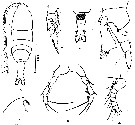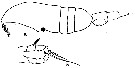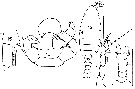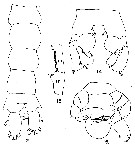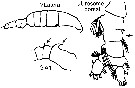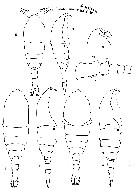|
|
 |
|
Calanoida ( Order ) |
|
|
|
Metridinidae ( Family ) |
|
|
|
Pleuromamma ( Genus ) |
|
|
| |
Pleuromamma abdominalis (Lubbock, 1856) (F,M) | |
| | | | | | | Syn.: | Diaptomus abdominalis Lubbock,1856;
Pleuromma abdominale : Claus, 1863 (p.195); Brady, 1883 (p.46, figs.F,M); Giesbrecht, 1892 (p.347, 357, 774, figs.F,M);
? Pleuromamma abdominalis var. scutullata : Harding, 1974 (p.141, tab.3, gut contents); | | | | Ref.: | | | Giesbrecht & Schmeil, 1898 (p.109, Rem. F,M); Thompson & Scott, 1903 (p.235, 249); Esterly, 1905 (p.174, figs.F, Rem.F,M); Farran, 1908 b (p.61); A. Scott, 1909 (p.122, Rem.); Lysholm & Nordgaard, 1921 (p.24); Sars, 1925 (p.201); Farran, 1908 b (p.61, Rem.); Wolfenden, 1911 (p.289); Pesta, 1920 (p.527); Farran, 1926 (p.272); Mori, 1929 (p.175, figs.F); Rose, 1929 (p.29); Farran, 1929 (p.209, 259); Sewell, 1932 (p.264); Steuer, 1932 a (p.9, figs.F,M); Wilson, 1932 a (p.125, figs.F,M); Rose, 1933 a (p.180, figs.F,M); Jespersen, 1934 (p.101); Hardy & Gunther, 1935 (p.167); Farran, 1936 a (p.110, Rem.); Mori, 1937 (1964) (p.69, figs.F,M); Jespersen, 1940 (p.47); Sewell, 1947 (p.168, fig.44); Farran, 1948 f (n°17, p.3, figs.F); Davis, 1949 (p.53, figs.F,M); Brodsky, 1950 (1967) (p.306, figs.F,M); Marques, 1953 (p.110); Chiba & al., 1957 (p.308); 1957 a (p.12); Vervoort, 1957 (p.123, Rem.); Tanaka, 1960 (p.51, form typica); Grice, 1962 (p.215, figs.F,M, Rem.); Gaudy, 1962 (p.93, 99, Rem.: p.108, Pl. IX: Juv., Tableau development7: ); Tanaka, 1963 (p.23, Rem.F,M); Paiva, 1963 (p.54); Chen & Zhang, 1965 (p.68, figs.F,M); Vervoort, 1965 (p.107, Rem.); Owre & Foyo, 1967 (p.71, figs.F,M, Rem.); Mazza, 1967 (p.186, Rem.); Vidal, 1968 (p.34, figs.F); Ramirez, 1969 (p.73, figs.F,M, Rem.); Bradford, 1970 a (p.357, Rem.); Vaupel-Klein, 1970 (p.4, 37); Corral Estrada, 1970 (p.174, Rem.); Bradford, 1972 (p.44, figs.F,M, Rem.); Razouls, 1972 (p.94, Annexe: p.70, figs.F); Kos, 1972 (Vol. I, figs. M, Fesct.); Dawson & Knatz, 1980 (p.5, figs.F,M); Björnberg & al., 1981 (p.642, figs.F,M); Gardner & Szabo, 1982 (p.334, figs.F, M); Zheng & al., 1982 (p.43, figs.F,M); Roe, 1984 (p.358); Baessa-de-Aguiar, 1989 (1992) (p.118, figs.M); J.S. Park & Mauchline, 1994 (p.108 & suiv., fig.F); J.S. Park, 1995 (p.212, figs.); Chihara & Murano, 1997 (p.838, tab.7, Pl.131: F,M); Cuoc & al., 1997 (p.656, figs.F: genital complex); Bradford-Grieve & al., 1999 (p.884, 948, figs.F,M); Bradford-Grieve,1999 b (p.119, figs.F,M, Rem., figs.178, 192); Conway & al., 2003 (p.91, figs.F,M, Rem.); G. Harding, 2004 (p.30, figs.F,M); Mulyadi, 2004 (p.61, figs.F,M, Rem.); Conway, 2006 (p.18, copepodides 1-6, Rem.); Avancini & al., 2006 (p.92, Pl. 61, figs.F,M, Rem.); Vives & Shmeleva, 2007 (p.375, figs.F,M, Rem.); Fornshell & Ferrari, 2010 (p.753, figs.1, 2, 3, variability); Hirai & al., 2015 (p.77, genetic diversity, endemism, world-wide). | 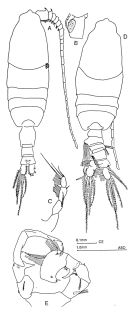 issued from : J.M. Bradford-Grieve in The Marine Fauna of New Zealand: Pelagic Calanoid Copepoda. National Institute of Water and Atmospheric Research (NIWA). NIWA Biodiversity Memoir, 111, 1999. [p.119, Fig.80]. Female (28°52'S, 178°05'W): A, habitus (dorsal); B, genital somite (right lateral side); C, P5. Male: D, habitus (dorsal); E, P5. Female characteristics: Genital swelling centrally placed on genital segment. - Pigment spot usually on left side, rarely on right side. - A1 proximal segments with several small and 2 large denticles (on segments 1 and 2), 1 is straight and the other incurved, these dentiçles often vary in size and direction. - P5 4-segmented with 3 free segments; distal segments with 3 apical setae of unequal length. male characteristics: Pigment spot, genital aperture, and denticles on the inner margin of endopod segment 1 of P2 situated on the left. - Right A1 geniculate, proximal segments denticulate. - Urosome asymmetrical, with long thick bundles of bristles. - Left P5 with a wide distal segment.
|
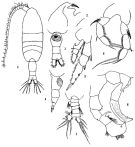 issued from: Q.-c Chen & S.-z. Zhang in Studia Marina Sinica, 1965, 7. [Pl.22, 1-8]. habitus (dorsal); 2, forehead (lateral); 3, urosome (ventral); 4, idem (lateral right side); 5, right P2 (posterior); 6, P5 (posterior). Male: 7, urosome (dorsal); 8, P5 (posterior).
|
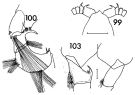 issued from : F.C. Ramirez in Contr. Inst. Biol. mar., Buenos Aires, 1969, 98. [p.70, Lam. XIII, figs.99, 100, 103, ]. Female (from off Mar del Plata): 99, forehead (dorsal). Male: 100, P5; 103, P2 (partial).; Scale bars in mm: 0.5 (99); 0.05 (100, 103)
|
 issued from : J. Corral Estrada in Tesis Doct., Univ. Madrid, A-129, Sec. Biologicas, 1970. [Lam.47, figs.5-6]. As Pleuromamma abdominalis typica. Male (from Canarias Is.): 5, P5; 6, urosome (dorsal).
|
 issued from : J. Corral Estrada in Tesis Doct., Univ. Madrid, A-129, Sec. Biologicas, 1970. [Lam.47, figs.7-8]. As Pleuromamma abdominalis edentata. Female: 7, proximal segments of A1; 8, P5.
|
 issued from : R.B.S. Sewell in The John Murray Expedition, 1933-34, Scientific Reports, VIII (1), 1947. [p.166, Fig.44, F]. Process on the posterior aspect of the 2nd basal segment of P1. Remarks: The shape of the hook-like or spine-like process shows some variation in different genera, but it is undoubtedly homologous throughout the whole series. The function of this organ is unknown.
|
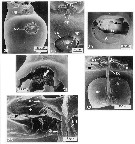 issued from : C. Cuoc, D. Defaye, M. Brunet, R. Notonier & J. Mazza in Mar. Biol., 1997, 129. [p.657, Figs.25, 26, 30, 31, 33, 35]. Scanning electron micrographs of genital area of females (from coast of Portugal). 25, lateroventral view of the genital double-somite; 26, outer genital area of inseminated females; 30, copulatory pore ( cp) and seminal receptacle ( sr) of virgin female after partial elimination of epicuticular membrane (star), note opening of seminal duct (arrow); 31, seminal contents (arrow) of inseminated female after removal of spermatophoral plug; 33, inner dorsal view of genital area; 35, inner dorsal view of parts of genital area. m: mucle; d2bd blind duct; : closed gonopores; : copulatory pore; di: seminal duct; : egg-laying duct; gp: gonoporal plategs: gonoporal slits; sp: spermatophoral plug; sr: seminal receptacle.
|
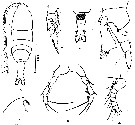 issued from : Z. Zheng, S. Li, S.J. Li & B. Chen in Marine planktonic copepods in Chinese waters. Shanghai Sc. Techn. Press, 1982 [p.44, Fig.25-1]. Female: a, habitus (dorsal); b, forehead (lateral); c, urosome (ventral); d, proximal segments of A1; e, P2; f, basipodite, exopala segment 1 and endopod. Scale bar in mm.
|
 issued from : Z. Zheng, S. Li, S.J. Li & B. Chen in Marine planktonic copepods in Chinese waters. Shanghai Sc. Techn. Press, 1982 [p.45, Fig.25-2]. Male: h, habitus (dorsal); i, forehead (lateral); j, urosome (ventral); k, grasping segments of A1; l, P2; m, P5. Scale bar in mm.
|
 issued from : T. Mori in The pelagic Copepoda from the neighbouring waters of Japan, 1937 (2nd edit., 1964). [Pl.34, Figs.6-9]. Female: 6, habitus (dorsal); 8, P5. Male: 7, habitus (dorsal); 9, P5 (posterior).
|
 issued from : T. Mori in The pelagic Copepoda from the neighbouring waters of Japan, 1937 (2nd edit., 1964). [Pl.35, Figs.6-7]. Male: 6, P1; 7, P2 (posterior).
|
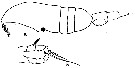 issued from : C.O. Esterly in Univ. Calif. Publs. Zool., 1905, 2 (4). [p.175, Fig.33, a-b]. Female (from San Diego Region): a, habitus (lateral); b, P5. Pigment knob on right or left side. Nota Male: Pigment knob, genital opening and hooks on inner border of 1st segment of endopod of P2, on left side
|
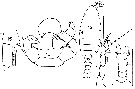 issued from : J.M. Bradford in Mem. N. Z. Oceonogr. Inst., 1972, 54. [p.43, Fig.4, (12-16)]. Female (from Kaikoura, New Zealand): 12, habitus (dorsal); 13, P5. Male: 14, urosome (dorsal); 15, right A1 (segments 17-18); 16, P6. Scale bars: 1 mm (12, 14); 0.1 mm (15, 16). Nota: All females are of the forma typica (Steuer, 1932). The males captured are forma abyssalis sub-forma thermophila (Steuer, 1932), with an almost symmetrical urosome. The presence of spines on the first two segments of A1 distinguish the females.
|
 issued from : C. Razouls in Th. Doc. Etat Fac. Sc. Paris VI, 1972, Annexe. [Fig.52]. Female (from Banyuls, G. of Lion): A, urosome (dorsal); B, genital segment and 2nd urosomal segment (lateral, right side); C, P5; D, proximal segments of A1.
|
 issued from : G.D. Grice in Fish. Bull. Fish and Wildl. Ser., 1962, 61. [p.216, Pl.21, Figs.6-8]. As Pleuromamma abdominalis f. typica. Female (from equatorial Pacific): 6, proximal part of A1. Male: 7, urosome (dorsal); 8, P5. Remarks: According to Grice, the female of forma typica and forma edentata are distinguished by the teeth on the 1st and 2nd segments of A1: in forma typica these segments each has a large tooth (fig.6) while in forma edentata these segments have only small teeth (see fig.10 in edentata).
|
 issued from : G.D. Grice in Fish. Bull. Fish and Wildl. Ser., 1962, 61. [p.216, Pl.21, Figs.9-11]. As Pleuromamma abdominalis f. edentata. Female (from equatorial Pacific): 9, habitus (lateral); 10, proximal part of A1; 11, P5.
|
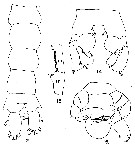 issued from : G.D. Grice in Fish. Bull. Fish and Wildl. Ser., 1962, 61. [p.216, Pl.21, Figs.12-15]. As Pleuromamma abdominalis f. abyssalis. Male (from equatorial Pacific): 12, urosome (dorsal); 13, segments 17 and 18 of right A1; 14, P2 (distal end of exopods and endopods not shown); 15, P5.
|
 issued from : J. Fornshell & F.D. Ferrari in Crustaceana, 2010, 83 (6). [p.759, Fig.1]. Attenuations on the proximal segments of left A1 (dorsal view) for six females from 1.oo)N, 150.00°W (A-F), and thee females from 7.50àS, 15000°W (G-I) Proximal is right, ventral is up. Scale bar : 0.1 mm.
|
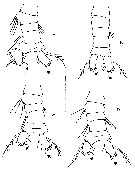 issued from : J. Fornshell & F.D. Ferrari in Crustaceana, 2010, 83 (6). [p.761, Fig.2]. Males, dorsal view of urosome. A, specimen attribuable to forma abdominalis, with strongly asymmetrical urosome and re-curved seta on left caudal ramus (from 2.62°S, 119.03°W); B, specimen attribuable to forma abyssalis with quasi-symmetrical urosome, and simple lateral seta on the left caudal ramus (from 8.88°N, 119.00°W); C, specimens intermediate between forma abdominalis and forma abyssalis with slightly asymmetrical urosome and simple seta on the left caudal ramus (from 2.62°S, 119.03°W); D, specimen intermediate between forma abdominalis and forma abyssalis with slightly asymmetrical urosome (from 7.00°N, 150.37°W). Anterior is up, apical setae of caudal ramus with elliptical cutoff; arrowhead to dorsal seta of caudal ramus; setules, denticles, and spinules much less dense than natural. Scale bar: 0.5 mm.
|
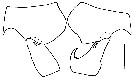 issued from : J. Fornshell & F.D. Ferrari in Crustaceana, 2010, 83 (6). [p.762, Fig.3]. Left and right basis and endopod of male P2 of forma abdominalis (specimen from 12.00°S, 150.00°W). Scale bar: 0.1 mm.
|
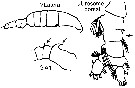 issued from : G. Harding in Key to the adullt pelagic calanoid copepods found over the continental shelf of the Canadian Atlantic coast. Bedford Inst. Oceanogr., Dartmouth, Nova Scotia, 2004. [p.30]. Female & Male. Nota: A1 with strong curved spine on segments 1 and straight spine on segment 2 (arrowed).
|
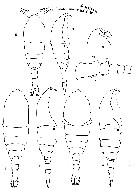 issued from : N.A. Sedova inZool. Zh., 2006, 85 (6). [Fig.4] Female (from Kamchatka Peninsula): 1-2, habitus (dorsal and lateral, respectively); 3-4, habitus (female with latero-dorsal folds). Male: 5-6, habitus (dorsal and lateral, respectively); 7, proximal segments of A1; 8, forehead (lateral).
|
 issued from : N.A. Sedova inZool. Zh., 2006, 85 (6). [Fig.5] Female: 1, P1; 2, P2; 3, P3; 4, P4; 5, P5; 6, P5 (with structure of terminal setae)
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.33, Fig.52]. As Pleuromma abdominale. Female: 52, habitus (lateral).
|
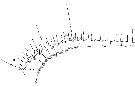 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.32, Fig.5]. As Pleuromma abdominale. Female: A1 (segments 1 to 14, ventral view).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.32, Fig.5]. As Pleuromma abdominale. Female: A1 (segments 15 to 25, ventral view).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.32, Figs.15, 16]. As Pleuromma abdominale. Female: 15, median part of A2 (posterior view); 16, Mx2 (posterior view). Re = exopod; Ri = endopod; L = lobe.
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.32, Figs.22, 23]. As Pleuromma abdominale. Female: 22, P5 (posterior view); 23, exopod of P3 (outer part; anterior view).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.33, Fig.44]. As Pleuromma abdominale. Female: 44, forehead (lateral). R = rostrum.
|
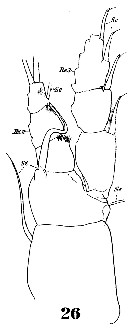 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.32, Fig.26]. As Pleuromma abdominale. Female: 26, P1 (anterior view).
|
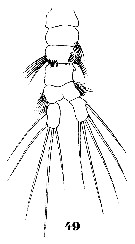
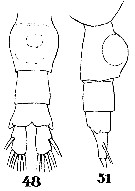 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.33, Figs.48, 51]. As Pleuromma abdominale. Female: 48, urosome (ventral); 51, same (lateral).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.32, Fig.3]. As Pleuromma abdominale. Male: 3, P5 (posterior view).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.33, Figs.32, Fig.13]. As Pleuromma abdominale. Male: 13, A1 (segments 1 to 14).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.32, Fig.13]. As Pleuromma abdominale. Male: A1 (segments 15 to 25).
|
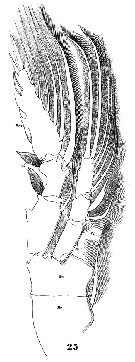 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.32, Fig.25]. As Pleuromma abdominale. Male: 25, right P2 (postrior view)
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.32, Figs.28, 29, 30]. As Pleuromma abdominale. Male: 28, P4 (anterior view); 29, exopod of left P3 (posterior view); 30, left P2 (anterior view).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.33, Figs.43, 46]. As Pleuromma abdominale. Male: 43, thoracic segmets (lateral); 46, forehead (lateral)
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.33, Fig.49]. As Pleuromma abdominale var. abyssale. Male: 49, urosome (ventral)
|
 issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1, Crustacea, 1: Copepoda. Copepods of the Florida Current. 1967. [p.73, Fig.476]. Female: 476, A1 (proximal segments).
|
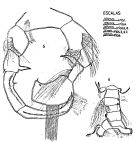
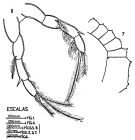
 issued from : P.E. Lapernat & C. Razouls in Vie Milieu, 2002, 52 (1). [p.21, Pl. II, Fig.1]. Masticatory edge of Md gnathobase female (from off Malta, Mediterranean Sea).
|
 issued from : Mulyadi in Published by Res. Center Biol., Indonesia Inst. Sci. Bogor, 2004. [p.62, Fig.34]. Female (from Flores Sea): a, habitus (dorsal); b, proximal segments of A1; c, forehead (lateral); d, posterior end of last thoracic segment andgenital complex (lateral right side); e, P5. Male: f, habitus (dorsal); g, forehead (lateral); h, P2; i, P5.
|
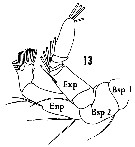 Issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1, Crustacea, 1: Copepoda. Copepods of the Florida Current, 1967. [p.12, Fig.13]. Female: 13, A2.
|
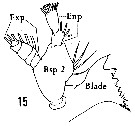 Issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1, Crustacea, 1: Copepoda. Copepods of the Florida Current, 1967. [p.12, Fig.15]. Female: 15, Md. Blade = Coxa with gnathobase bearing teeth and dorsal seta; Bsp 2 = Basis; Enp = endopod; Exp = exopod.
|
 Issued from : M.G. Mazzocchi in Guida al Riconoscimento del plancton dei Mari Italiani, Vol. II, 2006. [p.69, Tav. 61]. Redrawn after Rose, 1933; Steuer, 1933. Female: a, habitus (lateral); b-c, genital segment (ventral and lateral, respectively); d, P5. Male: e, forehead (dorsal); f, geniculate segment of right A1; g, urosome (dorsal); h, P5.
|
 Issued from : M.C. Kos in Field guide for plankton. Zool Institute USSR Acad., Vol. I, 1972. After Steuer, 1932. As Pleuromamma abdominalis f. abyssalis. Male: 1, abdomen (dorsal); 2, same (dorsal); 3, P2.
|
 Issued from : M.C. Kos in Field guide for plankton. Zool Institute USSR Acad., Vol. I, 1972. As Pleuromamma abdominalis f. typica. After Steuer, 1932. Female: 1-2, forehead (dorsal, and lateral, respectively); 3, abdomen (dorsal); 4, P5. Male: 5, forehead (lateral); 6, abdomen (dorsal); 7, P2; 8, P5.
| | | | | Compl. Ref.: | | | Cleve, 1904 a (p.195); Pearson, 1906 (p.23); Esterly, 1916 (p.171, 179, nutrition); Rose, 1924 d (p.479); 1925 (p.152); Hardy & Gunther, 1935 (1936) (p.167, Rem.); Wilson, 1942 a (p.202); Massuti Alzamora, 1942 (p.111); Lysholm & al., 1945 (p.32); Sewell, 1948 (p.329, 503, 508, 514, 516, 519, 525, 530, 532, 547, 567); Moore, 1949 (p.53); C.B. Wilson, 1950 (p.289); Kott, 1957 (p.5, 19); King & Hida, 1955 (p.11); Yamazi, 1958 (p.150, Rem.); Wickstead, 1962 (p.550, food & feeding); Fagetti, 1962 (p.27); Fish, 1962 (p.22); Ganapati & Shanthakumari, 1962 (p.8, 15); Ahlstrom & Thrailkill, 1963 (p.57, Table 5, abundance); V.N. Greze, 1963 a (tabl.2); Duran, 1963 (p.119); Grice, 1963 a (p.496); Gaudy, 1963 (p.25, Rem.); Björnberg, 1963 (p.49, Rem.); Deevey, 1964 (p.589, Table 1, lengths variation); Unterüberbacher, 1964 (p.27); De Decker, 1964 (p.16, 24, 28); De Decker & Mombeck, 1964 (p.13); Shmeleva, 1965 b (p.1350, lengths-volume-weight relation); Pavlova, 1966 (p.44); Furuhashi, 1966 a (p.295, vertical distribution in Oyashio/Kuroshio transitional area, Table 8, 9); Mullin, 1966 (p.546, Table I, III, diet); Mazza, 1966 (p.71); 1967 (p.329, 367); Ehrhardt, 1967 (p.738, 887, figs.51-53, geographic distribution, Rem.); Fleminger, 1967 a (tabl.1); Grice & Hulsemann, 1967 (p.17); Séguin, 1968 (p.488); De Decker, 1968 (p.45); Evans, 1968 (p.14); Berdugo & Kimor, 1968 (p.447); Vinogradov, 1968 (1970) (p.268); Morris, 1970 (p.2301); Dowidar & El-Maghraby, 1970 (p.269); Timonin, 1971 (p.281, trophic group); Carli, 1971 (p.372, tab.1, 2); Gueredrat, 1971 (p.300, fig.10, Table 1, 2); Deevey, 1971 (p.224); Shih & al., 1971 (p.42); Lee & al., 1971 (p.1150); Boucher & Thiriot, 1972 (p.47, Tableau 4); Bainbridge, 1972 (p.61, Appendix Table III: occurrence); Apostolopoulou, 1972 (p.328, 355); Razouls S., 1972 b (p.2, respiration); ; Nival & al., 1972 (p.63, respiration); Binet & al., 1972 (p.69); Roe, 1972 (p.277, tabl.1, tabl.2); 1972 b (p.547, Rem.); Björnberg, 1973 (p.342, 388); Guglielmo, 1973 (p.399); S. Razouls, 1974 (147, oxygen rate); de Bovée, 1974 (p.109, 124); Corral Estrada & Pereiro Muñoz, 1974 (tab.I); Harding, 1974 (p.141, Table 3, gut contents); Gaudy, 1975 (p.109, fig.1, Table I, 4, respiration); Vives & al., 1975 (p.46, tab.II, III, IV, XII); Vives, 1976 (p.104); Peterson & Miller, 1976 (p.14, Table 1, 3, abundance vs interannual variations); Timonin & Voronina, 1977 (p.291); Tranter, 1977 (p.596); Deevey & Brooks, 1977 (p.256, tab.2, Station "S"); Peterson & Miller, 1977 (p.717, Table 1, seasonal occurrence); Carter, 1977 (1978) (p.36); Frontier, 1977 a (p.15); Arashkevich, 1978 (p.118, Table: diets); Dessier, 1979 (p.89, 206); Vaissière & Séguin, 1980 (p.23, tab.2); Andronov & Maigret, 1980 (p.71); Svetlichnyi, 1980 (p.28, Table 1, passive submersion); Hayward, 1980 (p.295, Table 2, vertical distribution, feeding); Haury & Wiebe, 1982 (p.915, horizontal micro-distribution); Kovalev & Shmeleva, 1982 (p.84); Gaudy & Boucker, 1983 (p.37, Table 1, 3, 5, fig.1, 2, Rem.: metabolism); Scotto di Carlo & Ianora, 1983 (p.150); Dessier, 1983 (p.89, Tableau 1, 2, Rem., %); Guangshan & Honglin, 1984 (p.118, tab.); Tremblay & Anderson, 1984 (p.5); De Decker, 1984 (p.316, 359: chart); Binet, 1984 (tab.2, 3); Cummings, 1984 (p.163, Table 2); Sameoto, 1984 (p.767, vertical migration); Regner, 1985 (p.11, Rem.: p.35); Petipa & Borichenko, 1985 (tab.2); Longhurst, 1985 (tab.2); Brenning, 1985 a (p.24, Table 2); Sazhina, 1985 a (p.491, tab.2); Petipa & Borichenko, 1985 (tab.1,2); Wishner & Allison, 1986 (tab.2); Brenning, 1986 (p.9, spatial distribution, T-S Diagram, Rem.); Chen Y.-Q., 1986 (p.205, Table 1: abundance %, fig.11, Table 2: vertical distribution); Saraswathy, 1986 (p.189); Brinton & al., 1986 (p.228, Table 1, Rem.); Madhupratap & Haridas, 1986 (p.105, tab.1); Rudyakov, 1986 (tab.1); Ambler & Miller, 1987 (tab.2, 3, 4, 5); Jimenez-Perez & Lara-Lara, 1988; Lozano Soldevilla & al., 1988 (p.59); Dessier, 1988 (tabl.1); Hernandez-Trujillo, 1989 a (tab.1); Cervantes-Duarte & Hernandez-Trujillo, 1989 (tab.3); Madhupratap & Haridas, 1990 (p.305, fig.4: vertical distribution night/day; fig.7: cluster); Heinrich, 1990 (p.18); Timonin, 1990 (p.479); Suarez & al., 1990 (tab.2); Scotto di Carlo & al., 1991 (p.270); Arinardi, 1991 (p.295); Hattori, 1991 (tab.1, Appendix); Suarez & Gasca, 1991 (tab.2); Hernandez-Trujillo, 1991 (1993) (tab.I); Baessa De Aguiar, 1991 (1993) (p.107); Huntley & Lopez, 1992 (p.201, Table B1, growth rate, temperature-dependent production); Suarez, 1992 (App.1); Heinrich, 1992 (p.86); Jiyalal Ram & Goswami, 1993 (p.129, tab.IV); Ashjian & Wishner, 1993 (p.483, abundance, species group distributions); Seguin & al., 1993 (p.23, 26, Rem.); Hays & al., 1994 (tab.1); Kouwenberg, 1994 (tab.1); Shih & Young, 1995 (p.70); Kotani & al., 1996 (tab.2); Pakhomov & McQuaid, 1996 (p.271, abundance, distribution, seabirds); Errhif & al., 1997 (p.422); Hure & Krsinic, 1998 (p.102, 112); Barange & al., 1998 (p.1663, Table 2, 5, abundance vs STC region); Gilabert & Moreno, 1998 (tab.1, 2); Alvarez-Cadena & al., 1998 (tab.3, 4); Suarez-Morales, 1998 (p.345, Table 1); Suarez-Morales & Gasca, 1998 a (p.110); Mauchline, 1998 (tab.30, 33, 63, 64); Siokou-Frangou, 1999 (p.476); Hernandez-Trujillo, 1999 (p.284, tab.1); 1999 a (p.154); Lapernat, 2000 (tabl.3,4); Razouls & al., 2000 (p.343, Appendix); Pakhomov & al., 2000 (p.1663, Table 1, 2, transect Cape Town-SANAE antarctic base); Fernandez-Alamo & al., 2000 (p.1139, Appendix); Haury & al., 2000 (p.69, Table 1); Pakhomov & al., 2000 (p.1663, Table 2, transect Cape Town-SANAE antarctic base); Suarez-Morales & al., 2000 (p.751, tab.1); Seridji & Hafferssas, 2000 (tab.1); Lopez-Salgado & al., 2000 (tab.1); Moraitou-Apostolopoulou & al., 2000 (tab.I); Haury & al., 2000 (p.69, Fig.7); Madhupratap & al., 2001 (p. 1345, vertical distribution vs. O2, figs.4, 5: clusters, p.1353); Holmes, 2001 (p.19); Hunt & al., 2001 (p.374, tab.1, 2); Rebstock, 2002 (p.71, Table 3, 5, 6, Fig.2, climatic variability); Hernandez-Trujillo & Suarez-Morales, 2002 (p.748, tab.1); Beaugrand & al., 2002 (p.1692); Beaugrand & al., 2002 (p.179, figs.5, 6); Labat & al., 2002 (p.741, tab.3); Vukanic, 2003 (139, tab.1); Hwang & al., 2003 (p.193, tab.2); Hsiao & al., 2004 (p.326, tab.1); Hsieh & al., 2004 (p.397, tab.1); Pusch & al., 2004 (251, tab.3); Gallienne & al., 2004 (p.5, tab.3); CPR, 2004 (p.59, fig.175); Beaugrand, 2004 (p.3, fig.5); Lo & al., 2004 (p.89, tab.1); Fernandez & al., 2004 (p.501, tab.5); Shimode & al., 2005 (p.113 + poster); Berasategui & al., 2005 (p.313, fig.2); Zuo & al., 2006 (p.159, tab.1, abundance, fig.8: stations group); Hwang & al., 2006 (p.943, tabl. I) ; Dias & Araujo, 2006 (p.55, Rem., chart); Lavaniegos & Jiménez-Pérez, 2006 (p.151, tab.2, 3, Rem.); Zervoudaki & al., 2006 (p.149, Table I); Mackas & al., 2006 (L22S07, Table 2); Hooff & Peterson, 2006 (p.2610); Koppelmann & Weikert, 2007 (p.266: tab.3); Hwang & al., 2007 (p.24); Dur & al., 2007 (p.197, Table IV); Khelifi-Touhami & al., 2007 (p.327, Table 1); Cabal & al., 2008 (289, Table 1); Neumann-Leitao & al., 2008 (p.799: Tab.II, fig.6); Morales-Ramirez & Suarez-Morales, 2008 (p.520); Ayon & al., 2008 (p.238, Table 4: Peruvian samples); Fernandes, 2008 (p.465, Tabl.2); Lopez Ibarra, 2008 (p.1, Table 1, figs.11, 16: abundance, Table 3: N, C isotopes, fig.17, 18, 19, 20, 22, 24,, 25, 26, Annexe 2, Table 4: index trophic); Gaard & al., 2008 (p.59, Table 1, N Mid-Atlantic Ridge); Raybaud & al., 2008 (p.1765, Table A1); Tseng L.-C. & al., 2008 (p.46, table 2, abundance vs moonsons); Lan Y.C. & al., 2008 (p.61, Table 1, % vs stations); Tseng L.-C. & al., 2008 (p.153, Table 2, fig.5, occurrence vs geographic distribution, indicator species); C.-Y. Lee & al., 2009 (p.151, Tab.2); Galbraith, 2009 (pers. comm.); Zhang W & al., 2009 (p.263); Licandro & Icardi, 2009 (p.17, Table 4); Park & Ferrari, 2009 (p.143, Table 5, Appendix 1, biogeography); C.E. Morales & al., 2010 (p.158, Table 1); Brugnano & al., 2010 (p.312, Table 3, fig.8); Hafferssas & Seridji, 2010 (p.353, Table 3); Lidvanov & al., 2010 (p.356, Table 3); Williamson & McGowan, 2010 (p.273, Table III, Pacific central gyres: N & S); Schnack-Schiel & al., 2010 (p.2064, Table 2: E Atlantic subtropical/tropical); Hidalgo & al., 2010 (p.2089, Table 2); Dias & al., 2010 (p.230, Table 1, fig.5 b); Mazzocchi & Di Capua, 2010 (p.426); Medellin-Mora & Navas S., 2010 (p.265, Tab. 2); Nowaczyk & al;, 2011 (p.2159, Table 2); Tseng L.-C. & al., 2011 (p.47, Table 2, occurrences vs mesh sizes); Hsiao S.H. & al., 2011 (p.475, Appendix I); Hsiao & al., 2011 (p.317, Table 2, indicator of seasonal change); Andersen N.G. & al., 2011 (p.71, Fig.3: abundance); Selifonova, 2011 a (p.77, Table 1, alien species in Black Sea); Tutasi & al., 2011 (p.791, Table 2, 3, abundance distribution vs La Niña event, Rem. p.799); Pillai H.U.K. & al., 2011 (p.239, Table 3, vertical distribution); Batten & Walne, 2011 (p.1643, Table I, abundance vs temperature interannual variability); Shiganova & al., 2012 (p.61, Table 4); Uysal & Shmeleva, 2012 (p.909, Table I); Brugnano & al., 2012 (p.207, Table 2, 3); Takenaka & al., 2012 (p.1669, fig.2, 3, Table 1, bioluminescence); Mulyadi & Rumengan, 2012 (p.202, Rem.: p.204); Salah S. & al., 2012 (p.155, Tableau 1); Miloslavic & al., 2012 (p.165, Table 2, transect distribution); Hidalgo & al., 2012 (p.134, Table 2, 3, figs.6, 8, occurrence vs hydrology); Jang M.-C & al., 2012 (p.37, abundance and seasonal distribution); Takahashi M. & al., 2012 (p.393, Table 2, water type index); Minutoli & Guglielmo, 2012 (p.91, carbon requirement vs vertical distribution); Belmonte & al., 2013 (p.222, Table 2, abundance vs stations); Teuber & al., 2013 (p.28, Table 1, respiration rates); Teuber & al., 2013 (p.1, Table 1, 2, 3, abundance vs oxygen minimum zone, respiration rates, enzyme activity); Palomares-Garcia & al., 2013 (p.1009, Table I, V, fig.7, abundance vs environmental factors); in CalCOFI regional list (MDO, Nov. 2013; M. Ohman, comm. pers.); Kobari & al., 2013 (p.78, Table 2); Tseng & al., 2013 (p.507, seasonal abundance); Hwang & al., 2014 (p.43, Appendix A: seasonal abundance); Wishner & al., 2013 (p.122, diel vertical migration); Hirai & al., 2013 (p.1, Table I, molecular marker); Schukat & al., 2013 (p.1, Table 1, 2, fig.2, respiration, ingestion); Sano & al., 2013 (p.11, Table 2, fig.5, feeding habits); Mendoza Portillo, 2013 (p.37: Fig.7, seasonal dominance); Lidvanov & al., 2013 (p.290, Table 2, % composition); Bonecker & a., 2014 (p.445, Table II: frequency, horizontal & vertical distributions); Lopez-Ibarra & al., 2014 (p.453, fig.6, biogeographical affinity); Bode & al., 2015 (p.268, Table 1, 2, figs.3, 4, chemical components, trophic level, geographic zone); Dias & al., 2015 (p.483, Table 2, abundance, biomass, production); Kiko & al., 2016 (p.2241, fig.5, Table 3, respiration, ammonium excretion); Zakaria & al., 2016 (p.1, Table 1) ; Benedetti & al., 2016 (p.159, Table I, fig.1, functional characters); El Arraj & al., 2017 (p.272, table 2, spatial distribution); Benedetti & al., 2018 (p.1, Fig.2: ecological functional group); Belmonte, 2018 (p.273, Table I: Italian zones); Chaouadi & Hafferssas, 2018 (p.913, Table II, III, fig.4, 5: occurrence, abundance, seasonal variation); Dias & al., 2018 (p.1, Table 4: % vs. season); Hure M. & al., 2018 (p.1, Table 1: abundance, % composition); Bode & al., 2018 (p.840, Table 1, respiration & ingestion rates, depth); Acha & al., 2020 (p.1, Table 3: occurrence % vs ecoregions, Table 5: indicator ecoregions). | | | | NZ: | 22 | | |
|
Distribution map of Pleuromamma abdominalis by geographical zones
|
| | | | | | | | | | | | | | | | | |  issued from : M. Saraswathy in Mahasagar-Bull. Nat. Inst. Oceanogr., 1986, 19 (3). [p.188, Fig.1D]. issued from : M. Saraswathy in Mahasagar-Bull. Nat. Inst. Oceanogr., 1986, 19 (3). [p.188, Fig.1D].
Distribution of P. abdominalis in the Indian Ocean (upper water column of 200 m).
Triangle: South West Moonsoon season: April 16 to October 15 (white triangle = negative stations; black = animals collected). Circle: North East Moonsoon season: October 16 to April 15 (white circle = negative stations; black = numbers of ianimals collected).
Nota: Mainly distributed in the Equatorial region and along the southern half of the east coast of Africa, in the Agulhas current region. The presence of this form in the upper 200 m seems to be related with the SW Moonsoon season. |
 issued from : M. Saraswathy in Mahasagar-Bull. Nat. Inst. Oceanogr., 1986, 19 (3). [p.191, Fig.2C]. issued from : M. Saraswathy in Mahasagar-Bull. Nat. Inst. Oceanogr., 1986, 19 (3). [p.191, Fig.2C].
Distribution of P. abdominalis var. abyssalis in the Indian Ocean (upper water column of 200 m).
Triangle: South West Moonsoon season: April 16 to October 15 (white triangle = negative stations; black = animals collected). Circle: North East Moonsoon season: October 16 to April 15 (white circle = negative stations; black = numbers of ianimals collected).
Nota: Scattered occurrences mainly in the Equatorial belt, SE Bay of Bengal, a few from the area south of 30°S from near Australia. |
 issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1, Crustacea, 1: Copepoda. Copepods of the Florida Current. 1967. [p.72, Table 28]. issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1, Crustacea, 1: Copepoda. Copepods of the Florida Current. 1967. [p.72, Table 28].
Vertical distribution of Pleuromamma abdominalis at the ''40-Mile station'' in the Florida Current (± 25°35'N, 79°27'W).
SL 53: 18 V 1958; SL 55: 21 VII 1958. A: during midday; B;: during midnight. |
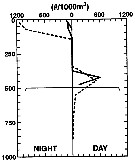 issued from : L. Haury, C. Fey, C. Newland & A. Genin in Progress in Oceanography, 2000, 45 [p.83, Fig.7]. issued from : L. Haury, C. Fey, C. Newland & A. Genin in Progress in Oceanography, 2000, 45 [p.83, Fig.7].
Vertical distribution of Pleuromamma abdominalis typica female from day and night tows taken over (solid lines) and away from (dashed lines) the seamount Fieberling Guyot (32°25'N, 127°47'W).
The horizontal line at 500 m marks the approximate depth of the summit plain. Note the reduction in abundance at night over the seamount. |
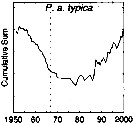 issued from : G.A. Rebstock in Global change Biology, 2002, 8. [p.77, Fig.2 i]. issued from : G.A. Rebstock in Global change Biology, 2002, 8. [p.77, Fig.2 i].
Climatic regime shifts and decadal-scale variability in calanoid copepod populations of Pleuromamma abdominalis typicaoff southern California (31°-35°N, 117°-122°W.
Cumulative sums of nonseasonal anomalies from the long-term means of copepod abundance from years 1950 to 2000.
A negative slope indicates a period of below-average anomalies; a positive slope indicates a period of above-average anomalies. Abrupt changes in slope indicate step changes.
The October 1966 cruise (prior to the increase in sampling depth), March 1976 cruise (prior to the 1976-77 climatic regime shift), and October 1988 cruise (prior to the hypothesized 1989 climatic regime shift) are marked with vertical lines. |
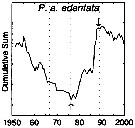 issued from : G.A. Rebstock in Global change Biology, 2002, 8. [p.77, Fig.2 k]. issued from : G.A. Rebstock in Global change Biology, 2002, 8. [p.77, Fig.2 k].
Climatic regime shifts and decadal-scale variability in calanoid copepod populations of Pleuromamma abdominalis edentataoff southern California (31°-35°N, 117°-122°W.
Cumulative sums of nonseasonal anomalies from the long-term means of copepod abundance from years 1950 to 2000.
A negative slope indicates a period of below-average anomalies; a positive slope indicates a period of above-average anomalies. Abrupt changes in slope indicate step changes. Step changes are marked with arrows (upward-pointing for increases, downward-pointing for decreases).
The October 1966 cruise (prior to the increase in sampling depth), March 1976 cruise (prior to the 1976-77 climatic regime shift), and October 1988 cruise (prior to the hypothesized 1989 climatic regime shift) are marked with vertical lines. |
 issued from : A.A. Shmeleva in Bull. Inst. Oceanogr., Monaco, 1965, 65 (n°1351). [Table 6: 26 ]. Pleuromamma abdominalis (from South Adriatic). issued from : A.A. Shmeleva in Bull. Inst. Oceanogr., Monaco, 1965, 65 (n°1351). [Table 6: 26 ]. Pleuromamma abdominalis (from South Adriatic).
Dimensions, volume and Weight wet. Means for 50-60 specimens. Volume and weight calculated by geometrical method. Assumed that the specific gravity of the Copepod body is equal to 1, then the volume will correspond to the weight. |
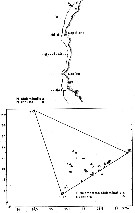 issued from : U. Brenning in Wiss. Z. Wilhelm-Pieck-Univ. Rostock - 35. Jahrgang 1986. Mat.-nat. wiss. Reihe, 5. [p.8, Figs.5, 6]. issued from : U. Brenning in Wiss. Z. Wilhelm-Pieck-Univ. Rostock - 35. Jahrgang 1986. Mat.-nat. wiss. Reihe, 5. [p.8, Figs.5, 6].
Spatial distribution and T-S diagram for Pleuromamma abdominalis and P. robusta from 8° S - 26° N; 16°- 20° W.
SO: Southern Surface Water (S °/oo: 34,50; T°C: 29,0); ND: Northern Water of the Surface Layer (S °/oo: 37,5; T°C: 21,0); SD: Southern Deep Water of the surface layer (S °/oo: 35,33; T°C: 13,4). See commentary in Temora stylifera and Brenning (1985 a, p.6). |
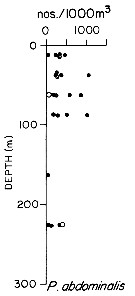 issued from : T.L. Hayward in Mar. Biol., 1980, 58. [p.299, Fig.2]. issued from : T.L. Hayward in Mar. Biol., 1980, 58. [p.299, Fig.2].
Plots of day (open circle) and night (filled circle) depth distributions of Pleuromamma abdominalis in the North Pacific central gyre (main sampling location: 25°N, 155°W with drogue), September 1968. |
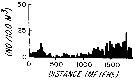 issued from : L.R. Haury & P.H. Wiebe in Deep-Sea Res., 1982, 29 (7A). [p.918, Fig.1, C]. issued from : L.R. Haury & P.H. Wiebe in Deep-Sea Res., 1982, 29 (7A). [p.918, Fig.1, C].
Abundance horizontal distribution of Pleuromamma abdominalis: non-random spatial structure.
Samples collected with a Longhurst-Hardy Plankton Recorder (LHPR) in the northern Sargasso Sea (34°10'N; 71°35'W) at three depth stata.
Nota: The pattern reveal that dominant variations occurred on scales of tens to hundreds of meters. The fluctuations in abundance are evident on different scales simultaneously.
After the authors, the abundance of species was not significantly nor consistently correlated to either temperature or alinity on the scales resolved by the tows, thus the LHPR data indicate that the physical environment or depth changes along the horizontal tows, was not, in general, a direct causative factor for the patterns observed. |
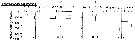 Issued from : P.-E. Lapernat in DEA Océanogr. Biol., Univ. P. & M. Curie, Paris VI. July 5, 2000. [Fig.13]. Issued from : P.-E. Lapernat in DEA Océanogr. Biol., Univ. P. & M. Curie, Paris VI. July 5, 2000. [Fig.13].
Verical distribution of Pleuromamma abdominalis at eutrophic (Mauritania coast: 20°32' N, 18°36' W) (1 from night and 2 from day) and oligotrophic sites (off NW Cape Verde Islands: 21° N, 31° W) (3 from night and 4 from day) in females (F) and males (M) (ind. per m3) in the day (white circle) and night (black circle).
Nota: Sampling in the water column 0-1000 m, one during the day and another during the night with BIONESS multiple-net: 0-75; 75-150; 150-250; 250-350; 350-450; 450-550; 550-700; 700-850; 850-965 m. In May-June 1992. |
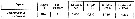 Issued from S. Razouls in XXIII rd Congress of Athens, 3-11 November 1972. [p.2]. Oxygen consumed by individual (adult) in the Banyuls Bay and equivalent carbon asked. Issued from S. Razouls in XXIII rd Congress of Athens, 3-11 November 1972. [p.2]. Oxygen consumed by individual (adult) in the Banyuls Bay and equivalent carbon asked.
(1) Hydrological season in the stability period: Eté = Summer: 18-20 °C; Hiver = Winter: 13-10°C.
Espèces = species; Saison = Season; Lg céph.= cephalothoracic length; an = individual. |
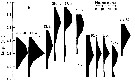 Issued from : G. Deevey in J. mar. biol. Ass. U.K., 1964, 44. [p.593, Fig. 1, B] Issued from : G. Deevey in J. mar. biol. Ass. U.K., 1964, 44. [p.593, Fig. 1, B]
Histogram showing the length distribution, plotted as numbers measured of females of Pleuromamma abdominalis from 1958-1959, at Station "S' (32°8' N, 64°42' W), 15 miles south-east of Brermuda. Samples by plankton net, oblique hauls from 500 m to the surface. |
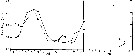 Issued from : G. Deevey in J. mar. biol. Ass. U.K., 1964, 44. [p.595, Fig. 2, A] Issued from : G. Deevey in J. mar. biol. Ass. U.K., 1964, 44. [p.595, Fig. 2, A]
The observed variations in mean cephalothorax length of Pleuromamma abdominalis females, compared with the calculated lengths basede on the phytoplankton data ( amount of Chlorophyll a values present in the upper 100 m during the month preceding each length measurement of the copepod adults.
Simple and partial correlation coefficients for the mean lengths of females and Chlorophyll a of the upper 100 m and surface temperatures averaged for the month preceding each mean lengthy measurement:
Number of pairs : 19
Simple correlations:
: XY = -0.758; XZ = 0.866; YZ = -0.927.
Partial correlations:
XY, Z constant = 0.238; XZ, Y constant = 0.667.
X: length; Y: temperature; Z: Clorophyll.
All values highly significant (P <0.01) except XY, Z constant. |
 Issued from : G. Deevey in J. mar. biol. Ass. U.K., 1964, 44. [p.592, Table 1] Issued from : G. Deevey in J. mar. biol. Ass. U.K., 1964, 44. [p.592, Table 1]
Mean cephalothorax lengths and numbers measured of females and the mean surface temperature and Chlorophyll a of the month preceding each measurement at Station 'S' (SE off Bermuda). |
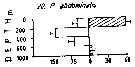 Issued from : M. Madhupratap & P. Haridas in J. Plankton Res., 12 (2). [p.311, Fig.4]. Issued from : M. Madhupratap & P. Haridas in J. Plankton Res., 12 (2). [p.311, Fig.4].
Vertical distribution of calanoid copepod (mean +1 SE), abundance No/100 m3. 20- Pleuromamma abdominalis.
Night: shaded, day: unshaded.
Samples collected from 6 stations located off Cochin (India), SE Arabian Sea, November 1983, with a Multiple Closing Plankton Net (mesh aperture 300 µm), in vertical hauls at 4 depth intervalls (0-200, 200-400, 400-600, 600-1000 m). |
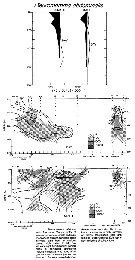 Issued from : Y.-Q. Chen in CalCOFI Rep., 1986, XXVII. [Fig.11, p.219]. Issued from : Y.-Q. Chen in CalCOFI Rep., 1986, XXVII. [Fig.11, p.219].
Vertical abundance of females, males and late copepodites.
Nota: The numbers of this species were the lowest for the genus Pleuromamma during the Krill Expedition in the Pacific east tropical (23°N to 3°S). Females were distributed at most of the stations except stations 16 and 17 (see chart, Fig.1).
Females usually occurred between 200 and 600 m, but at stations 18-20 they occurred at depths below 400 m in day samples.
The maximum concentration was 1000 individuals/1000 m3 and was found between 300 and 450 m (samples with a bongo net: mesh apertures 330 µm and 220 µm).T
The shallow depths of females and males in night samples suggest pronounced diel vertical migrations over an extensive vertical range. The ranges of vertical distribution of females and males were similar
The mean percentage combined was 1.8% (day: 0.6, night: 3.0) for all samples with the bongo net .
See in Subeucalanus subtenuis Chart figs.1, Hydrographic conditions Figs.12, 13). |
 Issued from : M. Bode, R. Koppelmann, L. Teuber, W. Hagen & H. Auel inGlobal Biogeochemical Cycles, 2018, 32. [p.844, Table 1). Issued from : M. Bode, R. Koppelmann, L. Teuber, W. Hagen & H. Auel inGlobal Biogeochemical Cycles, 2018, 32. [p.844, Table 1).
Cf. explanations of these measures in Calanoides natalis from the same authors.
Compare with Pleuromamma xiphias and P. robusta. |
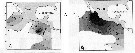 Issued from : G.A. Lopez Ibarra in Tesis, Inst. Politec. Nac., CICIMAR, 2008. [p.48, Fig. 22 and p.51, Fig.24] Issued from : G.A. Lopez Ibarra in Tesis, Inst. Politec. Nac., CICIMAR, 2008. [p.48, Fig. 22 and p.51, Fig.24]
Spatial distribution of the isotopic values from the dominant species for localities (see map for the different zones: a to f). A: 13C; B: 15N.
Nota: Sampling in Julio to December 2003, with a Bongo net (333 µm mesh aperture) from obliquely drawing from 200 m depth to surface.
Cf. Map of the 6 zones sampled, legend and remarks by the same author in Subeucalanus subcrassus. |
 Issued from : G.A. Lopez Ibarra in Tesis, Inst. Politec. Nac., CICIMAR, 2008. [p.37, Table 3]. Issued from : G.A. Lopez Ibarra in Tesis, Inst. Politec. Nac., CICIMAR, 2008. [p.37, Table 3].
Summary of abundance and steady isotopics 15N and 13C in Subeucalanus subcrassus.
C = carnivore ; T = tropical biogeographical affinity; zones (a-f) = Cf. fig.1 in the same author.
Compare with Subeucalanus subcrassus and other species (Tabla 3).
Sometimes considered as omnivore.
Compare with Pleuromamma robusta and P. gracilis. |
| | | | Loc: | | | Cosmopolite (tropical, temperate); excepted: Antarctic (sometimes sub-Antarctic: Atlant. (SW), South Georgia (rare), off W Prince Edward).
Subtropical convergence, off Hatteras Cape, Indian, Pacif. (SW, SE, equatorial), Drake Passage, sub-Arctic (only Iceland, Shetland and Faroe Is.), Canadian Atlantic coast, British Columbia, Oregon (off Newport), equatorial Indian Ocean, Batten Island, Indonesia-Malaysia, Flores Sea, China Seas (East China Sea, South China Sea), Taiwan Strait, off SW Taiwan, Taiwan, NE Taiwan, Korea Strait, Japan, Sagami Bay, S Hokkaido, Kuroshio Current, Pacific (central gyres: N & S), off Washington coast, Oregon (off Newport), California, W Baja California, Gulf of California, W Central America, Peru, Chile (S, off Santiago, Concepcion), Sargasso Sea, off Bermuda: Station ‘’ S’’ (32°10’N, 64°30’W), off E Cape Cod | | | | N: | 339 ? | | | | Lg.: | | | (22) F: 4,36-2,4; M: 4,3-2,68; (26) F: 3,39-3,18; M: 3,37-3; (34) F: 3,06-2,95; 2,76-2,52; M: 3,21-2,76; 2,68-2,4; (35) F: 4,5-2,4; (38) F: 3,36; (45) F: 3-2,4; M: 3,35-3; (46) F: 3,7-2,7; M: 3,5-2,75; (59) F: 4,3-2,6; M: 4,3-2,6; (66) F: 3,1; (73) F: 3,27-3,1; M: 3,27-3,17; (91) F: 4-2,5; M: 4,3-2,5; (116) F: 3,88-2,96; M: 3,16; (142) F: 3-2,4; M: 3,3; (199) F: 3,36-2,81; M: 3,52-2,81; (205) F: 4,1; (207) F: 3,31-2,59; M: 3,54-2,93; (237) F: 2,5-3,5; M: 3,5-4; (254) F: 3,5; M: 3,1; (290) F: 2,45-2,85; M: 1,9-2; (432) F: 3,7-3,3; (449) F: 3,7-2,7; M: 3,5-2,75; (785) F: 2,75-2,64; M: 5,36; (786) F: 3,6-2,77; M: 3,4-3,18; (806) M: 3,1-2,7; (864) F: 3,75-4,35; (866) M: 2,7-4,3; (909) F:2,6-4,1; M: 2,9-3,3; (920) F: 3,07; M: 3,12; (991) F: 2,4-4,36; M: 2,68-4,3; (1023) F: 2,84; M: 3,33; (1096) F: 2,6-3,3; M: 2,7; (1122) F: 3,3; M: 3,25; (1230) M: 2,49-3,62; {F: 2,40-4,50; M: 2,40-4,30}
The mean female size is 3.174 mm (n = 55; SD = 0.6061), and the mean male size is 3.206 mm (n = 46; SD = 0.6529). The size ratio (male : female) is 1.06 (n = 24; SD = 0.2277 . | | | | Rem.: | Oceanic. Epi-meso-bathypelagic. After Pearson (1906, p.24): vertical range down to near 4000 m. Considered as vertical migrator. Overall Depth Range in Sargasso Sea: 0-2000 m (Deevey & Brooks, 1977, Station "S"); ? Harding, 1974, off E Cape Cod (2000-1000 m). 0-457 m at Station S 2 in S Bösö (E middle Japan).
After Brinton & al., 1986 (p.246, Table 1); Wilson's (1950) Gulf of California records of P. robusta are probably based on misidentification of abdominalis.
For Fornshell & Ferrari (2010, p.763) the widespread distribution of morphs attribuable to forms typica and forma edentata, their co-occurrences, and the presence of specmens exhibiting morphologies incrementally graduated between the two forms, suggest that these two morphological forms of P. abdominalis are examples of a continuous variability within this species. Thus the authors do not believe that forma edentata should be treated as a subspecies of P. abdominalis. However it is important to continue to register the morphological variation among males of P. abdominalis. In situations where groups of females or males can be clearly categorized, they should be denoted simply as morphs.
After Lapernat & Razouls (2002, p.19) the Itoh's index value = 657.3 (number of teeth: 8) on the right Md, and 672.2 on the left Md (number of teeth: 8).
Timonin (1971, p.282) considers the trophic interrelations in the equatorial and tropical Indian Ocean, and divides the plankters into 6 trophic groups from the litterature and the results of studies of mouth-parts structure and intestine content. This species is omnivorous.
In vertical tow 4000-3000 m (Harding, 1974).
Steuer, 1931 (p.8, 9) définit 3 formes: typica , edentata , abyssalis :
Pleuromamma abdominalis abdominalis (Lubbock,1856) (F,M)
Syn.: P. abdominalis typica Steuer, 1931 (p.8, fig.F); 1932 a (p.12, figs.F,M); 1933 (p.5, figs.F,M); Dakin & Colefax, 1940 (p.85, figs.F); Vervoort, 1957 (p.124); Tanaka, 1960 (p.51); Grice, 1962 (p.215, figs.F,M); Grice & Hart, 1962 (tab.3); Grice, 1963 a (p.496); Grice & Hulsemann, 1965 (p.224); Vervoort, 1965 (p.106, Rem.); Carter, 1977 (1978) (p.36); Jimenez-Perez & Lara-Lara, 1988; Haury & al., 2000 (p.69, Table 1, Fig.7); Rebstock, 2001 (tab.2); 2002 (p.71, Table 3, Fig.2, climatic variability); Mackas & al., 2006 (L22S07, Table 2); P. abdominalis abyssalis-thermophila Dakin & Colefax, 1940 (p.85, figs.M)
Ref.: Gaudy, 1962 (p.108, 133, figs.juv.,F); Mazza, 1962 (p.336, figs.F,M); Vervoort, 1965 (p.106, Rem.); Mazza, 1967 (p.186, Rem. juv.); Grice & Hulsemann, 1968 (tab.2); Park, 1970 (p.477); Longhurst, 1985 (tab.2); Lapernat, 1999 (p.23, 55), Lapernat & Razouls, 2001 (tab.1)
Loc.: Antarct. (rare), sub-Antarct.(rare), South Africa, Atlant., Congo, Caribbean Sea, Sargasso Sea, N Madeira, W Ireland, Medititerranean Sea, off Malta, Red Sea, Indian, Indonesia, Pacif., Australia (Nouvelle-Galles du Sud), New Zealand, California, G. of California, off W Guatemala, off Juan Fernandez Is.
Lg.: (16) F: 3,28-2,6; M: 3,15-2,74; (101) F: 3,13-2,85; M: 3,13-2,79; (207) F: 3,31-2,59; M: 3,54-2,93; (340) F: 3,2; 2,9; M: 4,25; 3
Pleuromamma abdominalis abyssalis Steuer,1931 (F,M)
Ref.: Steuer, 1931 (p.9, figs.M); 1932 a (p.13, figs.F,M); 1933 (p.8, figs.M); Farran, 1936 a (p.110, Rem.); Grice, 1962 (p.215, figs.M); Vervoort, 1965 (p.107)
Ref. compl.: Saraswathy, 1986 (p.192); Morales-Ramirez & Suarez-Morales, 2008 (p.520); Wishner & al.,2013 (p.122, diel vertical migration)
Loc.: South Africa (W & S), Faroe, Indian, Pacif., W Costa Rica
Lg.: (34) F: 2,76-2,52; M: 2,68-2,4; (101) M: 3,06
Pleuromamma abdominalis edentata Steuer,1931 (F)
Ref.: Steuer, 1931 (p.8, fig.F); 1932 a (p.12, figs.F); 1933 (p.7, figs.F); Grice, 1962 (p.215, figs.F); Vervoort, 1965 (p.107); Vidal, 1968 (p.34, figs.F); Corral Estrada, 1970 (p.174, Rem.); Park, 1970 (p.477); Carter, 1977 (1978) (p.36); Dessier, 1979 (p.206); Longhurst, 1985 (tab.2); Jimenez-Perez & Lara-Lara, 1988; Haury & al., 2000 (p.69, Table 1); Rebstock, 2001 (tab.2); Rebstock, 2002 (p.71, Table 3, 5, 6, Fig.2, climatic variability); Mackas & al., 2006 (L22S07, Table 2); Morales-Ramirez & Suarez-Morales, 2008 (p.520)
Loc.: South Africa (W & S, SE), G. of Guinea, Caribbean Sea, Indian, Pacif. (equatorial), California, G. of California, off Guatemala W, W Costa Rica
Lg.: (101) F: 3,06-2,75; (180) F: 3,31-3,29; M: 3,4; 3,33; (187) F: 3,35
After Ehrhardt (1967, p.888, 894) the species is found in the southwest Tyrrhenian Sea in water salinity between 37.21-38.51 p.1000 (mainly between 37.22- 37.79) and for the Oxygen between 4.61-6.40 cm3/l (mainly between 5.18-5.42).
After Benedetti & al. (2018, p.1, Fig.2) this species belonging to the functional group 3 corresponding to large filter feeding herbivorous.
See in DVP Conway & al., 2003 (version 1)
: R. Stephen, 2007 : Data sheets of NIO, Kochi, India (on line).
After Sano & al (2013, p.14, Table 2) atomic C:N ratio (mean ±SD) = 4.7 ±0.2, n = 9, in Sagami Bay (21-27 April 2009). | | | Last update : 28/10/2022 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed February 12, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |