|
|
 |
|
Calanoida ( Order ) |
|
|
|
Metridinidae ( Family ) |
|
|
|
Metridia ( Genus ) |
|
|
| |
Metridia pacifica Brodsky, 1950 (F,M) | |
| | | | | | | Syn.: | Metridia sp. Esterly, 1924;
Metridia lucens pacifica Brodsky,1948;
Metridia lucens : Sato, 1913; Campbell, 1929 (p.316, Rem.); Mori, 1937 (1964) (p.67); Davis, 1949 (p.48, figs.F, p.66: Rem.); Tanaka, 1953 (p.143); Cameron, 1957 (? part., p.170, 183);
Cf.: in Shih & al., 1971 (p.148, Rem.);
no M. pacifica : Mazzocchi Ianora, 1991 | | | | Ref.: | | | Brodsky, 1950 (1967) (p.295, figs.F,M); Tanaka, 1963 (p.21, figs.F,M, Rem.); Furuhashi, 1965 a (p.49, figs.F); Park, 1968 (p.557: Rem.); Vaupel-Klein, 1970 (p.4, 32, figs.M, Rem. | 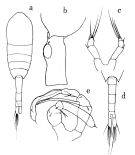 issued from : O. Tanaka in Publs Seto Mar. Biol. Lab., 1963, XI (1). [p.22, Fig.159]. Female: habitus (dorsal); b, last thoracic segment and genital somite (left lateral side); c, P5. Nota: The urosome segments and furca in the proportional lengths as 42:23:15:20. Male: d, urosome (dorsal); e, P5. Nota: The urosome segments and furca in the proportional lengths as 15:18:17:17:14:19 = 100.
|
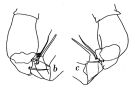 Issued from : J.C. von Vaupel-Klein in Zool. Verh. Leiden, 1970, 110. [p.30, Fig.13,b-c]. Male: b, c, part of right P5 (from two different specimens, viewed from different angles). Nota: The prehensile A1 is found on the left or the right side, without a distinct preference for either of these sides.
|
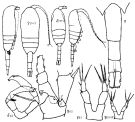 Issued from : K.A. Brodskii in Calanoida of the Far Eastern Seas and Polar Basin of the USSR. Opred. Fauna SSSR, 1950, 35 (Israel Program for Scientific Translations, Jerusalem, 1967) [p.295, Fig.201]; Female (from NW Pacific): habitus (dorsal and right lateral side); urosome; S2, P2; S1, P5 (NW Pacific); S5J, P5 (Sea of Japan). Male: habitus (dorsal and lateral right side); S5, P5.
|
 issued from : C.O. Esterly in Univ. Calif. Publs Zool., 1924, 26 (5). [p.97, Fig. H). As Metridia sp. Female (from San Francisco Bay): 2, A1; 4, habitus (lateral); 6, last thoracic segment (lateral, left side; from a second specimen); 7, habitus (dorsal); 9, basal portion of A1; 10, P2; 12, P3; 13, urosome (dorsal; from a second specimen); 14,2nd basipodal segment, 1st segment of exopod and entire endopod (the foot opposite to one shown in 10). Male: 1, A1; 3, last thoracic segment and first two segments of abdomen (lateral, right side); 5, forehead (lateral); 8, habitus (lateral); 11, P3; 15, P5 (right foot at left drawing); 16, A2.
|
 issued from : B.K. Sullivan, C.B. Miller, W.T. Peterson & A.H. Soeldner in Mar. Biol., 1975, 30. [p.180, Fig.5, A]. Metridia pacifica female (from 50°N, 145°W): A, SEM of right Md (posterior surface); B, detail of dorsal end.
|
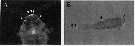 Issued from : Y. Takenaka, A. Yamaguchi, N. Tsuruoka, M. Torimura, T. Gojobori & Y. Shigeri in Mol. Biol. Evol., 2012, 29 (6). [p.1670, Fig.1]. Metridia pacifica (from off south of Hokkaido, Japan). A: Fluorescent microscopic image of bioluminescent copepoda illuminated with ultraviolet. Arrowheads indicate the position of putative luminous glands on head. B: Bright-field and fluorscent image; putative luminous glands were on the legs and abdomen adjacent to the head.
| | | | | Compl. Ref.: | | | Heinrich, 1962 (p.465, life history, production); Ahlstrom & Thrailkill, 1963 (p.57, Table 5, abundance); Brodsky, 1964 (p.105, 107); Vinogradov, 1968 (1970) (p.44, 49, 51, 54, 56, 57, 65, 109, 256, 291); Mullin C.H., 1968 (p.29, egg laying v.s. hours); Morris, 1970 (p.2301); Shih & al., 1971 (p.148, 205); Peterson & Miller, 1975 (p.642, 650, Table 3, interannual abundance); Takahasshi & Ikeda, 1975 (p.2189, excretion vs. food concentration); Ikeda, 1976 (p.51, respiration rate); Peterson & Miller, 1976 (p.14, Table 1, 2, abundance vs interannual variations); Reeve & al., 1977 (p.105, mercury & copper vs. respiration & excretion); Peterson & Miller, 1977 (p.717, Table 1, seasonal occurrence, Rem.); Star & Mullin, 1981 (p.1322, abundance); Mackas & Sefton, 1982 (p.1173, Table 1); Vidal & Whitledge, 1982 (p.77, respiration v.s. latitude); Dagg & al., 1982 (p.45, Table 3, abundance, ingestion excretion); Gordon & al., 1985 (p.89, Table 2, 3, 4, fish diet); Batchelder, 1985 (p.949, figs.1-9, Table 1-2, Rem.: life history); 1986 (p.227, fig.1, 2, genital system female); Rudyakov, 1986 (tab.1); Mackas & Anderson, 1986 (p.115, Table 2); Mackas & Burns, 1986 (p.383, feeding); Vidal & Smith, 1986 (p.523, Table 1, biomass); Smith & Vidal, 1986 (p.215, Table 4, 6, fig.4, 7, abundance): Ohman, 1988 (p.143, Table 1: lipid content); Odate & Maita, 1988 (p.228, Rem.: p.230); Hattori, 1989 (p.39); Springer & al., 1989 (p.359, Fig.5, 10, 11, Table 1); Batchelder & Miller, 1989 (p.113, population dynamic, modelling); Coyle & al., 1990 (p.763); Hirakawa & al., 1990 (tab.3); Haury & al., 1990 (p.447, Table 1 fig.9, shearing flow effects vs. vertical distribution); Shih & Marhue, 1991 (tab.2, 3); Hattori, 1991 (tab.1, Appendix); Hirakawa, 1991 (p.373); Huntley & Lopez, 1992 (p.201, Table 1, eggs, temperature-dependent production); Shih & Young, 1995 (p.70); Kotani & al., 1996 (tab.2); Suarez-Morales & Gasca, 1998 a (p.110); Ohman & al., 1998 (p.1709, organic composition, vertical distribution, ETS activity, egg production, dormancy: Table 3); Mauchline, 1998 (tab.21, 47, 58, 63); Ban & al., 1998 (p.42, abundance vs. phytoplankton bloom); Russell & al., 1999 (p.77, tab.1); Dolganova & al., 1999 (p.13, tab.1); Bragina, 1999 (p.196); Goldblatt & al., 1999 (p.2619, Rem.: p.2635); Mackas & Tsuda, 1999 (p.335, Table 1); Mackas & Yelland, 1999 (p.2941, Table 1); Gomez-Gutiérrez & Peterson, 1999 (p.637, Table II, abundance); Pinchuk & Paul, 2000 (p.4, table 1, % occurrence); Kosobokova & Hirche, 2000 (p.2029, tab.2); Haury & al., 2000 (p.69, Table 1); Mackas & al., 2001 (p.685, Tab. 6,: interannual changes in species composition); Rebstock, 2001 (tab.2, 4); 2002 (p.71, Table 3, Fig.4, climatic variability); Napp & al., 2002 (p.5991, Table 1, fig.7, interannual variation); Yamaguchi & al., 2002 (p.1007, tab.1); Mackas & Galbraith, 2002 a (p.423, Table 2); Peterson & Keister, 2003 (p.2499, interannual variability); Yamaguchi & al., 2004 (p.480, tab.2); Park, W & al., 2004 (p.464, tab.1); Kang & al., 2004 (p.1524); Mackas & al., 2004 (p.875, Table 2: form pseudopacifica); Miller C.B., 2005 (p.219, 220, Rem.); Shimode & al., 2005 (p.113 + poster); Choi & al., 2005 (p.710: Tab.III); Ashjian & al., 2005 (p.1380: tab.2); Coyle, 2005 (p.77, fig.7,9); Mackas & al., 2005 (p.1011, 1021, tab.2, 3); Liu & Hopcroft, 2006 (p.769, growth, development); Lavaniegos & Jiménez-Pérez, 2006 (tab.2, 3, Rem.); Mackas & al., 2006 (L22S07, Table 2); Ware & McQueen, 2006 (p.28, Table B1, weight ranges); Hooff & Peterson, 2006 (p.2610); Mackas & al., 2007 (p.223, climatic change index); Deibel & Daly; 2007 (p.271, Table 4, Rem.: Arctic polynyas); Ferrari & Dahms, 2007 (p. 64, Rem.); Humphrey, 2008 (p.84: Appendix A); Liu & Hopcroft, 2008 (p.930: fig.7); Takenada & al., 2008 (p.28, bioluminescence, luciferas, biol. mol.); Kobari & al., 2008 (p.1648, coppodids I-VI, depth distribution); Takahashi & al., 2008 (p.222, Table 2, grazing impact); Peterson, 2009 (p.73, Rem.: p 78); Galbraith, 2009 (pers. comm.); Hopcroft & al., 2009 (p.9, Table 3); Tsuda & al., 2009 (p.2767, figs.14, 15, vertical distribution, iron fertilization); Oba & al., 2009 (p.684, bioluminescence); Chiba & al., 2009 (p.1846, Table 1, occurrence vs temperature change); Takahashi & al., 2009 (p.1777, vertical distribution, downward transport of carbon); Kobari & al., 2010 (p.1703: feeding, fig.3); Yamaguchi & al., 2010 (p.1679, figs.4, 10, Table 1, population structure); Yamaguchi & al., 2010 (p.1691, figs.2, 4, 9, Table 2, vertical distribution); Kosobokova & Hopcroft, 2010 (p.96, Table 1); Homma & Yamaguchi, 2010 (p.965, Table 2); Hopcroft & al., 2010 (p.27, Table 1, 2, fig.9); Kosobokova & al., 2011 (p.29, Table 2, Rem.: polar Basin); Batten & Walne, 2011 (p.1643, Table I, abundance vs temperature interannual variability); Saito & al., 2011 (p.145, abundance, size vs. temperature); Yamaguchi & al., 2011 (p.13, seasonal variation, life history); Sato & al., 2011 (p.1230, vertical distribution); Homma & al., 2011 (p.29, Table 2, 3, 4, 5, abundance, feeding pattern: suspension feeders); Matsuno & al., 2011 (p.1349, Table 1, fig.5, abundance vs years); Matsuno & al., 2012 (Table 2); DiBacco & al., 2012 (p.483, Table S1, ballast water transport); Takenaka & al., 2012 (p.1669, fig.1, 2, 3, Table 1, bioluminescence); Volkov, 2012 (p.474, Table 6, 7, 8, abundance); Takahashi M. & al., 2012 (p.393, Table 2, water type index); in CalCOFI regional list (MDO, Nov. 2013; M. Ohman, comm. pers.); Kobari & al., 2013 (p.78, Table 2); Ohashi & al., 2013 (p.44, Table 1, 2, 3, 4, Rem., p.48); Questel & al., 2013 (p.23, Table 3, interannual abundance & biomass, 2008-2010); Peijnenburg & Goetze, 2013 (p.2765, genetic data); Pierson & al., 2013 (p.49, vertical migration vs seasonal environment); Barton & al., 2013 (p.522, Table 1: metabolism, diapause, population dynamic, feeding mode, biogeo); Eisner & al., 2013 (p.87, Table 3: abundance vs water structure); Bode M. & al., 2014 (p.11, Rem.); Coyle & al., 2014 (p.97, table 3, 10, 11, 12, 14, 15); Chiba S. & al., 2015 (p.968, Table 1: length vs climate); Smoot & Hopcroft, 2016 (p.1, Rem.: p.5: occurrence); Ohtsuka & Nishida, 2017 (p.565, Table 22.1); Record & al., 2018 (p.2238, Table 1: diapause); Hirai & al., 2020 (p.1, Fig. 5: cluster analysis (OTU), spatial distribution). | | | | NZ: | 6 | | |
|
Distribution map of Metridia pacifica by geographical zones
|
| | | | | |  issued from : H.P. Batchelder in Deep-Sea Res. Part. A, 1985, 32 (8). [p.956, Fig.8]. issued from : H.P. Batchelder in Deep-Sea Res. Part. A, 1985, 32 (8). [p.956, Fig.8].
A, B: Percentages of Metridia pacifica adult males and females, respectively, between given depths at Station ''P'' (50°N, 145°W).
C: Number by m2 of adult males (solid circles) and adult females (open circles) from 0 to 2000 m.
Dashed vertical lines delimit times of ghanged sex ratios. |
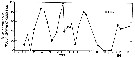 issued from : H.P. Batchelder in Deep-Sea Res. Part. A, 1985, 32 (8). [p.959, Fig.9]. issued from : H.P. Batchelder in Deep-Sea Res. Part. A, 1985, 32 (8). [p.959, Fig.9].
Percentages of reproductively mature adult females of Metridia pacifica at Station ''P''.
Nota: Abundances of the life stages were followed in the eastern subarctic Pacific from February 1980 to March 1981. All stages were present throughout most of the year, but spawning was most concentrted during late winter, summer, and autumn;
Three cohorts appeared to be completed during the study period. The three cohorts developed at the same rate, each requiring 3 to 4 months.
The recommencement of reproduction in February and March, following a hiatus in female reproduction from November to January, initiates a cohort and provides an impetus for a cycling of the population age structure. |
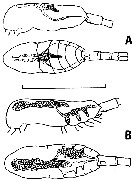 issued from : H.P. Batchelder in J. Crustacean Biol., 1986, 6 (2). [p.229, Fig.1]. issued from : H.P. Batchelder in J. Crustacean Biol., 1986, 6 (2). [p.229, Fig.1].
Metridia pacifica (from Station ''P'': 50°N, 145°W). Drawings of formolin preserved and fast green stained individuals.
A: lateral and dorsal view of genital system in ''immature'' adult females.
B: same in ''mature'' adult female. Eggs within the oviducts are 50-60 µm in diameter (eggs shown in one side only)
Scale bar = 2.0 mm. |
 issued from : H.P. Batchelder in J. Crustacean Biol., 1986, 6 (2). [p.230, Fig.2]. issued from : H.P. Batchelder in J. Crustacean Biol., 1986, 6 (2). [p.230, Fig.2].
Percentage of adult female population of Metridia pacifica (from Station ''P'') within the 0-100 m depth interval that was reproductively mature during 1980 to early 1981. |
 issued from : A. Yamaguchi, K. Ohgi, T. Kobari, G. Padmavati & T. Ikeda in Bull. Fish. Sci. Hokkaido Univ., 2011, 61 (1). [p.18, Fig.5 (part.)]. issued from : A. Yamaguchi, K. Ohgi, T. Kobari, G. Padmavati & T. Ikeda in Bull. Fish. Sci. Hokkaido Univ., 2011, 61 (1). [p.18, Fig.5 (part.)].
Schematic diagram showing life cycle timing (mating, spawning, quiescence or diapause) of Metridia pacifica, one of five dominant copepods in the Oyashio region, western subarctic Pacific (Site H: 41°30'N-42°30'N, 145°00'E-146°00'E).
Compare with Eucalanus bungii, Neocalanus cristatus, Neocalanus flemingeri and Neocalanus plumchrus. |
 Issued from : A. Yamaguchi, K. Ohgi, T. Kobari, G. Padmavati & T. Ikeda in Bull. Fish. Sci. Hokkaido Univ., 2011, 61 (1). [p.20, Fig.6 (part.)]. Issued from : A. Yamaguchi, K. Ohgi, T. Kobari, G. Padmavati & T. Ikeda in Bull. Fish. Sci. Hokkaido Univ., 2011, 61 (1). [p.20, Fig.6 (part.)].
Schematic diagram showing life cycle timing (mating, spawning, quiescence or diapause) of Metridia pacifica, one of five dominant copepods in the subarctic Pacific (except Oyashio region, western subarctic Pacific (Site H: See Fig.5).
Compare with Eucalanus bungii, Neocalanus cristatus, Neocalanus flemingeri and Neocalanus plumchrus. |
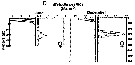 Issued from : M.D. Ohman, A.V. Drits, M.E. Clarke & S. Plourde in Deep-Sea Res. II, , 1998 , 45. [p.1719, Fig. 4 D]. Issued from : M.D. Ohman, A.V. Drits, M.E. Clarke & S. Plourde in Deep-Sea Res. II, , 1998 , 45. [p.1719, Fig. 4 D].
Vertical distribution of copepod in the San Diego Trough in June and December. Adult female of Metridia pacifica.
Dark symbols illustrate nightime distributions, open symbols illustrate daytime distributions, each plotted at the mid-point of the sampling interval. Sampling extended deeper in December than in June.
Sampling out in the San Diego Trough (near 32°50'N, 117°40'W) in 6-14 June 1992 and 15-22 December 1992.
For the authors, this species showed no evidence of dormancy in the site. The coherent diel vertical migration of all females in December as well as in June, together with their sustained grazing activity, relatively high metabolic enzyme activity, and relatively low lipid stores, are all consistent with active population growth. Perhaps this species show less evidence of dormancy in this locality than further north in their geograpphic range, like in Dabob Bay (Osgood & Frost, 1994) and still further north in the subarctic Pacific (Batchelder, 1985). |
 Issued from : M.D. Ohman, A.V. Drits, M.E. Clarke & S. Plourde in Deep-Sea Res. II, , 1998 , 45. [p.1730, Table 3]. Issued from : M.D. Ohman, A.V. Drits, M.E. Clarke & S. Plourde in Deep-Sea Res. II, , 1998 , 45. [p.1730, Table 3].
Differential dormancy of co-occuring copepods in the California Current System in the San Diego Trough.
Indices used to differentiate actively growing from dormant animals included developmental stage structure and vertical distribution; activity of aerobic metabolic enzymes (Citrate Synthase and Electron Transfer System complex); investment in depot lipids (wax esters and triacylglycerols); in situ grazing activity from gut fluorescence, and egg production rates. |
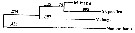 Issued from : A. Bucklin, B.W. Frost & T.D. Kocher in Mar. Biol., 1995, 121. [p.661, Fig.4]. Issued from : A. Bucklin, B.W. Frost & T.D. Kocher in Mar. Biol., 1995, 121. [p.661, Fig.4].
Phylogenetic relationships among three Metridia species based on sequence data for a 387 base pair region of the mitochondrial 16S rRNA gene and determined by neighbor joining (Saitou & Nei, 1987). Statistical methods for phylogenetic reconstruction are identical to those for calanus spp..
Numbers on horizontal branches are branch lengths; numbers at branchpoints (in iralics) are percentages of trees that show that branchpoint among 1000 bootstrapped replicates.
Nannocalanus is a outgroup by comparison.
M. pacifica from Dabob Bay, WA (Puget): 47°46'N, 122°50'W (B. Frost collector); M. lucens from Gulf of Maine (GOM): 42°30'N, 69°48'W (E. Durbin collector); M. longa from Gulf of St. Lawrence (STL): 48°40'N, 68°35'W ( J. Runge collector). |
 Issued from : E.A. Ershova, E.R. Hopcroft, K.N. Kosobokova, K. Matsuno, R.J. Nelson, A. Yamaguchi & L.B. Eisner in Oceanography, 2015, 18 (3). [p.110, Fig.7]. Issued from : E.A. Ershova, E.R. Hopcroft, K.N. Kosobokova, K. Matsuno, R.J. Nelson, A. Yamaguchi & L.B. Eisner in Oceanography, 2015, 18 (3). [p.110, Fig.7].
Abundance (ind. per m3) of Metridia pacifica (b) in the Chukchi Sea .
Color circle spot: stations where taxon was present during 1946-2012 (< 1 ind.per m3); x: stations where taxon was not found. |
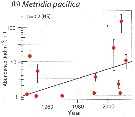 Issued from : E.A. Ershova, E.R. Hopcroft, K.N. Kosobokova, K. Matsuno, R.J. Nelson, A. Yamaguchi & L.B. Eisner in Oceanography, 2015, 18 (3). [p.112, Fig.10]. Issued from : E.A. Ershova, E.R. Hopcroft, K.N. Kosobokova, K. Matsuno, R.J. Nelson, A. Yamaguchi & L.B. Eisner in Oceanography, 2015, 18 (3). [p.112, Fig.10].
Mean abundances of Metridia pacifica (b) in the Chukchi Sea north of 70°N and east of 175°W.
Bars indicate 95% confidence interval.
Solid line indicates fitted linear trend. |
 Issued from : E.A. Ershova, E.R. Hopcroft, K.N. Kosobokova, K. Matsuno, R.J. Nelson, A. Yamaguchi & L.B. Eisner in Oceanography, 2015, 18 (3). [p.109, Fig.6]. Issued from : E.A. Ershova, E.R. Hopcroft, K.N. Kosobokova, K. Matsuno, R.J. Nelson, A. Yamaguchi & L.B. Eisner in Oceanography, 2015, 18 (3). [p.109, Fig.6].
Interannual variability of Pacific copepod Metridia pacifica (c) abundance in the Chukchi Sea during 1946-2012.
Each symbol represents one cruise; bars indicate 95% confidence interval. Dashed line shows fitted trend over averaged data.
Blue circle: June; green circle: July: yellow circle; August; purple circle: September. |
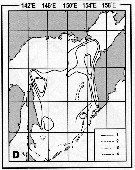 Issued from : A.I. Pinvhuk & A.J. Paul in Univ. Alaska Sea Grant College Progr., 2000. [p.30, Fig.35D]. Issued from : A.I. Pinvhuk & A.J. Paul in Univ. Alaska Sea Grant College Progr., 2000. [p.30, Fig.35D].
Distribution of Metridia pacifica biomass (mg.m3) in the 0-100 m layer in the Okhotsk Sea.
1: September-October 1652; 2: June-July 1951; 3: June-July 1650; 4: August-September 1949. (from Lubny-Gertsik, 1959). |
| | | | Loc: | | | East China Sea, E Korea, Japan (Izu, Onagawa, Ishikari Bay, Funka Bay, Toyama Bay, off Sanriku), Honshu, Kuroshio region, S. Hokkaido, Oyashio region, Station 'H' (41°30'N, 145°30'E), Japan Sea, Okhotsk Sea, S Kuril Is., Aleutian Basin, Station Knot, Pacif. (central subarctic), Bering Sea, S Aleutian Basin, S Aleutian Is., Arct. (Beaufort Sea, Makarov Basin, Chukchi Sea, off NW Alaska, Canada Basin), St. Lawrence Island, Anadyr Strait, Shpanberg Strait, Station "P", Alaska (Auke Bay, Icy Strait), British Columbia, Hecate Strait, Fjord system (Alice Arm & Hastings Arm) , Portland Inlet, Saanich Inlet, Vancouver Is., Puget Sound, off Washington coast, Oregon (Yaquina, off Newport), San Francisco Bay, off Monterey Bay, California, W BaJa California. | | | | N: | 125 | | | | Lg.: | | | (22) F: 3,1-2,6; M: 2,1-2; (26) F: 3,45-2,67; M: 2,38; (59) F: 3,2-2,4; M: 2,6-2,3; (145) F: 2,6; M: 2,1; (205) F: 3,3-2,9; M: 2,05-1,65; (208) F: 3,3-2,9; M: 2,1; {F: 2,40-3,45; M: 1,65-2,60}
The mean female size is 2.947 mm (n = 11; SD = 0.3479), and the mean male size is 2.142 mm (n = 9; SD = 0.2663). The size ratio (male : female) is 0.74 (n = 6; SD = 0.0991).
Chiba S. & al., 2015 (p.971, Table 1: Total length female (June-July) = 3.1 mm [optimal SST (°C) = 8.6]. | | | | Rem.: | epi-bathypelagic. Considered as vetical migrator.
Vaupel Klein (1970, p.32, 33) does not consider this species as a synonym of M. lucens, contrary to Fleminger (1967 a);
According to Mazzocchi & al. (1995, p.51) the systematic literature is contradictory and insufficient to clearly distinguish between M. pacifica and M. lucens. Cf. Comments in Bradford-Grieve (1999 b, p.115-116).
For Heinrich (1962, p.465) the species is a common herbivorous copepod in the western region of the Bering Sea.
Observed in ballasts of ships at San Francisco.
After Miller (2005, p.220) Metridia is a strong diel migrator, all species have a resting stage as 5th copepodite (C5); this stage loads with large amounts of nutriment stored as lipid wax, then dscends to depths below 400 m as a refuge from late summer warming and predation.
For Kosobokova & al. (2010, in press) Metridia pacifica is an expatriate species from Pacific to the Arctic Ocean Basins, because the reproduction is not assumed in polar waters.
For Kosobokova & Hopcroft (2011, Table 3) this species is an Pacific form expatriate in Arctic waters.
Batchelder (1985) stressed the variability for which 1 to 4 generations were observed in the subarctic region, depending on the geographical location, due to local temporal differences in environmental parameters.
Feeding mode after Barton & al. (2013, Table 1) : 'cruising mode'; the separation between 'feeding current' (hovering) and 'cruising' mode is gradual. A neutrally buoyant zooplankter that beats its feeding appendages will cruise through the water, while a negatively buoyant individual that beats the appendages in the same way will produce a feeding current. Most species are intermediate in their feeding mode but may be more towards one end of the continuum. Some species may switch between 'ambush feeding' and 'feeding-current' feeding or cruise feeding. | | | Last update : 12/12/2020 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed February 01, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |















