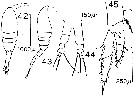|
|
 |
|
Calanoida ( Order ) |
|
|
|
Metridinidae ( Family ) |
|
|
|
Metridia ( Genus ) |
|
|
| |
Metridia longa (Lubbock, 1854) (F,M) | |
| | | | | | | Syn.: | Calanus longus Lubbock,1854;
Metridia armata Boeck,1864;
Cf.: in Shih & al., 1971 (p.148) | | | | Ref.: | | | Giesbrecht, 1892 (p.339, 345, figs.F,M); Giesbrecht & Schmeil, 1898 (p.106); Sars, 1900 (p.99, figs.F,M); 1902 (1903) (p.112, figs.F,M); T. Scott, 1902 (p.454, figs.F,M, Rem.); Lysholm, 1913 (p.6); Willey, 1920 a (p.15); Lysholm & Nordgaard, 1921 (p.23); Sars, 1925 (p.198); Campbell, 1929 (p.316, Rem.); Wilson, 1932 a (p.120, figs.F,M); Rose, 1933 a (p.177, figs.F,M); Jespersen, 1934 (p.93, fig.25, 26, Rem.); 1940 (p.44, fig.5); Lysholm & al., 1945 (p.32); Farran, 1948 c (n°14, p.3, figs.F); ? Davis, 1949 (p.49, fig.F, Rem.); Brodsky, 1950 (1967) (p.292, figs.F,M); Marques, 1953 (p.109); Vidal, 1971 a (p.13, 23, 115, figs.F,M); Arcos, 1975 (p.17, figs.F,M); Szabo & Gardner, 1986 (p.1560: Appendix, Rem.); Lapota & al., 1988 (p.314, fig. Nauplius, bioluminescence); Kurbjeweit & Buchholtz, 1991 (p.168, A1 structures); Razouls, 1994 (p.142, figs.F,M, Rem.); Karlson & Bamstedt, 1994 (p.79, fig.2: Md); Bucklin & al., 1995 (p.655, 658: phylogeny); Bradford-Grieve & al., 1999 (p.884, 948, figs.F,M); G. Harding, 2004 (p.32, figs.F,M); Ferrari & Dahms, 2007 (p.35, Rem. N, biolum.); Vives & Shmeleva, 2007 (p.362, figs.F,M, Rem.); Dalpadado & al., 2008 (p.2266, Fig.2: Md, Table 2, 3); Ershova & Kosobokova, 2012 (p.676, figs.F,M: genital system, morphotypes). | 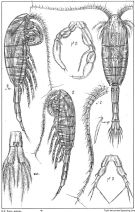 issued from : G.O. Sars in An Account of the Crustacea of Norway. Vol. IV. Copepoda Calanoida. Published by the Bergen Museum, 1903. [Pl.LXXV]. Female & Male. Nota: Sens.app = aesthetasc on A1 male.
|
 issued from : G.O. Sars in An Account of the Crustacea of Norway. Vol. IV. Copepoda Calanoida. Published by the Bergen Museum, 1903. [Pl.LXXVI]. Female. Nota: R = rostrum (frontal view); M = Md; m = Mx1; mp1 = Mx2; mp2 = Mxp.
|
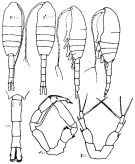 Issued from : K.A. Brodskii in Calanoida of the Far Eastern Seas and Polar Basin of the USSR. Opred. Fauna SSSR, 1950, 35 (Israel Program for Scientific Translations, Jerusalem, 1967) [p.292, Fig.198]. Female (from Arctic): habitus (dorsal and lateral left side); Do, urosome (dorsal); S5, P5. Male: habitus (dorsal and lateral left side); S5, P5.
|
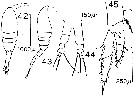 issued from : D.F.R. Arcos in Gayana, Zool., 1975, 32. [Lam.V, Figs.42-45]. Female (from Bahia de Concepcion, Chile): 44, P5; 45, P2. Male: 42-43, habitus (dorsal and lateral, respectively).
|
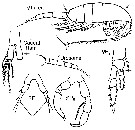 issued from : G. Harding in Key to the adullt pelagic calanoid copepods found over the continental shelf of the Canadian Atlantic coast. Bedford Inst. Oceanogr., Dartmouth, Nova Scotia, 2004. [p.32]. Female & Male. Nota: Metasome posteriorly truncated (arrowed); cephalosome not vaulted in lateral view (arrowed); caudal rami three times as long as broad.
|
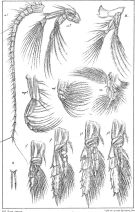
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.33, Fig.9]. Female: 9, A1 (proximal segments).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.33, Figs.20, 27]. Female: 20, P5; 27, P3 (inner setae missing).
|
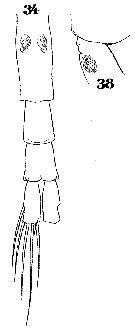 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.33, Figs.34, 38]. Female: 34, urosome (ventral); 38, thoracic segment 5 and genital segment (lateral).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.33, Figs.13, 23]. Male: 13, geniculate segments of A1; 23, P5 (anterior view). Ps = left leg; Pd = right leg; B1 = basipodite 1 ( = coxa); B2 = basipodite 2 (= basis); Re = exopod.
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. - Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.32, Fig.10]. Female: 10, exopodial segment 1 of P2 (anteriot view).
|
 Issued from : T. Scott in Rep. Fishery Bd Scotl., 1902, 20 (3). [Pl. XXII, Figs. 1-4]. Female (from Shetland Islands): 1, habitus (dorsal); 3, P5. Male: 2, habitus (dorsal); 4, P5.
|
 Issued from : E.A. Ershova & K.N. Kosobokova in Biol. Bull., 2012, 39 (8). [p.680, Fig.3]. Male (from White Sea): a, P5 ''left-sided morph''; b, P5 ''right-sided morph. Ventral view for a and b..
|
 Issued from : E.A. Ershova & K.N. Kosobokova in Biol. Bull., 2012, 39 (8). [p.680, Fig.4]. Male (from White Sea): a, Slit-like gonopore ''left-sided morph''; b, same ''right-sided morph''. Ventral view . Slit-like gonopore indicated by arrows.
|
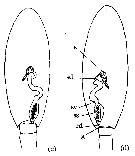 Issued from : E.A. Ershova & K.N. Kosobokova in Biol. Bull., 2012, 39 (8). [p.679, Fig.2]. Schematic structure of the male genital system of M. longa from White Sea (dorsal view): c, ''left-sided morph''; d, ''right-sided morph''. s: seminal gland; sd: spermaduct; sv: seminal vesicle; ss: spermatophore sac; ed: ejaculatory duct; g, gonopore. The black mass inside the spermatophore sas is the spermatophore.
|
 Issued from : E.A. Ershova & K.N. Kosobokova in Biol. Bull., 2012, 39 (8). [p.681, Fig.5]. Schematic structure of the female genital segment of M. longa from White Sea (ventral view): a, with left-sided insemination; b, with right-sided insemination; c, with bilateral insemination. cp: copulatory pore; sp: spermatheca; cd: copulatory duct; g, gonopore. Nota: The authors emphasizes that the vast majority of the females in the studied population in all seasons of the year had only one filled sepermatheca, either the right or the left. The females with bilateral insemination also occurred, but did not exceed 2.7%. The ratio of females with the right and left filled spermathecae and ‘’right-handed’’ and left-handed’’ males suggests thart the male morphotype determines in which spermathecae male gonads are most likely to get into. The presence of females with two filled spermathecae implicates insemination by two males of different morphotypes. Since each spermatheca is connected to only one of the two oviducts, it is assumed that a half of the eggs produced by unilateral inseminated females remains not fertilized. The morphology of genital structures and litterature data on the egg production of M. longa indicate that almost a half of eggs produced by females is not viable and thus wasted.
|
 Issued from : C. Razouls in Ann. Inst. océanogr., Paris, 1994, 70 (1). [p.142]. Caractéristiques morphologiques de Metridia longa femelle et mâle adultes. Terminologie et abbréviations: voir à Calanus propinquus. La station ''Kerfix'' est située à l'entrée du Golfe du Morbihan ( Ïles Kerguelen)
| | | | | Compl. Ref.: | | Mrazek, 1902 (p.515, 523); Pearson, 1906 (p.23, Rem.); Damas & Koefoed, 1907 (p.396, tab.II, III); Jespersen, 1939 (p.60, Rem., Table 25, 26, 27, 28, 29, 30); Bogorov, 1939 b (p.706); Wilson, 1942 a (p.194); Sewell, 1948 (p.349, 512, 514, 526, 548, 559, 567, 568); C.B. Wilson, 1950 (p.264); Gundersen, 1953 (p.1, 22, Table 19, seasonal abundance); King & Hida, 1955 (p.11); Østvedt, 1955 (p.15: Table 3, p.17, 71); Fagetti, 1962 (p.26); Grice, 1962 a (p.101); 1963 a (p.496); M.W. Johnson, 1963 (p.89, Table 1, 2); Lacroix & Bergeron, 1963 (p.59, Tableau IV); De Decker & Mombeck, 1964 (p.13); Brodsky, 1964 (p.105, 107); Sherman & Schaner, 1965 (p.618, parasitism); Grice & Hulsemann, 1965 (p.224); Harding, 1966 (p.17, 65, 66); Furuhashi, 1966 a (p.295, vertical distribution vs mixing Oyashio/Kuroshio region); Matthews, 1967 (p.159, Table 1, Rem.); Haq, 1967 (p.40, nutrition); Pertsova, 1967 (p.240); Maclellan D.C., 1967 (p.101, 102: occurrence); Dunbar & Harding, 1968 (p.319); Conover & Corner, 1968 (p.49, 57, respiration & nitrogen excretion); Corner & Cowey, 1968 (p.393, Table 7, respiration rate); Vinogradov, 1968 (1970) (p.61, 65, 90, 266); Paulmier, 1971 (p.168); Shih & al., 1971 (p.40, 205); Matthews & Sands, 1973 (p.19, Table 4); Björnberg, 1973 (p.337, 387); Kolosova, 1975 (p.92,fig.3, Table 1); Landry, 1975 a (p.434, Rem.: p.437, fig.3); Skjoldal & Bamstedt, 1977 (p.197, adenosides); Deevey & Brooks, 1977 (p.256, tab.2, Station "S"); Hopkins & al., 1978 (p.261, Table 3, Rem.: DSL); Kolosova, 1978 (p.320); Pipe & Coombs, 1980 (p.223, figs. 1, 2, 4, table 1, vertical distribution); Kosobokova, 1980 (p.84, caloric value); Bamstedt & Skjoldal, 1980 (p.304, weight-RNA); Vives, 1982 (p.293); Peruyeva, 1982 (p.471, feeding); Buchanan & Sekerak, 1982 (p.41, vertical distribution); Tande & Grønvik, 1983 (p.43, gonad maturation, sex-ratio); Huntley & al., 1983 (p.143, Table 2, 3); Hassel, 1983 (p. 1, fig.6, abundance, distribution); Lapota & Losee, 1983 (p.52, bioluminescence); Bamstedt, 1983 (p.291, RNA variation); Peruyeva, 1984 (p.613, Table 1, feeding); Norrbin & Bamstedt, 1984 (p;47, Table 2, 3: calorific value); Grønvik & Hopkins, 1984 (p.93, life history); Hopkins C. & al., 1984 (p.77, life history); Tremblay & Anderson, 1984 (p.5); Sameoto, 1984 (p.213, Table 1, fig.4, 9); 1984 a (p.767, vertical migration); Bamstedt & Ervik, 1984 (p.843, size, enzymes, chemical composition); Bamstedt, 1985 (p.607, excretion rate); Bamstedt & Tande, 1985 (p.259, Table 2: literature data respiration & excretion); Sameoto & al., 1986 (p.53); Groendahl & Hernroth, 1986 (tab.1); Lapota & al., 1986 (p.887); Mikhailovsky, 1986 (p.83, Table 1, ecological modelling); Hirche, 1987 (p.347, activity, respiration v.s. temperature); Falk-Petersen & al., 1987 (p.115, lipid composition); Tiselius, 1988 (p.215, grazing); Conover & al., 1988 (p.267, 268); Head & al., 1988 (p.333, Table 1, 2, gut analysis, defecation rate); S.L. Smith, 1988 (p.145, vertical distribution, ice-edge effect); Bamstedt, 1988 (p.15, protein content); Bamstedt & Tande, 1988 (p.31, respiration, excretion); Runge & Ingram, 1988 (p.280, grazing); Kosobokova, 1989 (p.27); Citarella, 1989 (p.123, abundance); Ikeda & Skjoldal, 1989 (p.173, oxygen consumption, N & P excretion, O:N vs. body weight); Estep & al., 1990 (p.235, grazing); S.L. Smith, 1990 (p.59, egg production, lgut content); Fransz & al., 1991 (p.9); Buskey & Stearns, 1991 (p.885, bioluminescence); Conover & al., 1991 (p.178); Hattori, 1991 (tab.1, Appendix); Hirche, 1991 (p.351); Hirche & al., 1991 (p.477, Fig.3, 7, Table 2); Conover & Huntley, 1991 (p.1, fig. 2, 5, Table 2, 3, 6, 8, 9, 10, 11, polar seas comparison); Hirche & Mumm, 1992 (p.S485, geographic distribution, egg production); Head, 1992 (p.583, gut pigment destruction); Buskey, 1992 (p.691, tab.1); Lapota & al., 1993 (p.665, bioluminescence); Mumm, 1993 (tab.1, fig.2); Conover & al., 1993 (p.303, Table 2, figs. 7 ,8, dry weight, %); Vinogradov & al., 1994 (tab.1); Richter, 1994 (tab.4.1a); Petryashov & al., 1995 (tab.1); Pedersen & al., 1995 (p.266, tabl.II); Ashjian & al., 1995, p.4371, Fig.4, 5, 6, Table 2, 4); Hays, 1995 (p.301, Table 1, vertical migration); 1995 a (p.1461, ontogeny); Krause & al., 1995 (p.81,Rem.: p.130; Kotani & al., 1996 (tab.2); Albers & al., 1996 (p.347, lipids vs. diet); Hanssen, 1997 (tab.3.1); Ashjian & al., 1997 (p.279, Table 1, 2, Figs. 3, 4D); Falkenhaug & al., 1997 (p.449, spatio-temporal pattern); Hirche & Kwasniewski, 1997 (p.299, Table 1, 5); Weslawski & Legezynska, 1998 (p.1238); Kosobokova & al., 1998 (tab.2); Mauchline, 1998 (tab.19, 21, 33, 38, 48, 58, 63, 64); Mumm & al., 1998 (p.189, Figs.3, 5); Conover & Gustavson, 1999 (p.41, tab.6); Thibault & al., 1999 (p.1391); B.W. Hansen & al., 1999 (p.233, seasonal abundance & biomass); Halvorsen & Tande, 1999 (p.279, figs.3, 5); Kosobokova & Hirche, 2000 (p.2029, tab.2); Beare & al., 2000 (p.1545, Arctic index indicatot); Razouls & al., 2000 (p.343, Appendix); Holmes, 2001 (p.18, Rem.); Fortier M. & al., 2001 (p.1263, fig.6, 7, diel vertical migration); Lischka & al., 2001 (p.186); Sameoto & al., 2002 (p.13); Ringuette & al., 2002 (p.5081, Table 1, Fig.7, population dynamic); Beaugrand & al., 2002 (p.1692); Beaugrand & al., 2002 (p.179, figs.5, 6); Auel & Hagen, 2002 (p.1013, tab.2, 3); Pertsova & Kosobokova, 2002 (p.226, interannual vatiation); Wexels Riser & al., 2002 (p.175, fecal pellets, Rem.: p.184); Kosobokova & al., 2003 (p.697, tab.2);
After Karnovsky & al., 2003 (p.289, Appendix 2) the mean dry mass (mg) female is 0.015. Ashjian & al., 2003 (p.1235, figs.); Kahle & Zauke, 2003 (p.409, metals concentration); Gislason & Astthorsson, 2004 (p.472, tab.1); CPR, 2004 (p.56, fig.164); Hopcroft & al., 2005 (p.198, table 2); Frangoulis & al., 2005 (p.254, Table I: C/N/P fecal pellet composition); Dmoch & Walczowski, 2005 (p.102 + poster); Blachowiak-Samolyk & al., 2006 (p.101, tab.1); Durbin & Casas, 2006 (p.2537, Table 2a, 2b); Willis & al., 2006 (p.39, Table 2, advection vs changes in community structure); Hop & al., 2006 (p.182, Table 4, 5: inter-annual variability); Kattner & al., 2007 (p.1628, Table 1, Rem.: p.1634, Table 1, seasonal pulsing phytoplankton vs dominance); Head & Sameoto, 2007 (p.2686, abundance vs interdecadal variability); Deibel & Daly; 2007 (p.271, Table 1, 2, 5, Rem.: Arctic polynyas); Blachowiak-Samolyk & al., 2007 (p.2716, Table 2); Walkusz & al., 2008 (p.1, Table 3, abundance); Lane & al., 2008 (p.97, Tab.4); Stepanyuk & al., 2008 (p.142, bioluminescence); Gaard & al., 2008 (p.59, Table 1, N Mid-Atlantic Ridge); Darnis & al., 2008 (p.994, Table 1, figs.8, 9); Falk-Petersen & al., 2008 (p.2275, depth distribution); Pasternak & al., 2008 (p.2245, Table 1, 2, 3, grazing); Blachowiak-Samolyk & al., 2008 (p.2210, Table 2, 3, 5, fig.4, biomass, composition vs climatic regimes); Daase & al., 2008 (p.192, vertical distribution); Galbraith, 2009 (pers. comm.); Campbell & al., 2009 (p.1274, Table 2, 3, figs.3, grazing); Dvoretsky & Dvoretsky, 2009 a (p.11, Table 2, abundance); Kosobokova & Hirche, 2009 (p.265, Table 4, fig.10: chart, biomass); Sampei & al. , 2009 (p.1894, in moored trap); Park & Ferrari, 2009 (p.143, fig.2, biogeography); Kosobokova & Hopcroft, 2010 (p.96, Table 1, fig.7); Arendt & al., 2010 (p.49, egg & faecal pellets production vs. environmental conditions); Dünweber & al., 2010 (p.11, biomass); Bucklin & al., 2010 (p.40, Table 1, Biol mol.); Hsiao & al., 2010 (p.179, Table III, trace metal concentration); Dvoretsky & Dvoretsky, 2010 (p.991, Table 2); 2011 a (p.1231, Table 2: abundance, biomass); Pepin & al., 2011 (p.273, Table 2, seasonal abundance); Kosobokova & al., 2011 (p.29, Table 2, figs.4, 6, Rem.: Arctic Basins); Tang & al., 2011 (p.77, composition & biomass); Arendt & al., 2011 (p.1526, clearance rate, , fecal pellet production, egg production); Pomerleau & al., 2011 (p.1779, Table III, IV, V, VI); Hirche & Kosobokova, 2011 (p.2359, Table 3, abundance, biomass %); Forest & al. 2011 (p.161, biomass, chemical composition); 2011 a (p.11418); 2012 (p.1301, figs.7, 8); Matsuno & al., 2012 (p.105, Table 1, 2, 3, fig.4, 7); Takenaka & al., 2012 (p.1669, fig.2, 3, Table 1, bioluminescence); Sigurdardottir, 2012 (p.1, Table 2.3); Alvarez-Fernandez & al., 2012 (p.21, Rem.: Table 1); Dvoretsky & Dvoretsky, 2012 (p.1321, Table 2, 3, 4, 5, abundance, biomass, production); Kjellerup & Kiørboe, 2012 (p.438, prey detection); Hidalgo & al., 2012 (p.134, Table 2); Markova & al., 2012 (p.98, luciferase); Sampei & al., 2012 (p.90, Table 1, abundance in sediment trap); Hsiao & Fang, 2013 (p.175, Table 2: Hg bioaccumulation); Gusmao & al., 2013 (p.279, fig.1, sex ratio vs predators, fig.4: seasonal variation of sex ratio); Kürten & al., 2013 (p.167, Table 1, C:N, fatty acid); Saiz & al., 2013 (p.17, Rem.: p.23, Table 2, abundance, distribution); Lidvanov & al., 2013 (p.290, Table 2, % composition); Dvoretsky & Dvoretsky, 2013 a (p.205, Table 2, % abundance); Arendt & al., 2013 (p.105, fig.3, abundance); Barton & al., 2013 (p.522, Table 1: metabolism, population dynamic, feeding mode, biogeo); Kouwenberg & al., 2014 (p.290, biogeography, Map 11); Schmid & al., 2016 (p.129, fig.3, identification vs imaging system); Smoot & Hopcroft, 2016 (p.1, Rem.: p.7); Record & al., 2018 (p.2238, Table 1: diapause); | | | | NZ: | 16 + 1 doubtful | | |
|
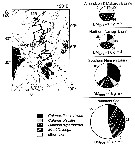
Distribution map of Metridia longa by geographical zones
|
| | | | | | | | | | | |  issued from : N. Mumm, H. Auel, H. Hanssen, W. Hagen, C. Richter & H.-J. Hirche in Polar Biol., 1998, 20. [p.192, Fig.1, p.194, Fig.3] issued from : N. Mumm, H. Auel, H. Hanssen, W. Hagen, C. Richter & H.-J. Hirche in Polar Biol., 1998, 20. [p.192, Fig.1, p.194, Fig.3]
Fig.1 after Diepenbroek & al., 1997; Station map, the dark line connects stations of different expeditions to a transpolar transect (AB: Amundsen Basin, BS: Barents Sea; GL: Greenland; GS: Greenland Sea; LR: Lomonov Ridge; MB: Makarov Basin; MJP: Morris Jessup Plateau; NB: Nansen Basin; NG: Nansen-Gakkel Ridge; SB: Spitsbergen; WSC: West Spitsbergen Current; YP: Yermak Plateau
Fig.3: Biomass share (% of total mesozooplankton dry mass) of Calanus finmarchicus, C. glacialis, C. hyperboreus, Metridia longa and other taxa in 0- to 500 m depth of different Arctic regions (DM total: mean total dry mass).
Note M. longa is a very important calanoid, with regard to both abundance and biomass. its abundance in the upper 500 m ranged generally around 1,200 ind. by m2 at the oceanic stations in the central Arctic Ocean. However, more than 8,000 ind by m2 occurred near the shelf break north of Spitsbergen. |
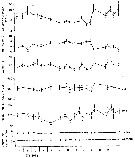 issued from : S. Grønvik & C.C.E. Hopkins in J. Exp. Mar. Biol. Ecol., 1984, 80. [p.102, Fig.9]. issued from : S. Grønvik & C.C.E. Hopkins in J. Exp. Mar. Biol. Ecol., 1984, 80. [p.102, Fig.9].
Metridia longa females (from 69°21'N, 19°06'E). Mean values with 95 % confidence limits for length, dry and wet weights, carbon (C) and nitrogen (N) as % dry weight and C/N ratio, November 1977 to November 1978.
Nota: Balsfjorden is a semi-enclosed fjord bounded by shallow sills (10-20 m; the species was sampled near Svartnes where the depth is ± 190 m, temperature range from 1-4 °C during most of the year, and light conditions vary from two months of midnight sun and two months of winter darkness. |
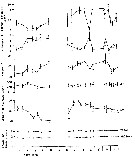 issued from : S. Grønvik & C.C.E. Hopkins in J. Exp. Mar. Biol. Ecol., 1984, 80. [p.101, Fig.8]. issued from : S. Grønvik & C.C.E. Hopkins in J. Exp. Mar. Biol. Ecol., 1984, 80. [p.101, Fig.8].
Metridia longa Males (from 69°21'N, 19°06'E). Mean values with 95 % confidence limits for length, dry and wet weights, carbon (C) and nitrogen (N) as % dry weight and C/N ratio, November 1977 to November 1978. |
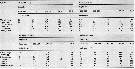 Issued from : S. Falk-Petersen & al. in Deep-Sea Res., 2008, 55. [p.2282, Table 5]. Issued from : S. Falk-Petersen & al. in Deep-Sea Res., 2008, 55. [p.2282, Table 5].
Arctic Ocean, Ice Stations 1 (82°N, 11°E) on 2 September 2004, and 2 (82°30'N, 21°E) on 4 September 2004: Depth distribution of mesozooplankton in the upper 1200 m. |
 Issued from : S. Falk-Petersen & al. in Deep-Sea Res., 2008, 55. [p.2281, Fig.8]. Issued from : S. Falk-Petersen & al. in Deep-Sea Res., 2008, 55. [p.2281, Fig.8].
Arctic Ocean, Ice Stations 1 (82°N, 11°E) on 2 September 2004, and 2 (82°30'N, 21°E) on 4 September 2004: Temperature (black profile), relative fluorscence values (red line), salinity (dotted line). |
 Issued from : K.W. Tang, T.G. Nielsen, P. Munk, J. Mortensen, E.F. Møller, K.E. Arendt, K. Tönnesson, T. Juul-Pedersen in Mar. Ecol. Prog. Ser., 2011, 434. [p.83, Fig.4] Issued from : K.W. Tang, T.G. Nielsen, P. Munk, J. Mortensen, E.F. Møller, K.E. Arendt, K. Tönnesson, T. Juul-Pedersen in Mar. Ecol. Prog. Ser., 2011, 434. [p.83, Fig.4]
Metridia longa (from the continental slope off Fyllas Bank to the inner part of Godthabsfjord, SW Greenland, corresponding to stations 0 to 20) in the summer (2008).
Contour plots of biomass (mg C/m3) of all developmental stages collected from 4 to 9 strata with a multinet samples (300 µm mesh aperture)
Dots are mid-points of sampling intervals. Numbers on top are stations. Hatched area = bottom topography.
Compare this distribution with the other dominant large zooplankton species Calanus glacialis, Calanus hyperboreus and Calanus finmarchicus for the same transect. |
 Issued from : K.W. Tang, T.G. Nielsen, P. Munk, J. Mortensen, E.F. Møller, K.E. Arendt, K. Tönnesson, T. Juul-Pedersen in Mar. Ecol. Prog. Ser., 2011, 434. [p.79, Fig.1] Issued from : K.W. Tang, T.G. Nielsen, P. Munk, J. Mortensen, E.F. Møller, K.E. Arendt, K. Tönnesson, T. Juul-Pedersen in Mar. Ecol. Prog. Ser., 2011, 434. [p.79, Fig.1]
Station positions along Godthabsfjord in southwestern Greenland. |
 Issued from : K.W. Tang, T.G. Nielsen, P. Munk, J. Mortensen, E.F. Møller, K.E. Arendt, K. Tönnesson, T. Juul-Pedersen in Mar. Ecol. Prog. Ser., 2011, 434. [p.81, Fig.2] Issued from : K.W. Tang, T.G. Nielsen, P. Munk, J. Mortensen, E.F. Møller, K.E. Arendt, K. Tönnesson, T. Juul-Pedersen in Mar. Ecol. Prog. Ser., 2011, 434. [p.81, Fig.2]
Contour plots of water temperature (°C), salinity, density (kg/m3) and chlorophyll a (mg/m3) along the transect of Godthabsfjord.
Distances were measured from Station o. Note the different contour line scales for different panels.
Hatched area in each panel represents bottom topography. |
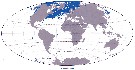 Issued from : J.H.M. Kouwenberg, C. Razouls & N. Desreumaux in Biogeographic Atlas of the Southern Ocean, Scient. Comm. Antarct. Res., Cambridge, 2014, 6.6. [p.294, Map 11]. Issued from : J.H.M. Kouwenberg, C. Razouls & N. Desreumaux in Biogeographic Atlas of the Southern Ocean, Scient. Comm. Antarct. Res., Cambridge, 2014, 6.6. [p.294, Map 11].
Distribution of Metridia longa. |
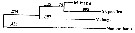 Issued from : A. Bucklin, B.W. Frost & T.D. Kocher in Mar. Biol., 1995, 121. [p.661, Fig.4]. Issued from : A. Bucklin, B.W. Frost & T.D. Kocher in Mar. Biol., 1995, 121. [p.661, Fig.4].
Phylogenetic relationships among three Metridia species based on sequence data for a 387 base pair region of the mitochondrial 16S rRNA gene and determined by neighbor joining (Saitou & Nei, 1987). Statistical methods for phylogenetic reconstruction are identical to those for calanus spp..
Numbers on horizontal branches are branch lengths; numbers at branchpoints (in iralics) are percentages of trees that show that branchpoint among 1000 bootstrapped replicates.
Nannocalanus is a outgroup by comparison.
M. pacifica from Dabob Bay, WA (Puget): 47°46'N, 122°50'W (B. Frost collector); M. lucens from Gulf of Maine (GOM): 42°30'N, 69°48'W (E. Durbin collector); M. longa from Gulf of St. Lawrence (STL): 48°40'N, 68°35'W ( J. Runge collector) |
 issued from : R.J. Conover & T.D. Siferd in Arctic, 1993, 45 (4). [p.307, Table 2]. issued from : R.J. Conover & T.D. Siferd in Arctic, 1993, 45 (4). [p.307, Table 2].
Numbers and dry weight biomass for the total water column (frpm Barrow Strait), before, during, and after the dark period 1984-1985 and 1985-1986 (Conover, Sifred and Harris, unpubl.).
Compare with Calanus hyperboreus, C. glacialis and Pseudocalanus acuspes in the same area of Barrow Strait.
For the authors, the life cycle is understood least well of the several dominant copepods at Resolute (Barrow Strait). While females show little gonad development until May or June, they are common in the population at any season/. Moreover, there is clear evidence for growth and development during the winter. Perhaps a portion of the population can complete a generation in one year.
In laboratory experiments, late-stage readily ingested the large, wax-ester-containing eggs of Calanus hyperboreus, which are abundant in the water column during late winter and spring (Conover, unpubl.). These would constitute an easily caught, highly nutritions energy source to facilitate dark-season growth in any opportunistics species. |
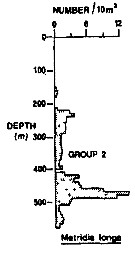 Issued from : R.K. Pipe & S.H. Coombs in J. Plankton Res., 1980, 2 (3). [p.230, Fig.4]. Issued from : R.K. Pipe & S.H. Coombs in J. Plankton Res., 1980, 2 (3). [p.230, Fig.4].
Metridia longa from Wyville Thomson Ridge (60°03.4' N, 07°03.9' W to 60°08' N, 07°02.9' W) on 24 April 1978: Vertical distribution.
Members of this group (Group 2) includes six calanoids species taken predominantly between 430 and 510 m; that is within the intermediate water between North Atlantic Oceanic water (temperatures between 8.0-8.7°C) and Norwegian Sea Deep water. This group contained alsoEukrohnia hamata and Thysanoessa longicaudata.
The group containedin addition to M. longa, Calanus hyperboreus, Heterorhabdus norvegicus, Pleuromamma robusta, Aetideus armatus, Gaetanus minor. Except of the bathypelagic copepods A. armatus, G. minor which are both cosmopolitan species, the organisms in this group are more commonly associated with arctic or boreal water (Fraser, 1961; jespersen, 1934; Einarsson, 1945); their occurrence as a group in the intermediate water suggests that this may possibly have originated from superficial water formed further to nthe north rather than being a mixture of North Atlantic Oceanic water and Norwegian Sea Deep water.
A computed coefficient of dissimilarity was used in association with a 'group-average' clustering strategy (see Clifford & Stephenson, 1975) to classify the plankton taxa on the basis of their vertical distribution after the indices of similarity and the results arranged diagramatically in the form of a dendogram (Fig.2). Classification was carried out using the Bray-Curtis measure which gives a coefficient of dissimilarity. |
| | | | Loc: | | | Antarct. (Peninsula), South Africa (E), Brazil, Angola, off Morocco-Mauritania, Bay of Biscay (Belon estuary), Gibraltar, Azores (rare), Sargasso Sea, off Bermuda: Station ‘’ S’’ (32°10’N, 64°30’W), off Cape Hatteras, G. of Maine, Bay of Fundy, Nova Scotia, Northumberland Strait, G. of St. Lawrence, Bradelle Bank, Newfoundland, off SE Nova Scotia, W Greenland, Nuuk, Disko Bay, Godthabsfjord, N Baffin Bay, Baffin Sea (Lancaster Sound), Fram Strait, Greenland Sea, Iceland, Faroe Is., Fram Strait, off Bear Island, Kongsfjorden, Kosterfjorden, Wyville Thomson Ridge (60°N), Spitsbergen, Balsfjorden, Barents Sea, Pechora Sea, Franz Josef Land, White Sea, Kara Sea, Laptev Sea, Chukchi Sea, Arct. (all polar Basins, Fletcher's Ice Is., Resolute Passage), Nansen Basin, SE Beaufort Sea, Devon Island, Amundsen Gulf, Barrow Strait, Canadian abyssal plain, Canada Basin, Lomonosov Ridge, Barrow Strait, Foxe Basin, Norway Sea, off E Shetland Is., Norway (Korsfjorden, Sognesjöen, Balsfjorden, Malangen fjord), Nordvestbanken, Raunefjorden, Skagerrak, North Sea, W Ireland, SW Indian, Japan (Onagawa, off Sanriku), S. Hokkaido, N Japan, Alaska, Bering Sea, British Columbia, off California, Hawaii, Pacif. (equatorial central), off NE Easter Is., S Galapagos, Chile, off Santiago | | | | N: | 239 | | | | Lg.: | | | (22) F: 4,5-4,1; M: 3,7-3,1; (45) F: 4,5-4; M: 4-3,5; (47) F: 4,1; M: 3,5; (59) F: 4,5-3,7; M: 4-3,1; (65) F: 4,3; M: 3,7; (75) F: 2,25; 1,64; M: 1,66; 1,59; (134) F: ± 4,3; (377) F: 4,8; M: 3,75; (787) M: 3,3; (796) F: 4-3,55; (1129) F: 3,7; M: 2,8; {F: 1,64-4,50; M: 1,59-4,00}
The mean female size is 4.158 mm (n = 13; SD = 0.3707), and the mean male size is 3.495 mm (n = 11; SD = 0.3863). The size ratio (male : female) is 0.83 (n = 7; SD = 0.0495). The body sizes from Marques (1953) (75) is not included because the great difference with the mean. | | | | Rem.: | Epi-Meso-bathypelagic.
Sampling depth (Antarct.) : 0-200 m. Sargasso Sea: 500-1500 m (Deevey & Brooks, 1977, Station "S"). 150-580 m (Pipe & Coombs, 1980, Arctic: 60°N).
For jespersen (1939, p.63) this species appears in far the greater number in the deepest water layers (chiefly in depths corresponding to hauls with about 700 m wire.
The dimensions given by Marques (1953) seem abnormally small, but the temperature is also higher than for the other geographical distribution data.
For Brodsky (1967, p.293) this species plays a role in the nutrition of planktophagous fishes.
See remarks in M. okhotensis.
According to Willey (1920, p.15), A.E. Nordenskiold (1881) points to: << during the wintering at Mussel Bay the small crustacea can live by millions in water-drenched snow at a temperature of from – 2° to – 10°. An exceedingly intense bluish-white flash of light appears, which in the spectroscope gives a one-coloured labrador-blue spectrum. When the temperature [of the snow-sludge] sinks below – 10° C, the power of this animal to emit light appears to cease >>.
After Karnovsky & al., 2003 (p.289, Appendix 2) the mean dry mass (mg) female is 0.287; CV: 0.120; CIv: 0.034.
The feeding mode after Barton & al. (2013, Table 1) is 'cruising' (See definition in Rem. Metridia pacifica). | | | Last update : 18/01/2021 | |
| | | | Metridia Luciferase, a naturally secreting bioluminiscent molecule, is isolated from this marine plankton, and is a potentially valuable tool for expression biologists.
Dr. Prem Raj Pushpakaran
http://myprofile.cos.com/drprpnitc, | |
|
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed January 06, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |