|
|
 |
|
Calanoida ( Order ) |
|
|
|
Lucicutiidae ( Family ) |
|
|
|
Lucicutia ( Genus ) |
|
|
| |
Lucicutia grandis (Giesbrecht, 1895) (F,M) | |
| | | | | | | Syn.: | Leuckartia grandis Giesbrecht, 1895 c (p.258, pl.4, fig.4: M);
no Leuckartia flavicornis (F) : Brady, 1883 (p.50, fig.F);
Lucicutia maxima : A. Scott, 1909 (p.127); Wolfenden, 1911 (p.318, figs.F); De Decker & Mombeck, 1964 (p.13);
Lucicutia challengeri Sewell, 1932 (p.290, figs.F,M); 1947 (p.174); 1948 (part., p.330, 503, 521, 530, 533, 538, 539, 547); Silas, 1972 (p.649, Rem.);
? L. bradyana Cleve, 1904 (p.204);
no Lucicutia grandis : Wolfenden, 1904 (p.121); 1905 a (p.8); 1911 (p.315); van Breemen, 1908 a (p.114); Farran, 1908 b (p.61, Rem.); Cépède, 1914 a (p.151); Lysholm & Nordgaard, 1921 (p.25); Sars, 1925 (p.208, figs.F,M); Farran, 1929 (p.209, 264); Wilson, 1932 a (p.128); Rose, 1933 a (p.193); Jespersen, 1934 (p.104); 1940 (p.51); Lysholm & al., 1945 (p.34); Brodsky, 1950 (1967) (p.328); Vervoort, 1957 (p.131); Grice, 1963 a (p.496); Grice & Hülsemann, 1965 (p.224); ? Morioka, 1972 a (p.314); Vaissière & Séguin, 1980 (p.23, tab.1); Kovalev & Shmeleva, 1982 (p.84); Hernandez & Suarez-Moralez, 1994 (p.169, fig.); Bradford-Grieve,1999 b (p.100, figs.F,M, Rem., figs.177, 191); Holmes, 2001 (p.17);
Lucicutia, aff. grandis : Gowing & Wishner, 1998 (p.2433, vertical distribution, gut contents). | | | | Ref.: | | | Giesbrecht & Schmeil, 1898 (p.111); ? Wilson, 1942 a (p.192, fig.F); 1950 (p.255); Hülsemann, 1966 (p.719, 721, figs.F,M, Rem.); Ali-Khan & Ali-Khan, 1982 (p.268, figs.F,M); Bradford-Grieve & al., 1999 (p.883, 945, figs.F,M); Markhaseva & Ferrari, 2005 (p.1078, figs.F,M, Rem.); Boxshall & Halsey, 2004 (p.132: F; p.134: M) | 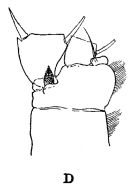 issued from : R.B.S. Sewell in The John Murray Expedition, 1933-34, Scientific Reports, VIII (1), 1947. [p.166, Fig.44, D]; As Lucicutia challengeri. . Process on the posterior aspect of the 2nd basal segment of P1. Remarks: The shape of the hook-like or spine-like process shows some variation in different genera, but it is undoubtedly homologous throughout the whole series. The function of this organ is unknown.
|
 Issued from : R.B.S. Sewell in Mem. Indian Mus., 1932, X (continued). [p.291, Fig.95]. As Lucicutia challengeri. Female (from N Indian): a, A2; b, Md (biting edge); c, Md (mandibular palp); d, Mx1; e, Mx2; f, Mxp; g, P1; h, P5. Male: j, P5.
|
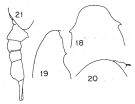 Issued from : K. Hülsemann in Bull. Mar. Sc., 1966, 16 (4). [p.708, Figs.18-21]. Female: 18, head (dorsal); 19, idem (lateral); 20, forehead and rostrum (lateral); 21, urosome (lateral right side). Nota: The females from Indian Ocean had only a swelling on the sides of the head instead of a strong spine-like protusion as did the females reported by Wolfenden (1911). The females described and figured by Wolfenden are identified with L. grandis.
|
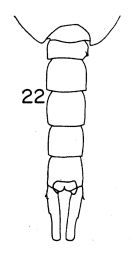 Issued from : K. Hülsemann in Bull. Mar. Sc., 1966, 16 (4). [p.710, Fig.22.]. Male: urosome (dorsal).
|
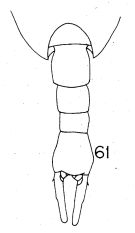 Issued from : K. Hülsemann in Bull. Mar. Sc., 1966, 16 (4). [p.716, Fig.61]. Female (from W Indian): 61, urosome (dorsal). Nota: The proportions of the abdominal segments and the furca are diagnostic.
|
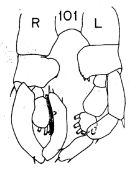 Issued from : K. Hülsemann in Bull. Mar. Sc., 1966, 16 (4). [p.724, Fig.101]. Male: 101, P5 (R = right leg, L = left leg).
|
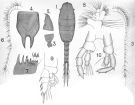 issued from : A. Scott in Siboga-Expedition, 1909, XIX a. [Plate XLI, Figs.1-10]. As Lucicutia maxima. Male (from Manipa Strait & Banda Sea): 1, habitus (dorsal); 2, forehead (lateral); 3, last thoracic and genital segments (left side); 4, rostrum; 5, right A1; 6, left A1; 7, Md (masticatory edge); 8, Mx1, 9, P1; 10, P5.
|
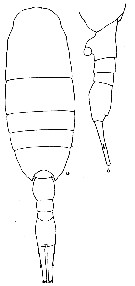 issued from : R.N. Wolfenden in Die Marinen Copepoden der Deutschen Südpolar-Expedition 1901-1903, 1911. [p.318, Fig.60]. As Lucicutia maxima. Female: a, habitus (dorsal); b, posterior part cephalothorax and urosome.
|
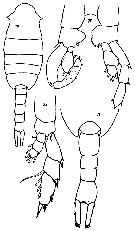 issued from : S. Ali-Khan & J. Ali-Khan in Crustaceana, 1982, 43 (3). [p;268, Figs.19-22]. Female (from 24°09'N, 64°27'E): 21, urosome (dorsal); 22, P5. Male: 19, habitus (dorsal); 20, P5. Nota: Both male and female heads with strong spine-like protrusions but none with swelling as observed by Hulsemann (1966) from Indian Ocean specimens.
|
 issued from : E.L. Markhaseva & F.D. Ferrari in J. Nat. Hist., 2005, 39 (15). [p.1082, Fig.1]. Female: A, habitus (dorsal); B, anterior part of cephalon (dorsal); C, idem (lateral); D, rostrum (anterior); E-F, urosome (dorsal); G, same (lateral, right side); H-J, same (lateral, left side); K, P5. Scale bars: 0.1 mm From Arabian Sea (17°14'N, 59°49'E): A, B, D, E, G, I, J. From tropical Indian Ocean (5°05'S, 57*35'E): C, F, H, K.
|
 issued from : E.L. Markhaseva & F.D. Ferrari in J. Nat. Hist., 2005, 39 (15). [p.1083, Fig.2]. Male: A, habitus (dorsal); B, urosome (lateral, left side); C, rostrum (anterior); D, A1 (segments 14-17); E, A1 (segments 18-21); F-G, left P5; H, exopod of right P5; I, right P5 (coxopod, basipod and endopod); J-K, right P5. Scale bars: 0.1 mm. (A-F, H-J; 0.5 mm (G, K) From Arabian Sea (17°14'N, 59'49'E): A-F, H-J. From Arabian Sea (13°50'N, 70°07'E): G-K
|
 issued from : J.M. Bradford-Grieve in The Marine Fauna of New Zealand: Pelagic Calanoid Copepoda. National Institute of Water and Atmospheric Research (NIWA). NIWA Biodiversity Memoir, 111, 1999. [p.102, Fig.66]. Female (41°47'S, 175°01'E): A, habitus (dorsal); B, urosome (right lateral side); C, P5. Male: D, habitus (dorsal); E, P5.
|
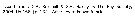 Lucicutia grandis Lucicutia grandis female: 1 - Characters following not combined : Prosome about 3 times longer than urosome. Cephalosome with slightly projecting and rounded anterior corners and well developed lateral spinous projections; anal somite about as long as wide; caudal rami 11.7 times longer than wide and bowed outwards at base, leaving elliptical space between rami proximally. 2 - P1 with 3-segmented endopod. 3 - Cephalosome with 1 pair of lateral spinous projections . 4 - Genital double-somite symmetrical (dorsal view). 5 - Anal somite almost as long as caudal ramus. 6 - Anal somite swollen.
|
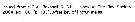 Lucicutia grandis Lucicutia grandis male: 1 - P1 with 3-segmented endopod. 2 -Cephalosome with 1 pair of strong lateral spinous projections. 3 - Caudal rami distinctly shorter than urosomal somites; anal somite shorter than wide. 4 - Inner margin of basis of left P5 protruding, bearing teeth, and on right leg protruded only in proximal half, without teeth.
| | | | | Compl. Ref.: | | | Pearson, 1906 (p.25); Grice & Hulsemann, ? 1965 (p.224); 1967 (p.17); Björnberg, 1973 (p.343, 387); Brinton & al., 1986 (p.228, Table 1); Gowing & Wishner, 1992 (tab.1); Padmavati & al., 1998 (p.349); Suarez-Morales & Gasca, 1998 a (p.110); Wishner al., 2000 (p.1576); Yamaguchi & al., 2002 (p.1007, tab.1); Kazmi, 2004 (p.228); Koppelmann & Weikert, 2005 (p.1173, vertical distribution); Ikeda & al., 2006 (p.1791,Table 2); Morales-Ramirez & Suarez-Morales, 2008 (p.520); Wishner & al., 2008 (p.163, Table 2, figs.8, 9, 10, zonation-oxycline, feeding: p.186); Gaard & al., 2008 (p.59, Table 1, N Mid-Atlantic Ridge); Galbraith, 2009 (pers. comm.); Homma & Yamaguchi, 2010 (p.965, Table 2); Hidalgo & al., 2010 (p.2089, Table 2); Mazzocchi & Di Capua, 2010 (p.426); Homma & al., 2011 (p.29, Table 2, 3, abundance, feeding pattern: suspension feeders); Takenaka & al., 2012 (p.1669, fig.2, 3, Table 1, bioluminescence); Belmonte, 2018 (p.273, Table I: Italian zones) | | | | NZ: | 10 + 6 doubtful | | |
|
Distribution map of Lucicutia grandis by geographical zones
|
| | | | | | | | |  Issued from : M.M. Gowing & K.F. Wishner in Deep-Sea Res. II, 1998, 45. [p.2440, Fig.2]. Issued from : M.M. Gowing & K.F. Wishner in Deep-Sea Res. II, 1998, 45. [p.2440, Fig.2].
Abundances of Lucicutia aff. L. grandis with depth and environmental variables measured with the MOCNESS sensors during the tows in Arabian Sea (16°02'N, 62°00'E).
Solid = males; clear = females; diagonal = immatures.
Nota: Samples during the Spring Intermonsoon (31 March-1 April 1995) and the late SW Monsoon (5 September 1995). |
 Issued from : M.M. Gowing & K.F. Wishner in Deep-Sea Res. II, 1998, 45. [p.2442 & 2446, Fig.3, 4]. Issued from : M.M. Gowing & K.F. Wishner in Deep-Sea Res. II, 1998, 45. [p.2442 & 2446, Fig.3, 4].
Mean percentages of food categories in gut-contents of Lucicutia aff. L. grandis from the Spring Intermonsoon and late SW Monsoon.
Data from all animals analysed at each depth were combined. n = number of animals.
Nota: The animals were clearly detritivorous, feeding on a variety of type of particles. The presence of metazoan remains suggests that the copepods are omnivorous. While pieces of cuticle could have been ingested as molts, the cnidarian nematocysts were sometimes associated with tissue, indicating carnivory. The results indicate that the diet of the population of Lucicutia included aggregates of material that had sunk from shallower depths.Intact cells in the gut contents indicate at least two types of aggregates, one originating in the oxygen minimum and the other in surface waters.
The animals consumed gram-negative bacteria including metal-precipitating bacteria, aggregates of probable gram-positive bacteria, virus-like particles and large virus-lake particles. |
| | | | Loc: | | | ? South Africa (E), S Atlant. (off Is. Tristan da Cunha E, off S Ascension Is.), ? Caribbean, ? NE Atlant. (30-60°); S Iceland-Azores (in Gaard & al., 2008 (Mid-Atlantic Ridge); ? Medit. (Ligurian Sea, Tyrrhenian Sea, Adriatic Sea), G. of Oman, N Arabian Sea, W Indian, ? Philippines, ? Japan, ? off SE Hokkaido, Bering Sea ( S Aleutian Basin), S Aleutian Is., Station Knot, ? Australia (Port Jackson), ? Is. Fiji, E Pacif. (off Equador), off British Columbia, Gulf of California, off W Mexico, W Costa Rica, Peru, Chile (N & S), New Zealand (E), Tasman Sea | | | | N: | 36 ? | | | | Lg.: | | | (10) F: 6,2; (21) F: 6,5-4,4; M: 4,9-3,9; (44) F: 5,54; M: 4,6-3,905; (47) M: 6; (402) F: 4-3,8; M: 3,6-3; (929) F: 4,7-4,9; M: 4,1-4,6; {F: 3,80-6,50; M: 3,00-6,00}
| | | | Rem.: | Bathypelagic. Pearson (1906, p.25): vertical range down to 1.300 m
Because of the confused synonymy, it is difficult to establish the geographical distribution of this species.
Not taken into account are the data from Sewell, 1948, cited here for the record.
According to Hülsemann (1966, p.719), Brady (1883) doubtfully identified male and female specimens with L. flavicornis. The length is given as 6.2 mm and the description and figures of the female agree well with the female of L. grandis; the male does agree with L. flavicornis (Claus) except for its size. Unfortunately, Brady does not distinguish between females and males at the collecting stations, thus it is not possible to use his records in the distribution of the species.
Sewell (1932) described L. challengeri which he identified with Brady's L. flavicornis. Sewell (p.294) stated that the P5 male agreed closely with Brady's form; however, description and figures show a resemblance with the P5 male of L. grandis as figured by Giesbrecht. Hülsemann examied one female and one male from the John Murray Expedition which were identified by Sewell as L. challengeri. Although Sewell stated (p.289) that L. challengeri is not identical with L. grandis, Hülsemann considers the two forms conspecific.
Cleve (1904) recognized the close relationship of L. bradyana with L. grandis. Because of differences between them (a relatively longer furca in bradyana in both sexes and a somewhat different construction of the 2nd basal segment of left P5 in the male) L. bradyana is doubtfully included in the synonymy.
For Markhaseva & Ferrari (2005, p.1079) the following combination of characters distinguishes the females of L. grandis from L. bradyana : anal somite swollen dorsally in lateral view (swollen ventrally in L. bradyana)) ; genital double-somite with conical plug (rounded in L. bradyana) ; medial seta at the exopodal segment 2 of P5 attenuate and thin at the tip (in L. bradyana this seta robust) ; rostral rami closely spaced (divergent in L. bradyana).
L. grandis differs from L. wolfendeni as follows : large anal somite swollen dorsally (anal somite of L. wolfendeni only slightly swollen dorsally) ; genital double-somite of L. grandis with a conical plug (L. wolfendeni with an elongate-oval plug) ; caudal rami of L. grandis are 1.0-1.3 times longer than anal somite (2.9-3.1 times longer than anal somite of L. wolfendeni ; rostral rami of L. wolfendeni are divergent and not closely spaced as in L. grandis.
The left P5 basipod of males of L. grandis which is not elongate medially-distally differs from both L. bradyana, in which there is an elongate medial-distal projection with varying number of terminal teeth, and L. wolfendeni in which the projection is present but less pronounced. The 3rd exopodal segment of the left P5 of L. grandis is oval-rounded, while for L. bradyana it is elongate-oval and for L. wolfendeni it is elongate-triangular. The 2nd endopodal segment of the right P5 of L. grandis is unarmed, while that of L. bradyana is densely hirsute. The inner margin of the right basipod of L. grandis has a small tooth-like indentation which differs from the distinct, proximal-facing lobe of L. wolfendeni.
L. grandis was described originally by Giesbrecht (1895) from a simple damaged male from 1°N, 83°W. | | | Last update : 09/12/2020 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed January 22, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |

















