10 - Caudal rami separate and divergent: anal somite shorter than preceding somite; genital swelling large.
|
 Lucicutia flavicornis Lucicutia flavicornis male: 1 - P1 with 3-segmented endopod. 2 - Cephalosome without lateral projections. 3 - Right A1 reaching at most 2 segments beyond tip of caudal rami. 4 - Caudal rami at most 7 times longer than wide. 5 - Inner margin of basis of both P5 without pointed process; endopod of right P5 -2 segmented. 6 - Caudal rami more than 4 times longer than wide. 7 - Body length less than 2.0 mm. 8 - A1 reaching beyond midlength of caudal rami; anal somite shorter than preceding somite; basis of left P5 with produced inner distal corner with 4-8 small teeth.
|
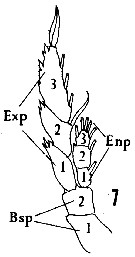 Issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1, Crustacea, 1: Copepoda. Copepods of the Florida Current, 1967. [p.11, Fig.7]. Femelle: 7, P5. Nota: Bsp = basipodite. 1 = Coxa; 2 = Basis. Enp = endopodite; Exp = Exopodite.
|
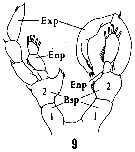 Issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1, Crustacea, 1: Copepoda. Copepods of the Florida Current, 1967. [p.11, Fig.9]. Male: 9, P5.
|
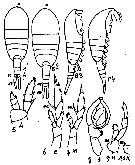 Issued from : M.C. Kos in Field guide for plankton. Zool Institute USSR Acad., Vol. I, 1972. . After Brodsky, 1962. Female: 1, habitus (dorsal) forma 'A'; 2, same, forma 'B'; 3, habitus (latera) forma 'A'; 4, same, forma 'B'; 5, P1; 6, P5, forma 'A'; 7, P5, forma 'B'; 8, right P5; 9, left P5; 10, inner left basis.
| | | | | Compl. Ref.: | | | Cleve, 1904 a (p.192); Pearson, 1906 (p.25); Rose, 1924 d (p.480); 1925 (p.152); Wilson, 1942 a (p.192); Massuti Alzamora, 1942 (p.94, Rem.); Sewell, 1948 (p.323, 503, 512); Moore, 1949 (p.56); C.B. Wilson, 1950 (p.255); Krishnaswamy, 1953 (p.126, Rem.: F, non M); Kott, 1957 (p.5, 18); Yamazi, 1958 (p.150, Rem.); Fagetti, 1962 (p.30); Cervigon, 1962 (p.181, tables: abundance distribution); Fish, 1962 (p.22); Ganapati & Shanthakumari, 1962 (p.8, 15); Grice & Hart, 1962 (p.287, table 4: abundance); Ahlstrom & Thrailkill, 1963 (p.57, Table 5, abundance); Gaudy, 1962 (p.93, 99, Rem.: p.111) ; Duran, 1963 (p.22); Giron-Reguer, 1963 (p.52); V.N. Greze, 1963 a (tabl.2); Shmeleva, 1963 (p.141); Grice, 1963 a (p.496); Gaudy, 1963 (p.26, Rem.); Björnberg, 1963 (p.51, Rem.); Deevey, 1964 (p.589, Table 3, lengths variation); De Decker & Mombeck, 1964 (p.13); Grice & Hulsemann, 1965 (p.224); Shmeleva, 1965 b (p.1350, lengths-volume -weight relation); Pavlova, 1966 (p.44); Furuhashi, 1966 a (p.295, vertical distribution in Kuroshio region, Table 8, 9); Neto & Paiva, 1966 (p.27, Table III); Mazza, 1966 (p.71); 1967 (p.329, 367); Ehrhardt, 1967 (p.740, 887, geographic distribution, Rem.); Fleminger, 1967 a (tabl.1); Grice & Hulsemann, 1967 (p.17); De Decker, 1968 (p.45); Delalo, 1968 (p.138); Evans, 1968 (p.13); Berdugo & Kimor, 1968 (p.448); Dowidar & El-Maghraby, 1970 (p.268); Itoh, 1970 (tab.1); Park, 1970 (p.477); Timonin, 1971 (p.281, trophic group); Deevey, 1971 (p.224); Gamulin, 1971 (p.381, tab.2); Carli, 1971 (p.372, tab.1, 2); Binet & al., 1972 (p.69); Roe, 1972 (p.277, tabl.1, tabl.2); Apostolopoulou, 1972 (p.328, 360); Bainbridge, 1972 (p.61, Appendix Table I: vertical distribution vs day/night, Table II: %); Björnberg, 1973 (p.344, 387); Guglielmo, 1973 (p.399); Corral Estrada & Pereiro Muñoz, 1974 (tab.I); Harding, 1974 (p.141, tab. 3, gut contents); de Bovée, 1974 (p.109, 124); Peterson & Miller, 1975 (p.642, 650, Table 3, interannual abundance); Patel, 1975 (p.659); Vives & al., 1975 (p.48, tab.II, III, IV); Vives, 1976 (p.104); Peterson & Miller, 1976 (p.14, Table 1, 2, 3, abundance vs interannual variations); Deevey & Brooks, 1977 (p.256, tab.2, Station "S"); Peterson & Miller, 1977 (p.717, Table 1, seasonal occurrence); Carter, 1977 (1978) (p.36); Bowman, 1977 (p.695, Rem.: parasite); Dessier, 1979 (p.187, 201, 206); Vaissière & Séguin, 1980 (p.23, tab.2); Rudyakov, 1982 (p.208, Table 2); Kovalev & Shmeleva, 1982 (p.84); Vives, 1982 (p.293); Scotto di Carlo & Ianora, 1983 (p.150); Dessier, 1983 (p.89, Tableau 1, 2, Rem., %); Tremblay Anderson, 1984 (p.5, Rem.); Stephen, 1984 (p.161, 169, Distribution vs thermocline & geographic); Guangshan & Honglin, 1984 (p.118, tab.); De Decker, 1984 (p.317, 353: chart); Cummings, 1984 (p.163, Table 2); Binet, 1984 (tab.3); Longhurst, 1985 (tab.2); Almeida Prado Por, 1985 (p.250); Regner, 1985 (p.11, Rem.: p.35); Moraitou-Apostolopoulou, 1985 (p.303, occurrence/abundance in E Mediterranean Sea, Rem.: p.309); Jansa, 1985 (p.108, Tabl.I, II, III, IV, V); Wishner & Allison, 1986 (tab.2); Brenning, 1985 a (p.24, Table 2): 1986 (p.11, spatial distribution, T-S diagram, Rem.); Brinton & al., 1986 (p.228, Table 1); Chen Y.-Q., 1986 (p.205, Table 1: abundance %, Table 2: vertical distribution); Madhupratap & Haridas, 1986 (p.105, tab.1); M. Lefèvre, 1986 (p.33); Rudyakov, 1986 (tab.1); Jimenez-Perez & Lara-Lara, 1988; Dessier, 1988 (tabl.1); Lozano Soldevilla & al., 1988 (p.59); Cervantes-Duarte & Hernandez-Trujillo, 1989 (tab.3); Suarez & al., 1990 (tab.2); Pancucci-Papadopoulou & al., 1990 (p.199); Madhupratap & Haridas, 1990 (p.305, fig.3: vertical distribution night/day; fig.7: cluster); Hirakawa & al., 1990 (tab.3); Othman & al., 1990 (p.565, Rem.); Hattori, 1991 (tab.1, Appendix); Shih & Marhue, 1991 (tab.2, 3); Yoo, 1991 (tab.1); Suarez & Gasca, 1991 (tab.2); Suarez, 1992 (App.1); Ashjian & Wishner, 1993 (p.483, abundance, species group distributions); Kimmerer, 1993 (tab.2); Seguin & al., 1993 (p.23, 26: Rem.); Kouwenberg, 1994 (tab.1); Shih & Young, 1995 (p.70); Palomares Garcia & Vera, 1995 (tab.1); Heinrich, 1995 (tab.1); Webber & Roff, 1995 (tab.1); Padmavati & Goswami, 1996 a (p.85, fig.3, Table 4, vertical distribution); Park & Choi, 1997 (Appendix); Hure & Krsinic, 1998 (p.64, 102); Madhupratap & al., 1996 (p.866); Kotani & al., 1996 (tab.2); Dower & Mackas, 1996 (p.837, seamount effects v.s. abundance); Suarez-Morales & Gasca, 1997 (p.1525); Padmavati & al., 1998 (p.349); Gilabert & Moreno, 1998 (tab.1, 2); Alvarez-Cadena & al., 1998 (tab.2,3,4); Noda & al., 1998 (p.55, Table 3, occurrence); Mauchline, 1998 (tab.30, 58); Suarez-Morales & Gasca, 1998 a (p.110); Siokou-Frangou, 1999 (p.476); Lapernat, 1999 (p.21, 55); Dolganova & al., 1999 (p.13, tab.1); Lavaniegos & Gonzalez-Navarro, 1999 (p.239, Appx.1); Neumann-Leitao & al., 1999 (p.153, tab.2); Onishchik, 1999 (p.76); El-Sherif & Aboul Ezz, 2000 (p.61, Table 3: occurrence); Razouls & al., 2000 (p.343, Appendix); Fernandez-Alamo & al., 2000 (p.1139, Appendix); Suarez-Morales & Gasca, 2000 (1247, tab.1); Lopez-Salgado & al., 2000 (tab.1); Moraitou-Apostolopoulou & al., 2000 (tab.I, fig.7); Haury & al., 2000 (p.69, Table 1); Madhupratap & al., 2001 (p. 1345, vertical distribution vs. O2, figs.4, 5: clusters, p.1353); Lapernat & Razouls, 2001 (p.123, tab.1); Rebstock, 2001 (tab.2); Dalal & Goswami, 2001 (p.22, fig.2); Holmes, 2001 (p.17); Lo & al., 2001 (1139, tab.I); Sameoto & al., 2002 (p.12); Zerouali & Melhaoui, 2002 (p.91, Tableau I); Yamaguchi & al., 2002 (p.1007, tab.1); Rebstock, 2002 (p.71, Table 3, 6, Fig.2, climatic variability); Keister & Peterson, 2003 (p.341, Table 1, abundance, cluster species vs hydrological events); Vukanic, 2003 (139, tab.1); Hwang & al., 2003 (p.193, tab.2); Shimode & Shirayama, 2004 (tab.2); Hsiao & al., 2004 (p.326, tab.1); Hsieh & al., 2004 (p.397, tab.1, p.399, tab.2); Wang & Zuo, 2004 (p.1, Table 2, dominance, origin); Rezai & al., 2004 (p.490, tab.2, p.495, tab.8); Chang & Fang, 2004 (p.456, tab.1); Lan & al., 2004 (p.332, tab.1); Lo & al.*, 2004 (p.218, fig.6); Pusch & al., 2004 (251, tab.3); Gallienne & al., 2004 (p.5, tab.3); Lo & al., 2004 (p.89, tab.1); Vukanic & Vukanic, 2004 (p.9, tab. 2); Kazmi, 2004 (p.228); Shimode & al., 2005 (p.113 + poster); Rezai & al., 2005 (p.157, Table 5: spatial & temporal variations); Markhaseva & Ferrari, 2005 (p.1098: Rem.); Lopez-Ibarra & Palomares-Garcia, 2006 (p.63, Tabl. 1, seasonal abundance vs El-Niño); Zuo & al., 2006 (p.163: tab.1); Isari & al., 2006 (p.241, tab.II); Hwang & al., 2006 (p.943, tabl. I); Sterza & Fernades, 2006 (p.95, Table 1, occurrence); Dias & Araujo, 2006 (p.53, Rem., chart); Lavaniegos & Jiménez-Pérez, 2006 (p.147, tab.2, 4, Rem.); Mageed, 2006 (p.171, Table 2, 4); Zervoudaki & al., 2006 (p.149, Table I); Hooff & Peterson, 2006 (p.2610); Rakhesh & al., 2006 (p.93, Table 2, spatial distribution); Koppelmann & Weikert, 2007 (p.266: tab.3); Khelifi-Touhami & al., 2007 (p.327, Table 1); Hwang & al., 2007 (p.24); Dur & al., 2007 (p.197, Table IV); Valdés & al., 2007 (p.104: tab.1); Jitlang & al., 2008 (p.65, Table 1); Ayon & al., 2008 (p.238, Table 4: Peruvian samples); McKinnon & al., 2008 (p.844: Table.1); Lopez Ibarra, 2008 (p.1, Table 1, 2, figs.11, 16: abundance, Table 3: N, C isotopes,Table 4: index trophic); Neumann-Leitao & al., 2008 (p.799: Tab.II, fig.6); Morales-Ramirez & Suarez-Morales, 2008 (p.513, 520); Wishner & al., 2008 (p.163, Table 2, fig.8, oxycline); Gaard & al., 2008 (p.59, Table 1, N Mid-Atlantic Ridge); Raybaud & al., 2008 (p.1765, Table A1); Lan Y.C. & al., 2008 (p.61, Table 1, % vs stations, Table 2: indicator species); Tseng L.-C. & al., 2008 (p.153, Table 2, fig.5, occurrence vs geographic distribution, indicator species); Tseng & al., 2008 (p.402, Table 2); Tseng L.-C. & al., 2008 (p.46, table 2, abundance vs moonsons); Pagano, 2009 (p.116); C.-Y. Lee & al., 2009 (p.151, Tab.2); Galbraith, 2009 (pers. comm.); Chiba & al., 2009 (p.1846, Table 1, occurrence vs temperature change); Miyashita & al., 2009 (p.815, Tabl.II); Tseng & al., 2009 (p.327, fig.5, feeding); Licandro & Icardi, 2009 (p.17, Table 4); Lan Y.-C. & al., 2009 (p.1, Table 2, % vs hydrogaphic conditions); Brugnano & al., 2010 (p.312, Table 3, fig.8); Williamson & McGowan, 2010 (p.273, Table 3, Pacific central gyres: N and S); Hernandez-Trujillo & al., 2010 (p.913, Table 2); Cornils & al., 2010 (p.2076, Table 3); Schnack-Schiel & al., 2010 (p.2064, Table 2: E Atlantic subtropical/tropical); Hidalgo & al., 2010 (p.2089, Table 2); Dias & al., 2010 (p.230, Table 1); Mazzocchi & Di Capua, 2010 (p.426); Medellin-Mora & Navas S., 2010 (p.265, Tab. 2); Fazeli & al., 2010 (p.153, Table 1); W.-B. Chang & al., 2010 (p.735, Table 2, abundance); Shanthi & Ramanibai, 2011 (p.132, Table 1); Hsiao S.H. & al., 2011 (p.475, 481: indicator species, Appendix I); Hsiao & al., 2011 (p.317, Table 2, indicator of seasonal change); Pillai H.U.K. & al., 2011 (p.239, Table 3, vertical distribution); Selifonova, 2011 a (p.77, Table 1, alien species in Black Sea); Guo & al., 2011 (p.567, table 2, indicator); Isari & al., 2011 (p.51, Table 2, abundance vs distribution); Tutasi & al., 2011 (p.791, Table 2, abundance distribution vs La Niña event); Andersen N.G. & al., 2011 (p.71, Fig.3: abundance); Shiganova & al., 2012 (p.61, Table 4); Jang M.-C & al., 2012 (p.37, abundance and seasonal distribution); Uysal & Shmeleva, 2012 (p.909, Table I); Salah S. & al., 2012 (p.155, Tableau 1); Miloslavic & al., 2012 (p.165, Table 2, transect distribution); Dorgham & al., 2012 (p.473, Table 3: abundance %; Table 4: abundance vs season); Tseng & al., 2012 (p.621, Table 3: abundance); Hidalgo & al., 2012 (p.134, Table 2); Belmonte & al., 2013 (p.222, Table 2, abundance vs stations); Palomares-Garcia & al., 2013 (p.1009, Table I, abundance vs environmental factors, very rare); in CalCOFI regional list (MDO, Nov. 2013; M. Ohman, comm. pers.); Tseng & al., 2013 (p.507, seasonal abundance); Tseng & al., 2013 a (p.1, Table 3, 4, abundance); Hirai & al., 2013 (p.1, Table I, molecular marker); Hwang & al., 2014 (p.43, Appendix A: seasonal abundance); Lidvanov & al., 2013 (p.290, Table 2, % composition); Oh H-J. & al., 2013 (p.192, Table 1, occurrence); Terbiyik Kurt & Polat, 2013 (p.1163, Table 2, seasonal distribution); Siokou & al., 2013 (p.1313, fig.4, 8, biomass, vertical distribution); Fernandez de Puelles & al., 2014 (p.82, Table 3, seasonal abundance); Bonecker & a., 2014 (p.445, Table II: frequency, horizontal & vertical distributions); Lopez-Ibarra & al., 2014 (p.453, fig.6, 8, Table 2, biogeographical affinity) ; Mazzocchi & al., 2014 (p.64, Table 3, 4, 5, spatial & seasonal composition %); Fierro Gonzalvez, 2014 (p.1, Tab. 3, 5, occurrence, abundance) ; Zaafa & al., 2014 (p.67, Table I, occurrence); Dias & al., 2015 (p.483, Table 2, abundance, biomass, production, Table 4: % vs. season); Zakaria & al., 2016 (p.1, Table 1); Benedetti & al., 2016 (p.159, Table I, fig.1, functional characters); Jerez-Guerrero & al., 2017 (p.1046, Table 1: temporal occurrence); Ohtsuka & Nishida, 2017 (p.565, Table 22.1); El Arraj & al., 2017 (p.272, table 2, seasonal composition); Benedetti & al., 2018 (p.1, Fig.2: ecological functional group); Belmonte, 2018 (p.273, Table I: Italian zones); Dias & al., 2018 (p.1, Tables 2, 4, 5: vertical distribution, abundance vs. season); Palomares-Garcia & al., 2018 (p.178, Table 1: occurrence); Hure M. & al., 2018 (p.1, Table 1: abundance, % composition, Rem.: p.12); Acha & al., 2020 (p.1, Table 3: occurrence % vs. ecoregions, Table 5: indicator ecoregions); Hirai & al., 2020 (p.1, Fig. 5: cluster analysis (OTU), spatial distribution). | | | | NZ: | 21 + 2 doubtful | | |
|
Distribution map of Lucicutia flavicornis by geographical zones
|
| | | | | | | | | | | | | | | 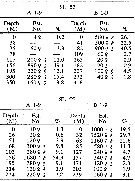 issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1, Crustacea, 1: Copepoda. Copepods of the Florida Current. 1967. [p.77, Table 34]. issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1, Crustacea, 1: Copepoda. Copepods of the Florida Current. 1967. [p.77, Table 34].
Vertical distribution of Lucicutia flavicornis at the ''40-Mile station'' in the Florida Current (± 25°35'N, 79°27'W).
SL 53: 18 V 1958; SL 55: 21 VII 1958. A: during midday; B;: during midnight. |
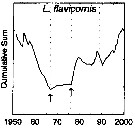 issued from : G.A. Rebstock in Global change Biology, 2002, 8. [p.77, Fig.2 b]. issued from : G.A. Rebstock in Global change Biology, 2002, 8. [p.77, Fig.2 b].
Climatic regime shifts and decadal-scale variability in calanoid copepod populations off southern California (31°-35°N, 117°-122°W.
Cumulative sums of nonseasonal anomalies from the long-term means of copepod abundance from years 1950 to 2000.
A negative slope indicates a period of below-average anomalies; a positive slope indicates a period of above-average anomalies. Abrupt changes in slope indicate step changes. Step changes are marked with arrows (downward -pointing for decreases).
The October 1966 cruise (prior to the increase in sampling depth), March 1976 cruise (prior to the 1976-77 climatic regime shift), and October 1988 cruise (prior to the hypothesized 1989 climatic regime shift) are marked with vertical lines. |
 issued from : A.A. Shmeleva in Bull. Inst. Oceanogr., Monaco, 1965, 65 (n°1351). [Table 6: 29 ]. Lucicutia flavicornis (from South Adriatic). issued from : A.A. Shmeleva in Bull. Inst. Oceanogr., Monaco, 1965, 65 (n°1351). [Table 6: 29 ]. Lucicutia flavicornis (from South Adriatic).
Dimensions, volume and Weight wet. Means for 50-60 specimens. Volume and weight calculated by geometrical method. Assumed that the specific gravity of the Copepod body is equal to 1, then the volume will correspond to the weight. |
 issued from : A.A. Shmeleva in Bull. Inst. Oceanogr., Monaco, 1965, 65 (n°1351). [Table 6: 30 ]. Lucicutia flavicornis var. I and II (from South Adriatic). issued from : A.A. Shmeleva in Bull. Inst. Oceanogr., Monaco, 1965, 65 (n°1351). [Table 6: 30 ]. Lucicutia flavicornis var. I and II (from South Adriatic).
Dimensions, volume and Weight wet. Means for 50-60 specimens. Volume and weight calculated by geometrical method. Assumed that the specific gravity of the Copepod body is equal to 1, then the volume will correspond to the weight. |
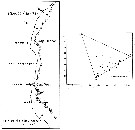 issued from : U. Brenning in Wiss. Z. Wilhelm-Pieck-Univ. Rostock - 35. Jahrgang 1986. Mat.-nat. wiss. Reihe, 5. [p.12, Figs.8, 9]. issued from : U. Brenning in Wiss. Z. Wilhelm-Pieck-Univ. Rostock - 35. Jahrgang 1986. Mat.-nat. wiss. Reihe, 5. [p.12, Figs.8, 9].
Spatial distribution and T-S Diagram for Lucicutia flavicornis from 8° S - 26° N; 16°- 20° W.
SO: Southern Surface Water (S °/oo: 34,50; T°C: 29,0); ND: Northern Water of the Surface Layer (S °/oo: 37,5; T°C: 21,0); SD: Southern Deep Water of the surface layer (S °/oo: 35,33; T°C: 13,4). See commentary in Temora stylifera and Brenning (1985 a, p.6). |
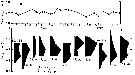 Issued from : G. Deevey in J. mar. biol. Ass. U.K., 1964, 44. [p.596, Fig.3, B] Issued from : G. Deevey in J. mar. biol. Ass. U.K., 1964, 44. [p.596, Fig.3, B]
Histogram showing the length distribution, plotted as numbers measured of females of Lucicutia flavicornis from 1958-1959, at Station "S' (32°8' N, 64°42' W), 15 miles south-east of Brermuda.
Samples by plankton net, oblique hauls from 500 m to the surface. |
 Issued from : G. Deevey in J. mar. biol. Ass. U.K., 1964, 44. [p.597, Table 3] Issued from : G. Deevey in J. mar. biol. Ass. U.K., 1964, 44. [p.597, Table 3]
Mean cephalothorax lengths and numbers measured of females.
See in Pleuromamma abdominalis for yhe mean surface temperature and Chlorophyll a of the month preceding each measurement at Station 'S' (SE of Bermuda).
Correlation coefficients for mean length with the temperature and chlorophyll of the preceding month were calculated. No correlation was found between the lengths and either the temperature or the chlorophyll. The variations in length noted are not related to the total phytoplankton cycle or to seasonal temperature changes. |
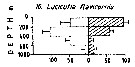 Issued from : M. Madhupratap & P. Haridas in J. Plankton Res., 12 (2). [p.310, Fig.3]. Issued from : M. Madhupratap & P. Haridas in J. Plankton Res., 12 (2). [p.310, Fig.3].
Vertical distribution of calanoid copepod (mean +1 SE), abundance No/100 m3. 10- Lucicutia flavicornis.
Night: shaded, day: unshaded.
Samples collected from 6 stations located off Cochin (India), SE Arabian Sea, November 1983, with a Multiple Closing Plankton Net (mesh aperture 300 µm), in vertical hauls at 4 depth intervalls (0-200, 200-400, 400-600, 600-1000 m). |
 Issued from : G.A. Lopez-Ibarra, S. Hernandez-Trijillo, A. Bode & M.J. Zetina-Rejon in Cah. Biol. Mar., 2014, 55. [p.459, Fig.8]. Issued from : G.A. Lopez-Ibarra, S. Hernandez-Trijillo, A. Bode & M.J. Zetina-Rejon in Cah. Biol. Mar., 2014, 55. [p.459, Fig.8].
Abundance recorded in the geographic zones with the highest canonical coefficient on the first two axes of the discriminant analysis.
See informations and figures in Pleuromamma robusta from the same authors. |
 Issued from : G.A. Lopez Ibarra in Tesis, Inst. Politec. Nac., CICIMAR, 2008. [p.37, Table 3]. Issued from : G.A. Lopez Ibarra in Tesis, Inst. Politec. Nac., CICIMAR, 2008. [p.37, Table 3].
Summary of abundance and steady isotopics 15N and 13C in Subeucalanus subcrassus.
O = main feeding: omnivore; T = tropical biogeographical affinity; zones (a-f) = Cf. fig.1 in the same author.
Compare with Subeucalanus subcrassus and other species (Tabla 3). |
| | | | Loc: | | | Cosmopolite (mainly tropical et sub-tropical, temperate: Atlant., Indian, Pacif.), also: Weddell Sea , sub-Antarct. (Indian), Angola, Baia Farta, Ivory Coast, off Rio de La Plata (shelfbreak), Brazil (S, off Rio de Janeiro, Campos Basin, Vitoria Bay, off Vitoria-Cabo de Sao Tomé, off Macaé, off Natal), Caribbean Colombia, E Costa Rica, off Bermuda (Station "S"), Sargasso Sea, off C. Hatteras, off E Cape Cod, off S Newfoundland, off E Nova Scotia, Flemish Cape, S Iceland, off W Ireland, Faroe Is., English Channel, South Africa (E & W), Great Meteor Seamount, G. of Guinea, Senegal-Mauritania, Morocco-Mauritania, Canary Is., Cap Ghir, Ibero-moroccan Bay, off W Rangier, Medit. (M'Diq, Alboran Sea, Algiers, Gulf of Annaba, Castellon, Baleares, Banyuls, Marseille, Monaco, Ligurian Sea,Tyrrhenian Sea, Milazzo, Messina, Gulf of Taranto, Malta, Adriatic Sea, Ionian Sea, Aegean Sea, Thracian Sea, Black Sea, Iskenderun Bay, Lebanon Basin, W Egyptian coast, Alexandria, Bardawill Lagoon), G. of Aqaba, off Sharm El-Sheikh, Red Sea, Gulf of Oman, G. of Aden, Arabian Sea, Sri Lanka, Mascarene Basin, off Madagascar S, Nosy Bé, Rodrigues Is. - Seychelles, Natal, India (Goa - Gujarat, Saurashtra coast, W, Lawson's Bay, Madras), Bay of Bengal, Andaman Sea, Nicobar Is., Batten Island, Australia (North West Cape), Straits of Malacca, Indonesia (SW Celebes), China Seas (Yellow Sea, East China Sea, South China Sea), Taiwan Strait, Taiwan (S, E, SW, W, Kaohsiung Harbor, NW, N: Mienhua Canyon, NE), S Korea, Geumo Is., Korea Strait, Japan Sea, Japan (Kuchinoerabu Is., Onagawa, Toyama Bay, off Sanriku, Honshu: Sagami Bay), Kuroshio zone, Station Knot, Bering Sea, Aleutian Is., Alaska, Cobb Seamount, British Columbia, Oregon (off Newport), California, W Baja California, Bahia Magdalena, Gulf of California, La Paz, G. de Tehuantepec, off W Guatemala , W Costa Rica, Clipperton Is., Pacif. (W equatorial), Pacific (central gyres: N and S), New Caledonia, S Pacif. (NPFZ), New Zealand (South Island SW, NE, off E, N & NE North Island), Australia (G. of Carpentaria, SE), Tasman Sea, Bahia Cupica (Colombia), Galapagos-Ecuador, Peru, Chile (N-S, off Santiago)
Nota: After Markhaseva & Ferrari (2005, p.1098) this species should not be considered an inhabitant of antarctic waters. | | | | N: | 387 | | | | Lg.: | | | (11) F: 1,466; (16) F: 1,57-1,25; M: 1,5-1,4; (21) F: 2-1,3; M: 1,7-1,3; (22) F: 1,75-1,37; M: 1,7-1,35; (26) F: 1,65-1,46; M: 1,7-1,34; (34) F: 1,8-1,63; 1,4-1,3; M: 1,68; (35) F: 1,5-1,4; (38) F: 1,58-1,47; M: 1,56-1,44; (46) F: 1,75-1,37; M: 1,7-1,35; (54) F: 1,82-1,66; M: 1,7-1,41; (59) F: 2,2-1,4; M: 1,7-1,35; (66) M: 1,36; (72) F: 2,04-1,38; M: 1,66-1,45; (73) F: 1,63-1,41; M: 1,57-1,49; (101) F: 1,9-1,26; M: 1,77-1,28; (104) M: 1,4; (116) F: 1,87; 1,8; M: 1,68; (142) F: 1,6; (145) F: 2,2; (150) F: 1,5-1,37; M: 1,47-1,37; (187) F: 1,6-1,5; M: 1,45-1,4; (199) F: 1,9-1,29; M: 1,75-1,29; (208) F: 1,56; M: 1,43; (237) F: 1,9-1,7; M: 1,25-1,5; (254) F: 1,5; (290) F: 1,5-1,7; M: 1,45-1,5; (327) F: 1,57-1,51; M: 1,54-1,39; (332) F: 1,35; (334) F: 1,75-1,4; M: 1,7-1,3; (335) M: 1,57-1,43; (336) M: 1,59; (340) F: 1,75-1,55; M: 1,45; (402) F: 1,41-1,32; M: 1,06; (449) F: 1,75-1,37; M: 1,7-1,35; (530) F: 1,5; M: 1,4; (785) F: 1,39; (795) F: 2,5; (909) F: 1,9-2; M: 1,55-1,7; (920) F: 1,54; M: 1,41-1,43; (991) F: 1,75-2; M: 1,155-1,70; (1023) F: 1,36-1,58; M: 1,47-1,50; (1068) F: 1,46-1,65; M: 1,34-1,70; (1108) F: 1,49-1,90; M: 1,44-1,92; (1230) F: 1,3-1,8; M: 1,3-1,7; {F: 1,25-2,50; M: 1,06-1,92} | | | | Rem.: | epi, meso & bathypelagic. Oceanic.
Sampling depth (Antarct., sub-Antarct.) : 0-300 m. Sargasso Sea: 0-1000 m (Deevey & Brooks, 1977, Station "S"); In vertical tow 4000-3000 m (Harding, 1974, Rem.: possible contaminant species from higher up the water column).
Grice (1962) emphasizes the existence of two size classes.
After Moraitou-Apostolopoulou (1985, p.309), due to the homothermal conditions in December on the coast of Israel, this deep water species appears in the planktonic community.
After Bradford-Grieve (1999 b, p.98) of all the forms mentioned by various authors, the large form is the most conservative and examination of Claus’ (1863) original description of Leuckartia flavicornis should retain the name Lucicutia flavicornis. The reason for this opinion is the large size of Claus’ specimens, the fact that right basipod 1 has a conspicuous rounded bump on the inner margin, and the female P5 has a long inner edge spine on the inner distal corner of exopod segment 2 (see Giesbrecht, 1892, figs.17, 19 and 29 in Plate 19). Examination of smaller forms from different oceans, which have been given the name L. flavicornis brings the conclusion that one or two undescribed species are involved at each locality and that these species may have much narrower geographic ranges than L. flavicornis sensu stricto. See L. flavicornis sp.
For C. Razouls, the only body size is not a good character, depending to chorologic factors, mixed generations.
A confusion is possible between this species and L. gemina. For Markhaseva & Ferrari, 2005 (p.1098) the southernmost locality was collected at 46° S in the SW Pacific (Bradford-Grieve, 1999), and should not be considered an inhabitant of antractic waters.
See remarks to L. gemina.
After Itoh (1970 a, fig., from co-ordonates) the Itho's index value of the mandibular gnathobase is 760, and after Lapernat & Razouls (2002, p.25): 709.7 (number of teeth: 9).
After Ehrhardt (1967, p.888, 894) the species is found in the southwest Tyrrhenian Sea in water salinity between 37.22-38.23 p.1000 (mainly between 37.77- 37.91) and for the oxygen between 4.45-5.90 cm3/l (mainly brtween 5.18-5.50).
Timonin (1971, p.282) considers the trophic interrelations in the equatorial and tropical Indian Ocean, and divides the plankters into 6 trophic groups from the litterature and the results of studies of mouth-parts structure and intestine content. This species is a coarse-filter feeder herbivorous.
After Benedetti & al. (2018, p.1, Fig.2) this species belonging to the functional group 4 corresponding to small filter feeding herbivorous and mixed feeding omnivorous (mostly broadcasters).
See in DVP Conway & al., 2003 (version 1)
R. Stephen: Data sheets of NIO, Kochi, India (on line). | | | Last update : 24/10/2022 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2025. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed December 30, 2025] © copyright 2005-2025 Sorbonne University, CNRS
|
|
 |
 |