|
|
 |
|
Calanoida ( Order ) |
|
|
|
Metridinidae ( Family ) |
|
|
|
Metridia ( Genus ) |
|
|
| |
Metridia gerlachei Giesbrecht, 1902 (F,M) | |
| | | | | | | Syn.: | Phyllopus Turqueti Quidor,1908 (p.4, figs.F,M) | | | | Ref.: | | | Giesbrecht, 1902 (p.27, figs.F,M); Wolfenden, 1911 (p.286); Brady, 1918 (p.25, figs.F,M); Farran, 1929 (p.209, 259); Vervoort, 1951 (p.120, Rem.); 1957 (p.120, figs.M, Rem.); Tanaka, 1960 (p.49, figs.F, M, juv.); 1964 (p.9); Bradford, 1971 b (p.24, figs.F,M, Rem.); Zvereva, 1972 (1975) (p.254, figs.F,M); Björnberg & al., 1981 (p.640, figs.F,M); Razouls, 1994 (p.140, figs.F,M); Bradford-Grieve & al., 1999 (p.884, 948, figs.F,M); Bradford-Grieve,1999 b (p.114, figs.F,M, Rem., figs.178, 192); Michels & Schnack-Schiel, 2005 (p.483, fig.4: Md); Eyun & al., 2007 (p.268, fig.1: molecular biology); Cheng F. & al., 2013 (p.119, molecular biology, GenBank); Xavier & al., 2020 (p.26, fig., Rem.). | 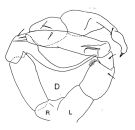 issued from : J.M. Bradford-Grieve in The Marine Fauna of New Zealand: Pelagic Calanoid Copepoda. National Institute of Water and Atmospheric Research (NIWA). NIWA Biodiversity Memoir, 111, 1999. [p.115, Fig.77]. Male (from Ross Sea): D, P5 (L = left leg; R = right leg). Nota: Right P5 with a strongly curved inner spine on exopodal segment 1 and left exopodal segment 2 with 3 inner edge spines. Remarks (p.114): Vervoort (1957) figures the inner edge spine on exopod segment 1 on male right P5 with small teeth on its outer border which is similar to M. lucens/pacifica. For Vervoort, that males of M. gerlachei can be separated from M. lucens by the vaulted cephalothorax which resembles that of the female; the geniculate A1 may be on the right or left side. The Southwest Pacific male P5 has small teeth on the terminal outer border of the long spine on right exopod segment 1. At the northern part of their range this species is quite difficult to tell apart from M. lucens/pacifica, especially where the female P5 is tending towards the M. gerlachei type; the rounded posterior metasome corners appear to be the main character distinguishing the species. In Ross Sea specimens the male P5 of M. gerlachei appears to differ from that of M. lucens/pacifica in the decoration, on the inner spine on right exopod segment 1 which is confined to near the tip, whereas in M. lucens/pacifica there are very small ''teeth'' along almost all of the border. The variability in male P5 needs to be described in more detail for the differences between M. gerlachei and M. lucens/pacifica.
|
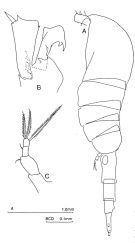 issued from : J.M. Bradford-Grieve in The Marine Fauna of New Zealand: Pelagic Calanoid Copepoda. National Institute of Water and Atmospheric Research (NIWA). NIWA Biodiversity Memoir, 111, 1999. [p.115, Fig.77]. Female (from 62°37'S, 169°51'E): A, habitus (left lateral side); B, segment 1 of exopod and endopod of P2; C, P5. Nota: Posterior metasomal corners rounded. - Caudal rami longer than anal segment and 3-3.5 times as long as wide. - P5 3-segmented; last 2 segments fused, 3.5 times as long as wide, with fusion indicated only by undulating borders of last segment; carrying a short outer edge spine and 3 long terminal setae.
|
 issued from : J.A. Zvereva in Issled. Fauny Moreï, 1972, 12 (20). [p.221, Fig.3, 5-6]. Female (from Antarctic): 5, P2. Male: 6, P5.
|
 issued from : W. Vervoort in B.A.N.Z. Antarctic Reseach Expedition, Report-Ser. B, Vol. III, 1957 [Fig.109]. Male (from 66°33'S, 45°32'E): a, habitus (lateral); b, distal part of left A1.
|
 issued from : W. Vervoort in B.A.N.Z. Antarctic Reseach Expedition, Report-Ser. B, Vol. III, 1957 [Fig.110]. Male: a, right P1 (anterior); b, left P2 (posterior); c, left P4 (posterior); d, P5 (rt = right leg; lt = left leg).
|
 issued from : J. Michels & S.B. Schnack-Schiel in Mar. Biol., 2005, 146. [p.488, Fig.5]. Mandibular gnathobase. a-c: Female (from Weddell and Bellingshausen Seas); d, Male. a, d: left gnathobase from cranial; b: right gnathobase from distal. V: ventral tooth, C1-C4: central teeth, D1-D3: dorsal teeth, B: dorsal bristle. Scale bars 0.020 mm.
|
 issued from : W. Giesbrecht in Copepoden. Res. voyage du S. Y. Belgica. Rapports scientifiques, Zoologie, 1902. [Taf. V, Figs.6-14]. Female (from S Peter Ist Island, Bellingshausen Sea): 6, habitus (dorsal); 7, idem (lateral); 8, anal segment and caudal rami (dorsal); 9, A1 (proximal segments); 11, P2 (basipodite 2); 12, exopodite 3 of P4; 13, P5. Male: 10, A1 (distal segments); 14, P5 (anterior).
|
 Issued from : J.M. Bradford in N.Z. Oceanogr. Inst., 1971, 206, Part 8, No 59. [p.22, Fig.86]. Female (from Ross Sea): 86, P5. Scale bar: 100 µm.
|
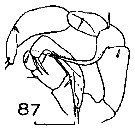 Issued from : J.M. Bradford in N.Z. Oceanogr. Inst., 1971, 206, Part 8, No 59. [p.22, Fig.87]. Male (from Ross Sea): 87, P5. Scale bar: 100 µm.
|
 Issued from : O. Tanaka in Spec. Publs. Seto mar. biol. Lab., 10, 1960 [Pl. XXII, 1-6]. Female (from 66°06'S- 67°04'S, 44°10'E-40°53'E): 1, habitus (lateral); 2, last thoracic segment and genital segment (lateral); 3, P2; 4, P5. Nota: Cephalothorax and abdomen in the proportional lengths 64 to 36. Abdomen 3-segmented; segments and caudal rami in the proportional lengths 41 : 23 : 15 : 21 = 100. A1 23-segmented, extends about level of the posterior margin of the cephalothorax. Male: 5, habitus (lateral); 6, P5. Nota: Cephalothorax and abdomen in the proportional lengths 55 to 45. Abdomen 5-segmented; segments and caudal rami in the proportional lengths 12 : 18 : 16 : 15 : 13 : 21 = 100. P5 5-segmented on each side. Distal segment of the left leg long and spoon-shaped with 2 apical and 2 marginal spines. The 3rd segment of the right leg prolonged into a long curved spine denticulated on the inner distal edge; 4th and 5th segments missing in the present specimen.
|
 Issued from : C. Razouls in Ann. Inst. océanogr., Paris, 1994, 70 (1). [p.140]. Caractéristiques morphologiques de Metridia gerlachei femelle et mâle adultes. Terminologie et abbréviations: voir à Calanus propinquus. Cette forme a été observé à la station ''Kerfix'' à l'entrée de la Baie du Morbihan (Ïles Kerguelen).
| | | | | Compl. Ref.: | | | Hardy & Gunther, 1935 (1936) (p.175, distribution charts); Ottestad, 1936 (p.11, 21, 38, vertical and antarctic distribution); Baker, 1954 (p.203, 211, fig.5); Vinogradov, 1968 (1970) (p.66, 69); Rudyakov, 1972 (p.886, Table 1: sinking rate); Björnberg, 1973 (p.337, 387); Heinrich, 1974 (fig.3); Voronina & Sukhanova, 1976 (1977) (p.614, food composition); Arashkevich, 1978 (p.118, Table: diets); Ikeda & Hing Fay, 1981 (1982) (p.921, metabolism, eammonia excretion, O:N ratio); Ikeda & Mitchell, 1982 (p.283, metabolism); Schnack, 1983 (p.63, feeding); Schnack & al., 1985 (p.256, fig.4); Hopkins, 1985 (p.197, Table 1, gut contents); Bamstedt & Tande, 1985 (p.259, Table 2: literature data respiration & excretion); Hubold, 1985 (p.43, Table 1, predation by fish); Dearborn & al., 1986 (p.1, predation by benthic star); Almeida Prado Por, 1986 (p.517); Kawaguchi & al., 1986 (tab.2); Reinhardt & Van Vleet, 1986 (p.149, lipid composition); Zmijewska, 1987 (tab.2a); Hopkins & Torres, 1988 (tab.1); Ward, 1989 (tab.2); Perissinotto, 1989 (p.505, Table 2, 3, abundance, diurnal variation); Perissinotto & al., 1990 (p.296, grazing); Øresland, 1990 (p.201, Table 1, predator); Atkinson & al., 1990 (tab.1); Tucker & Burton, 1990 (p.591, tab.1, fig.4, seasonal, spatial variations); Kellermann, 1990 (p.159, Table 1, predation); Rau & al., 1991 (p.1, isotopic forms vs feeding); Conover & Huntley, 1991 (p.1, Table 2, 3, 5, 7, 9, 10, polar seas comparison); Huntley & al., 1992 (p.172, diel vertical migration, grazing activity); Huntley & Escritor, 1992 (p.1027, spatial distribution, feeding behavior); Huntley & Lopez, 1992 (p.201, Table B1, growth rate, temperature-dependent production); Siegel & al., 1992 (p.18, tab.3,4); Vuorinen & Bonsdorff, 1992 (p.679, dispersion index); Freire & al., 1993 (tab.3); Kurbjeweit & al., 1993 (p.255, fig.2); Zmijewska, 1993 (p.73, seasonal and vertical distribution, life history); Hosie & Cochran, 1994 (p.21); Schnack-Schiel & Hagen, 1994 (p.1543, life cycle); Graeve & al., 1994 (p.915, lipid composition v.s. diet); Atkinson, 1994 a (p.1, feeding); Huntley & Nordhausen, 1995 (p.457, elemental composition, ammonium excretion rates, life history); Lopez & Huntley, 1995 (p.21, diel vertical migration, feeding); Metz & Schnack-Schiel, 1995 (p.71, feeding); Atkinson & Shreeve, 1995 (p.1291, vertical distribution, grazing); Atkinson & al., 1996 (p.195, feeding v.s. phytoplankton bloom); Albers & al., 1996 (p.347, lipids vs. diet); Hagen & Schnack-Schiel, 1996 (p.139, seasonal lipid); Froneman & al., 1996 (p.15, nutrition, grazing); Knox & al., 1996 (tab.1); Pakhomov & McQuaid, 1996 (p.271, abundance, distribution, seabirds); Zmijewska & al., 1997 (p.127); Froneman & al., 1997 (p.201, abundance, grazing); Errhif & al., 1997 (p.422); Vuorinen & al., 1997 (p.280); Fransz & Gonzalez, 1997 (p.395, weight-length, biomass vs. N-S transect); Beaumont & Hosie, 1997 (p.121, spatial distribution); Elwers & Dahms, 1998 (p.150, 151); Atkinson, 1998 (p.289, Table 1, biological data); Mauchline, 1998 (tab.21, 26, 27, 30, 33, 58, 64); Voronina, 1998 (p.375, biomass); Voronina & Kolosova, 1999 (p.71); Atkinson & Sinclair, 2000 (p.46, 50, 51, 54, 55, zonal distribution); Razouls & al., 2000 (p.343, tab. 3, 5, Appendix); Pakhomov & al., 2000 (p.1663, Table 1, 2, transect Cape Town-SANAE antarctic base); Voronina & al., 2001 (p.401); Chiba & al., 2001 (p.95, tab.4, 7); Hunt & al., 2001 (p.374, tab.1); Pasternak & Schnack-Schiel, 2001 (p.25); Li & al., 2001 (p.894, tab.1); Cabal & al., 2002 (p.869, Table 1, abundance); Kahle & Zauke, 2003 (p.409, metals concentration); Hunt, 2004 (p.1, 74, fig. 4.7, Rem.: p.97, fig.5.10: seasonal abundance); Fuentes & Schnack-Schiel, 2005 (p.253); Schultes & al., 2006 (p.21); Tsujimoto & al., 2006 (p.140, Table1); Hunt & Hosie, 2006 (p.1182, seasonal succession, indicator); Deibel & Daly, 2007 (p.271, Table 6a, 6b, 7a, Fig.5, Rem.: Antarctic polynyas); Kattner & al., 2007 (p.1628, Table 1); Schnack-Schiel & al., 2008 (p.1045: Tab.2, p.1030: fig.7); Schnack-Schiel & al., 2008 (p.1056, Table 1, 4); Kiko & al., 2008 (p.1000, Table 3); Park & Ferrari, 2009 (p.143, Table 2, fig.2, Appendix 1, biogeography); Takahashi & al., 2010 (p.1, Table 4, fig.7); Hidalgo & al., 2010 (p.2089, Table 2); Sartoris & al., 2010 (p.1860, fig.1, Rem.: cation percentages in hemolymph); Swadling & al., 2010 (p.887, Table 2, 3, A1, fig.6, abundance, indicator species); Hsiao & al., 2010 (p.179, Table III, trace metal concentration); Yang & al., 2011 (p.1065, fig.2c); Yang & al., 2011 a (p.921, Table 2, inter-annual variation 1999-2006); 2011 a (p.1614, Table 2, Fig.2A, 5, 6); Swadling & al., 2011 (p.118, Table 2, fig.5, interannual abundance); Ward & al., 2012 (p.78, Table A1, weight); Thompson G.A. & al., 2012 (p.127, Table 2, 3, fig.5); Makabe & al., 2012 (p.432, Table II, abundance vs mesh-size used); Michels & al., 2012 (p.369, Table 1, fig.9, occurrence frequency, vertical distribution); Yang G. & al., 2013 (p.1701, fig.4, Table 2, feeding); Ojima & al., 2013 (p.1293, Table 2, 3, abundance); Lee D.B. & al., 2013 (p.1215, Table 1, 2, 3, 4, abundance, composition, grazing); Hernandez-Leon & al., 2013 (p.196, fig.8, vertical distribution, , grazing, ETS activity); | | | | NZ: | 6 + 1 doubtful | | |
|
Distribution map of Metridia gerlachei by geographical zones
|
| | | | | | | | | 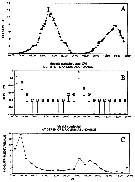 issued from : M.E. Huntley, S. Kaupp & M.D.G. Lopez in Antarftic J. U.S., 1992, 27. [p.172]. issued from : M.E. Huntley, S. Kaupp & M.D.G. Lopez in Antarftic J. U.S., 1992, 27. [p.172].
Diel vertical migration and herbivorous feeding by adult female Metridia gerlachei (from 64°12'S, 61°20'W; 25-26 December 1991).
A: Photosynthetically active radiation in relative units during the 37-hour sampling period.
B: Modal depth interval occupied by the adult female during the sampling period (numbers are the percentage of females in the o to 290 m water column found within the modal depth interval).
C: Mean gut pigment content of the adulut female found in the modal depth interval during the sampling period.
Nota: In late December females dominated the population of M. gerlachei (obtained in using MOCNESS with 333-meter mesh nets). These exhibited pronounced diel verical-migraton behavior, which imposed a similar rhythm on herbivorious grazing.
The population was essentially absent from the surface duringb daylight hours, most of it concentrated well below 200 meters (bottom depth at station is approximately 325 m).
At 20.OO, about6 hours before the onset of maximum darkness, the population began to rise perceptibly above 200 m and generally reached the upper portion of its nighttime excursion at 01.00 (local time). Almost immediately thereafter, the downward migration began, returning the population to its daytime depth range by the time surface-light intensity had increased to about 10 percent of its daily maximum (fig. A, B). The upper limit of the vertical excursion of the bulk of the population could not have been shallower than 15 m, and sometimes was deeper.
Based on these observations, the minimum migration velocity is 25 m per hour.
Grazing activity (as indicated by the level of gut fluorescence) was strongly tied to the nightly vertical migration event (fig. C). |
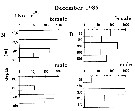 Issued from : M.I. Zmijewska in Oceanologia, 1993, 35. [Fig. 3.4]. Issued from : M.I. Zmijewska in Oceanologia, 1993, 35. [Fig. 3.4].
Diel changes in abundance distribution (ind./ m2) of Metridia gerlachei adult female and male (from 64°50'S, 61°50'W, Croker Passage, Antarctic Peninsula) during 3 austral seasons.
N = night; D = day. |
 Issued from : M.I. Zmijewska in Oceanologia, 1993, 35. [Fig. 3.4]. Issued from : M.I. Zmijewska in Oceanologia, 1993, 35. [Fig. 3.4].
Diel changes in abundance distribution (ind./ m2) of Metridia gerlachei adult female and male (from 64°50'S, 61°50'W, Croker Passage, Antarctic Peninsula) during 3 austral seasons.
N = night; D = day. |
 Issued from : M.I. Zmijewska in Oceanologia, 1993, 35. [Fig. 3.4]. Issued from : M.I. Zmijewska in Oceanologia, 1993, 35. [Fig. 3.4].
Diel changes in abundance distribution (ind./ m2) of Metridia gerlachei adult female and male (from 64°50'S, 61°50'W, Croker Passage, Antarctic Peninsula) during 3 austral seasons.
N = night; D = day. |
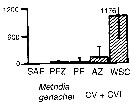 Issued from : A. Atkinson & J.D. Sinclair in Polar Biol., 2000, 23. [p.50, Fig.3] Issued from : A. Atkinson & J.D. Sinclair in Polar Biol., 2000, 23. [p.50, Fig.3]
Metridia gerlachei from Scotia Sea.
Median and interquartile ranges of copepods (nos /m2) in the five water zones; from north to south these are SAF Subantractic Front area, PFZ Polar frontal Zone, PF Polar Front area, AZ Antarctic Zone, WSC Weddell-Scotia Confluence area/ East Wind Drift.
Numbers on the plots are upper interquartiles where these could not be scaled. |
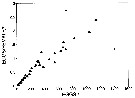 Issued from : M.E. Huntley & F. Escritor in Deep-Sea Res., 1992, 39 (6). [p.1047, Fig.16]. Issued from : M.E. Huntley & F. Escritor in Deep-Sea Res., 1992, 39 (6). [p.1047, Fig.16].
Predation rate (eggs /female/h) of Metridia gerlachei adult females on eggs of Calanoides acutus (eggs/l) under experimental conditions from animalsBransfield Strait (Antarctica).
Two data points (+) are considered outliers.
Nota: The females are clearly able to prey upon eggs of Calanoides acutus, and demonstrated no tendency for food saturation at concentrations up to 1200 eggs/litre.
Eggs of C. acutus are estimated to contain 0.24-0/68 ngC (see Lopez, 1991). These results suggest that, at a concentration of 1000 eggs/l, M. gerlachei females would ingest in the range of 5.6-14.3 µgC/day, for a daily ration of 4-11% body weight. |
 Issued from : M.E. Huntley & F. Escritor in Deep-Sea Res., 1992, 39 (6). [p.1046, Fig.15]. Issued from : M.E. Huntley & F. Escritor in Deep-Sea Res., 1992, 39 (6). [p.1046, Fig.15].
Metridia gerlachei late stage copepodites (CV and CVII): frequency distributions of daily ration (ù body carbon/day), determined on the basis of gut evacuation rate (from an experiment at Gerlache Strait, in December 1986 and March 1987)) andin situ gut pigment content.
Data are pooled from stations throughout the grid from December 1986 to February 1987.
The gut evacuation , measured by the gut fluorecence method in the Gerlache Strait, suggested a significant grazing rate on phytoplancton.
Daily rations were estimated for adult females collected from various stations throughout the study area in December to February. All stations used in this analysis were visited at night, and thus daily ration could be overestimated due to the more intense feeding at night.
The daily ingestion rate , I (µg C copepod by day) was estimated from the equation: I = kPgC; where k is the grazing constant estimated from the gut evacuation rate experiment (81.2 by day), Pg is the in situ gut pigment content (ng Chl a equivalents), and C is the carbon:chlarophyll ratio , estimated (here to 60 from O. Holm-Hansen, pers. comm.).
Frequency distributions of the daily ration of individual copepods indicate that the majority of animals ingested in the range of 40-100 µg C by day. This would represent a daily ration of about 30-75% of body weight if we assume a mean of 292 µg dry weight and a body carbon content of 45% (see Conover & Huntley, 1991). |
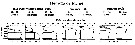 Issued from : S.B. Schnack-Schiel & W. Hagen in J. Plankton Res., 1994, 16 (11). [p.1557, Fig.12]. Issued from : S.B. Schnack-Schiel & W. Hagen in J. Plankton Res., 1994, 16 (11). [p.1557, Fig.12].
Vertical distribution of M. gerlachei from eastern Weddell Sea, as a percent of total numbers and temperature profile within the upper 1000 m. |
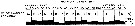 issued from A. de C. Baker in 'Discovery' Rep., 1954, 27. [p.215, Fig.5]. issued from A. de C. Baker in 'Discovery' Rep., 1954, 27. [p.215, Fig.5].
Occurrence of Metridia gerlachei in all longitudes around Antarctic zone of the Southern Ocean.
The percentage frequency of occurrence in samples taken within every 20° of longitude.
Nota: During the 'Discovery' investigations some thousands of plankton samples have been taken from stations spread over the whole of the Southern Ocean at all seasons of the year, the majority south of the Antarctic Convergence. An arbitrary selection of samples has been made from hauls between the surface and a depth of 250 m, which means that they have been taken from within the limits of the Antarctic surface water.
The data suggest that the Southern Ocean is an uninterrupted circumpolar belt with more or less uniform conditions prevailing in east and west directions, the range of planktonic species of the Antarctic surface water may be expected to extend as far as these uniform conditions persist, i.e to be circumpolar. |
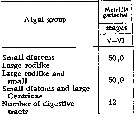 Issued from : N.M. Voronina & I.N. Sukhanova in Oceanology, 1977, 16 (6). [p.615]. Issued from : N.M. Voronina & I.N. Sukhanova in Oceanology, 1977, 16 (6). [p.615].
Frequency of encounter of various groups of diatom algae in the character of leading objects in the food pellet (% of number of digestive tracts with food).
Data from stations between 57°S-69°43'S.
The animals utilize all diatom species from 5 to 300 µm in size, the only animal food found there were tintinnids and radiolarians in negligible quantity. |
 Issued from : K.M. Swadling, So. Kawaguchi & G.W. Hosie in Deep-Sea Research II, 2010, 57. [p.898, Fig.6 (continued)]. Issued from : K.M. Swadling, So. Kawaguchi & G.W. Hosie in Deep-Sea Research II, 2010, 57. [p.898, Fig.6 (continued)].
Distribution of indicator species Metridia gerlachei from the BROKE-West survey (southwest Indian Ocean) during January-February 2006.
Sampling with a RMT1 net (mesh aperture: 315 µm), oblque tow from the surface to 200 m.
The survey area was located predominantly within the seasonal ice zone, and in the month prior to the survey there was considerable ice coverage over the western section but none over the east.
See map showing sampling sites in Calanus propinquus. |
 Issued from : A.C. Hardy & E.R. Gunther in Discovery Reports, 1935 (1936), 11. [p.171, Fig.79]. Issued from : A.C. Hardy & E.R. Gunther in Discovery Reports, 1935 (1936), 11. [p.171, Fig.79].
Charts showing the distribution of Metridia gerlachei in the upper layers of waters at stations in the 1926-7 surveys around South Georgia.
The squares represent the average numbers per 50 m vertical haul from 250 m (or less at shallow-water stations) to the surface with N 70 V nets. |
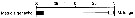 Issued from : E.T. Park & F.D. Ferrari in A selection from Smithsonian at the Poles Contributions to International Polar year. I. Krupnik, M.A. Lang and S.E. Miller, eds., Publs. by Smithsonian Institution Scholarly Press, Washington DC., 2009. [p.167, Fig.2]. Issued from : E.T. Park & F.D. Ferrari in A selection from Smithsonian at the Poles Contributions to International Polar year. I. Krupnik, M.A. Lang and S.E. Miller, eds., Publs. by Smithsonian Institution Scholarly Press, Washington DC., 2009. [p.167, Fig.2].
Distribution of selected pelagic calanoids Metridia gerlachei of the Southern Ocean and the closest relative in the subarctic region of the Arctic Ocean. |
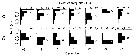 Issued from : J. Michels, S.B. Schnack-Schiel, A. Pasternak, E. Mizdalski, E. Isla & D. Gerdes in Polar Biol., 2012, 35. [p.378, Fig.9 (part., copepodid stages omitted.)]. Issued from : J. Michels, S.B. Schnack-Schiel, A. Pasternak, E. Mizdalski, E. Isla & D. Gerdes in Polar Biol., 2012, 35. [p.378, Fig.9 (part., copepodid stages omitted.)].
Vertical distribution of the adult females and males of Metridia gerlachei from East Weddell Sea shelf (70°48.558'S, 10°43.698'W) between 9 and 28 December 2003. The water depth at the sampling spots varied between 438 and 484 m.
The white dots represent the weighted mean depths. |
 Issued from : P. Ottestad in On Antarctic Copepods from the ''Norvegia'' Expedition 1930-1931. Scient. Results Norw. Antarct. Exped., 1936, 15. [p.20, Table 11, 12]. Issued from : P. Ottestad in On Antarctic Copepods from the ''Norvegia'' Expedition 1930-1931. Scient. Results Norw. Antarct. Exped., 1936, 15. [p.20, Table 11, 12].
Roman numerals = copepodid stages; depth in meters. Vertical hauls with standard plankton net (Rustad, 1930).
To determine the number of the various copepodite stages:
Stage I has 4 pair of legs and 2 abdominal segments.
Stage II has 5 pair of legs and 2 abdominal segments.
Stage III has 5 pair of legs and 3 abdominal segments.
Stage IV, M, F has 5 pair of legs and 4 or 5 abdominal segments.
Nota: An entirely different picture of the vertical distribution is obtained comparatively to C. acutus, C. propinquus and Rhincalanus gigas (Table 11 and 12). This species is found only exceptionally above 50 m. The greatest quantities both of the oldest and youngest stages are found between 400 and 100 m. There is, however, a striking difference between stations in the area between Kerguelen and Ross Sea on the one hand and those in Scotia Sea and the Weddell Sea on the other.. The three youngest stages (Table 12) do not occur at all above 50 m, and only a few individuals were taken between 100 and 50 m. |
 Issued from : P. Ottestad in On Antarctic Copepods from the ''Norvegia'' Expedition 1930-1931. Scient. Results Norw. Antarct. Exped., 1936, 15. [p.7, Fig.1]. Issued from : P. Ottestad in On Antarctic Copepods from the ''Norvegia'' Expedition 1930-1931. Scient. Results Norw. Antarct. Exped., 1936, 15. [p.7, Fig.1].
The position of the biological stations of ''Norvegica'' 1930-1931. |
 Issued from : K.M. Swadling, F. Penot, C. Vallet & al. in Polar Sci., 2011, 5. [p.125, Fig.5]. Issued from : K.M. Swadling, F. Penot, C. Vallet & al. in Polar Sci., 2011, 5. [p.125, Fig.5].
Mean abundance (ind. per 1000 m3) and standard errors of Metridia gerlachei collected each summer (January), 2004-2008 in the region covered the area from 139°E to 145°E from Terre Adélie to the Mertz Glacier Tongue.
Nota: The entire region sampled during 2004 to 2008 surveys was south of the continental slope and was under the influence of the westward flowing Antarctic Coastal Current. Macrozooplankton were collected by oblique tows of a Bongo net (500 µm mesh aperture) |
| | | | Loc: | | | Antarct. (Amundsen Sea, King George Is., Potter Cove, Gerlache & Bransfield Straits, Bellingshausen Sea, Peninsula, Drake Passage, Scotia Sea, Weddell Sea, off Halley Bay, SW Atlant., Syowa station, SE, Weddell Sea, Prydz Bay, Indian, Dumont d'Urville Sea, Lützow-Holm Bay, Pacif. (SW & SE), Ross Sea, McMurdo Sound, Station Davis, Prydz Bay), sub-Antarct. (Drake Passage, Scotia Sea, Indian), SW Atlant., Marion Is., off W Prince Edward Is., S Indian (in Wolfenden, 1911), Chile (N-S), S Tasman Sea, off S New Zealand (sub-Antarct.) | | | | N: | 157 | | | | Lg.: | | | (25) F: 4,01-3,38; M: 2,61-2,16; (31) F: 3,94-3,58; (33) F: 3,8; 4,25; M: 2,7-3; (66) F: 3,86-3,46; M: 2,39; (102) F: 4,3-3,65; M: 2,7-2,35; (114) F: 3,79-3,25; {F: 3,25-4,30; M: 2,16-2,70}
The mean female size is 3.773 mm (n = 12; SD = 0.3270), and the mean male size is 2.559 mm (n = 7; SD = 0.2794). The size ratio (male : female) is 0.66 (n = 4; SD = 0.0327). | | | | Rem.: | epi- to bathypelagic.
Sampling depth (Antarct., sub-Antarct.) : 0-1000 m.
After Ottestad (1936, p.38) according Mackintosh (1934) this species is a cold water form. This species deviates from the two Calanus propinquus and Calanoides acutus in several aspects. Its vertical migrations are considerable and it is, a patchy form. Moreover, both as regards horizontal and vertical distribution there is a great difference between this species and the two calanidae. Thus, it has been shown that M. gerlachei occurs in relatively great quantities at the stations north of South Orkney. The species is found preferably in deeper waters (between 100 and 300 m); the vertical distributions of the oldest and youngest are fairly identical than C. acutus (see Table 11 and 12).
This species is only known from the Antarctic and sub-Antarctic, it is one of the most abundant species, confined to the intermediate and deep waters south of the Antarctic Convergence (Tanaka, 1960, p.51); only C.B. Wilson (1950, p.264) reports the species near the Antilles, off Orénoque, off Rio de Janeiro S, Patagonia, off W. Colombia, Panama Canal; Vervoort (1957, p.121) assumes a confusion with M. lucens.
For Vervoort (1951, p.120), Wolfenden (1911) assumed that the specimens recorded by Cleve (1904) as M. lucens from South of the Cape Colony belong to the present form; this, however, is questionable, as M. lucens, a common form in boreal and temperate parts of the Atlantic and Pacific Oceans, penetrates far to the South. Moreover, M. gerlachei is at once recognized by the curiously shaped cephalothorax and it seems that Cleve must have been quite capable to discriminate between both forms.
After Atkinson (1998, p.295) this species has been classed as predominantly herbivorous in summer although laboratory experiments have found significant intake of protozoans and metazoans.
For Graeve & al. (1994, p.915) the trophic position of this species can be elucidated by means of the lipid composition (''marker lipids''); the level of wax esters was relatively low (27-42%), while the accumulation of triacylglycerols tended to be higher (19-22%); the fatty acid composition was characterized by very high amounts of these 22:6 and 20:5 acids; other important fatty acids were 18:1; the fatty alcohols consisted almost exclusively of the short-chain components 14:0 and 16:0. The omnivorous regime is clearly reflected by its lipid and fatty acid/alcohol pattern. | | | Last update : 17/06/2021 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2025. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed December 01, 2025] © copyright 2005-2025 Sorbonne University, CNRS
|
|
 |
 |

























