|
|
 |
| | | Calanoida Sars, 1902 ( Neocopepoda, Gymnoplea ) ( Classification et Phylogénie  ) ) | | Syn.: | Gymnoplea Giesbrecht, 1892 (p.41); Giesbrecht & Schmeil, 1898 (p.6, 7); Sars, 1901 a (1903) (p.2); Esterly, 1905 (p.113); van Breemen, 1908 a (p.5); Rose, 1933 a (p.17); Mori, 1937 (1964) (p.8); Kabata, 1979 (p.47, 49); Dudley, 1986 (p.10); Staborogatov, 1986 (p.1778); Huys & Boxshall, 1991 (p.316, 403, 419) | | Ref.: | Sars, 1902 (p.4); Gurney, 1931 a (p.82); Wilson, 1932 a (p.18, 540, clé G.); Rose, 1933 a (p.17); Brodsky, 1950 (1967) (p.79); Andronov, 1974 a (p.1002 & suiv.); Kabata, 1979 (p.48, 49); Bowman & Abele, 1982 (p.9); Marcotte, 1982 (p.188); 1986 (p.187); Brodsky & al., 1983 (p.85,139); Dudley, 1986 (p.7); Park,1986 (p.191); Boxshall, 1986 (p.204); Björnberg, 1986 (p.53, 232, Rem.N); Mauchline, 1988 (p.698); J.-S. Ho, 1990 (p.528); Huys & Boxshall, 1991 (p.48, 403, 406, 418, 419, Rem.); Mauchline, 1998 (p.51); Bradford-Grieve & al., 1999 (p.891, 901, Families Key); Boxshall & Halsey, 2004 (p.3; 37; 49; 12: families Key; p.38: Super-families Key); Vives & Shmeleva, 2007 (p.138, Families key.); Vives & Shmeleva, 2010 (p.27, 138 : Rem., families key); Bradford-Grieve & al., 2010 (p.291, phylogeny, families cladistic analysis)
For Bradford-Grieve & al., 2014 ( p.507, p.527: fig.12 phylogram of families); Renz & al., 2018 (p.24, Fig.15: Phylogeny); Laakmann & al., 2019 (p.330, figs. 2, 3, phylogenetic relationships); Hirai & al., 2020 (p.1, Fig.4: metabarcoding, Fig.8: OTUs distribution patterns) | | Rem.: | Andronov (1974 a) établit 9 Super-familles incluant les Platycopioidea. Park (1986) estime que les Spinocalanidae et les Epacteriscidae doivent être élevés au rang de superfamille. Les Platycopiidae étant isolés dans un Ordre différent, on recense 9 superfamilles dont certaines ont changées de dénomination en raison de la règle de priorité (Andronov, 1991, p.133): Arietelloidea [= Augaptiloidea (part.)], Bathypontioidea , Calanoidea [= Megacalanoidea], Clausocalanoidea [= Pseudocalanoidea (part.)], Diaptomoidea [= Centropagoidea], Eucalanoidea , Pseudocyclopoidea (Epacteriscoidea inclus par Bradford-Grieve & al., 2014), Ryocalanoidea , Spinocalanoidea.
Fosshagen & Iliffe, 2004 a (p.353) et Boxshall & Hasley (2004) ne reconnaissent pas la superfamille des Fosshagenioidea. Ces superfamilles sont définies in Boxshall & Hasley (2004, p.38).
Ferrari & Ueda (2005, p.333) suivant le Code international de nomenclature zoologique (ICZN, 1985) rétablissent l'antériorité des Centropagoidea , de même que (p.351) la structure de l'urosome du copépodite 5 chez la femelle de Fosshagenia ferrarii exclut cette forme des Centropagoidea et devrait rétablir la superfamille des Fosshagenoidea .
43 familles pélagiques ou saumâtre (les Calocalanidae et les Mecynoceridae sont intrégrés aux Paracalanidae) sont définies par Boxshall & Hasley (2004), soit par ordre alphabétique:
1 - Acartiidae, 2 - Aetideidae, 3 - Arctokonstantinidae, 4 - Arietellidae, 5 - Augaptilidae, 6 - Bathypontiidae, 7 - Boholinidae, 8 - Calanidae, 9 - Candaciidae, 10 - Centropagidae, 11 - Clausocalanidae, 12 - Diaixidae, 13 - Discoidae, 14 - Epacteriscidae, 15 - Eucalanidae, 16 - Euchaetidae, 17 - Fosshageniidae, 18 - Heterorhabdidae, 19 - Hyperbionychidae, 20 - Kyphocalanidae, 21 - Lucicutiidae, 22 - Megacalanidae, 23 - Mesaiokeratidae, 24 - Metridinidae, 25 - Nullosetigeridae, 26 - Paracalanidae, 27 - Parapontellidae, 28 - Phaennidae, 29 - Pontellidae, 30 - Pseudocyclopidae, 31 - Pseudocyclopiidae, 32 – Peudodiaptomidae, 33 – Rhincalanidae, 34 - Ridgewayiidae, 35 - Rostrocalanidae, 36 - Ryocalanidae, 37 - Scolecitrichidae, 38 - Spinocalanidae, 39 - Stephidae, 40 - Sulcanidae, 41 - Temoridae, 42 - Tharybidae, 43 - Tortanidae.
Les Diaptomidae dont les genres sont dulçaquicoles ne sont pas pris en compte ci-dessus.
Les Pseudodiaptomidae essentiellement dulçaquicoles et saumâtres, parfois côtiers, ne sont pas comptabilisés d'une manière exhaustive et indiqués dans la matrice de la répartition géographique.
Les Calanoida incertae sedis sont réunis dans une "groupe" arbitraire (inc. sed-calanoida).
Boxshall & Hasley (2004, p.188) considèrent la famille des Parkiidae comme synonyme des Scolecitrichidae faute d'une véritable analyse phylogénétique. Vyshkvartzeva (2004, p.176 & suiv.) conteste la position des auteurs précédents concernant le genre Parkius et rétablit le statut de la famille des Parkiidae.
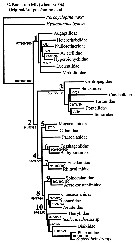
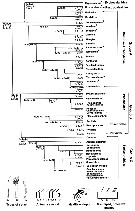
Ohtsuka & Huys (2001, p.461) dressent l'arbre phylogénétique des Superfamilles. | |
 issued from : S. Ohtsuka & R. Huys in Hydrobiologia, 2001, 453/454. [Fig. 15, p.461] Arbre phylogenetique des superfamilles des Calanoida, d'après Andronov (1974) et Park (1986). Chaque superfamille est définie par les caractères sexuels (apomorphies). Les flèches indiquent les synapomorphies |
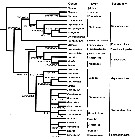
 issued from : L. Blanco-Bercial, J. Bradford-Grieve & A. Bucklin in Molecular Phylogenetics and Evolution, 2011. [p.103, Fig.2]. Simplified. Phylogram of families of the copepod Order Calanoida and outgroups. RAxML Maximum Likelihood tree, with nodes indicating percentage bootstrap recovery under three analyses: exclusion of all third codon positions in the protein-codifying genes (3rd pos.); and translation of protein-codifying genes to amino acid sequences (amono acid). The tree topology and the branch lengths shown correspond to the original sequence analysis.
See full text and fig.2: doi:10.1016/j.ympev.2011.01.008 The phylogeny agrees with previously-published morphological phylogenies (Andronov, 1974; Park, 1986), including e.g., enlargement of the Bathypontioidea to include the Fosshageniidae. |
 issued from : D.F. Figueroa in J. Crustacean Biol., 2011, 31 (1). [p.161, Fig.6]. Calanoida phylogenetic tree based on the 18S ribosomal RNA gene. Branch values correspond to bootstrap support for maximum parsimony, maximum likehood, neighbor joining, and Bayesian posterior probabilities ( -, indicates taht the branch failed the 50% bootstrap support. Nota: After the author, this particular genetic analysis suggests: 1- Centropagoidea is the sister branch to all other Calanoida; 2- Ridgewayiidae and Pseudocyclopidae likely share a common ancestor with Augaptiloidea; 3- Ridgewaiidae and Pseudocyclopidae should be included in the same superfamily, Pseudocyclopoidea; 4- Spinocalanoidea likely needs to be included in Clausocalanoidea to recover the monophyly of the latter superfamily. |
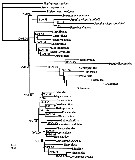 Issued from : J.M. Bradford-Grieve, G.A. Boxshall & L. Blanco-Bercial in Zool. J. Linnean Soc., 2014, 171. [p.527, Fig.12]. Phylogram of taxa of the copepod order Calanoida and out-groups. The topology and branch length correspond with the RAxML maximum-likelihood tree. Numbers at nodes indicate percentage of bootstrap recovery (when > 50%)/Bayesian posterior probability (when > 0.95, except for the Augaptiloidea/Centropagoidea node). For the super-family groupings, see Appendix S1 on line at the publisher's web-site. |
 Issued from : J.M. Bradford-Grieve, G.A. Boxshall, S.T. Ahyong & S. Ohtsuka in Invert. Syst., 2010, 24. [p.310, Fig.14]. Strict consensus of 66 trees, length 428, consistency index (CI) = 0.28, retention index (RI) = 0.72. The outgroup is a hypothetical calanoid ancestor. Calanoid copepod taxa used as exemplars in the cladistic analysis of calanoid families indicated in Table 1 (p.294). |
 Issued from : J.M. Bradford-Grieve, G.A. Boxshall, S.T. Ahyong & S. Ohtsuka in Invert. Syst., 2010, 24. [p.311, Fig.15]. Strict consensus of three trees after one round of successive weighting. Clade numbers above the line, jackknife supprt below. Outgroup is a hypothetical calanoid ancestor. Calanoid copepod taxa used as exemplars in the cladistic analysis of calanoid families indicated in Table 1 (p.294). |
 Issued from : J.M. Bradford-Grieve, G.A. Boxshall, S.T. Ahyong & S. Ohtsuka in Invert. Syst., 2010, 24. [p.294, Table 1]. Calanoid copepod taxa used as exemplars in the cladistic analysis of calanoid families. |
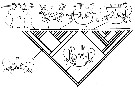 Issued from : J.M. Bradford-Grieve, G.A. Boxshall, S.T. Ahyong & S. Ohtsuka in Invert. Syst., 2010, 24. [p.316, Fig.17]. Diagramatic representation of new hypothesis of evolutionary trends in female genitalia (oviducts and shell ducts omitted). (A) hypothetical ancestor, Ridgewayiidae ( Brattstromia) and Epacteriscidae; (B) Pseudocyclopidae and Boholinidae; (C) Ridgewayiidae, Centropagoidea (except Acartia), Augaptilidae, Lucicutiidae, Heterorhabdidae,and Bathypontioidea?; (D) Augaptiloidea (Arietellidae, Hyperbionychidae, Nullosetigeridae, Metridinidae; (E) Acartiidae ( Acartia) - not in analysis- no operculum, only integumental fold with genital slit opening to outside: (F) Megacalanidae, Calanidae, Paracalanidae, Eucalanidae, Clausocalanoidea, (not Euchaetidae?). Large arrows indicate directions of transformations, small arrows indicate postulated places on tree (Fig. 16) where each type of configuration probably occurred (information from Cioc & al., 1997; Barthélémy & al., 1998; Barthélémy, 1999a, 1999b, and original data). cp = copiulatory pore; ga = genital atrium; go = genital operculum; gp = gonopore; gs = genital slit; ml = opercular muscles; m2 = gonoporal plate muscles; m3 = genital atrial muscles; sp = seminal pore; sr = seminal receptacle. |
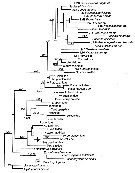 Issued from : S. Laakmann,, E.L. Markhaseva & J. Renz in Mol. Phylog. Evol., 2019, 130. [p.335, Fig. 2]. Phylogenetic relationships of all calanoid superfamilies and ''Bradfordian'' specimens with one individual per genus, respectively. Analyses are based on concatenated 18S (930 bp) - 28S (719 bp) - COI (547 bp) - cyt b (327) gene fragment (total length 2526 bp) and illustrated from maximum Likelihood Analysis Numbers on branches represent bootstrap values > 75% (first number) from Maximum Likelihood and posterior probabilities > 0.95 (second number) from Bayesian Inference Analysis. Grey names are sequences from Bradford-Grieve & al. (2014), black names are sequences for this study. Detailed settings for phylogenetic analyses are given in Appendix Table B (p.339-343) |
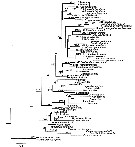 Issued from : S. Laakmann,, E.L. Markhaseva & J. Renz in Mol. Phylog. Evol., 2019, 130. [p.336, Fig. 3]. Phylogenetic relationships of representatives of all calanoid superfamilies and ''Bradfordian'' specimens from this study. Analyses are based on concateneded 18S (931 bp) - 28S (731 bp) - COI (547 bp) - cyt b (327 bp) gene fragment (total length 2536 bp) and illustrated from Maximum Likelihood Analysis. Numbers on branches represent bootstrap values > 75% (first number) from Maximum Likelihood and posterior probabilities > 0.95 (second number from Bayesian Inference Analysis. Grey names are sequences from Bradfoe-Grieve & al. (2014), black names are sequences from this syudy. Detailed settings for phylogenetic analyses are given in Appendix Table B (p.339-343 |
 Issued from : T.S. Jørgensen, B. Petersen, H.C.B. Petersen, P.D. Browne & al. in Genome Biol. Evol., 2019, 11 (5). [p.1442, Fig.1, D]. Within Copepoda, a 100-fold difference in genome size from the smallest Cyclopoid to the largest Calanoid can be seen. Within the order Calanoida, the genome sizes vary > 10-fold between the smallest Diaptomidae and the largest Calanidae. For the authors, DNA sequencing-based analyses suggest is a 14-fold difference in genome size between the six members of copepoda with available genomic information. This finding complements nucleus staining genome size estimations, where 100-fold difference has been reported within 70 species. The information confirms the evolution of genome size in Copepoda and expands the scope for evolutionary inferences in Copepoda by providing several levels of genetic information from a key planktonic crustacean species. See genome study for Acartia tonsa species (Calanoida: Diaptomoidea: Acartiidae) | | | | | Arietelloidea | | Syn.: | Augaptiloidea Blanco-Bercial & al., 2011 (p.1, 5, Table 1, Fig.2, Biol. mol, phylogeny) | | Ref.: | Vives & Shmeleva, 2010 (p.138, Rem.); Ohtsuka & Nishida, 2017 (p.583, Rem. bioluminescence) | | Rem.: | 5 familles : Arietellidae, Augaptilidae, Discoidae, Heterorhabdidae, Nullosetigeridae.
Selon Blanco-Bercial & al. (2011, Table 1): 8 families: Arietellidae, Augaptilidae, Discoidae, Heterorhabdidae, Hyperbionychidae, Lucicutiidae, Metridinidae, Nullosetigeridae. | | | | Bathypontioidea | | Ref.: | Vives & Shmeleva, 2010 (p.138, Rem.); Blanco-Bercial & al., 2011 (p.1, 5, Table 1, Fig.2, Biol. mol, phylogeny) | | Rem.: | 1 famille : Bathypontiidae. | | | | Calanoidea | | Syn.: | Megacalanoidea Blanco-Bercial & al., 2011 (p.1, 5, Table 1, Fig.2, Biol. mol, phylogeny) | | Ref.: | Vives & Shmeleva, 2010 (p.139, Rem.) | | Rem.: | 4 familles : Calanidae, Mecynoceridae, Megacalanidae, Paracalanidae. | | | | Clausocalanoidea | | Ref.: | Vives & Shmeleva, 2010 (p.139, Rem.); Blanco-Bercial & al., 2011 (p.1, 5, Table 1, Fig.2, Biol. mol, phylogeny); Laakman & al., 2019 (p.330: Rem concerning the ''Bradfordian'' families. | | Rem.: | 13 familles : Aetideidae, Clausocalanidae, Diaixidae, Euchaetidae, Kyphocalanidae, Mesaiokeratidae, Parkiidae, Phaennidae, Pseudocyclopiidae, Rostrocalanidae, Scolecitrichidae, Stephidae, Tharybidae.
|  Issued from : S. Laakmann,, E.L. Markhaseva & J. Renz in Mol. Phylog. Evol., 2019, 130. [p.331, Table 1]. Compilation of information on relationships among ''Bradfordian'' genera from Markhaseva & Ferrari (2005) and Markhaseva & al. (2014). Abbreviations: A2, antenna; Md, mandible; Mx1, maxillule; Mx2, maxilla; Mxp, maxilliped; P5, leg 5; w, worm-like sensory seta; b, brush-like sensory seta; s, sclerotized seta. |
 Issued from : S. Laakmann,, E.L. Markhaseva & J. Renz in Mol. Phylog. Evol., 2019, 130. [p.333, Fig. 1]. Relationships among ''Bradfordian'' genera. Nota: Relationships are based on number and type of setae on praecoxal endites of the Mxp; setation of five proximal segments of the exopod of A2; number and types of setae of the Mx2 zndopod. For Mxp, praecoxal endites sequence of 3 numbers separated by commas are number of setae on the proximal, middle and distal praecoxal endites (s: sclerotized seta; w: worm-like seta; b: brush-like seta. For A2 exopod: sequence of the 5 numbers separated by commas (artrodial membrane present) and dashes (arthrodial membrane absent) are segment/seta on the proximal five segments. For Mx2 endopod sequence of 3 numbers separated by commas are the number of sclerotized, worl-like, brush)like setae (compiled from Markhaseva & Ferrari, 2005; Markhaseva & Schulz, 2009; Markhaseva & al., 2008, 2014) Asterisk marks genera described since 2005. For the genera not yet completely or poorly described and not included in the morphological analysis see Table 1. The proposed evolutionary trends in the ''Bradfordian'' relationships consider: 1 - sensory setae arose asz transformation from sclerotized setae and are considered an apomorphic modification (derived from and differing from an ancestral condition); 2 - the loss of setae is a derived state and the transformation of setae from sclerotized into worm-like setae is a derived state, further, a brush-like seta is assumed to be derived from a worm-like seta (Ohtsuka & al., 2003), therefore a brush-like seta represent a more derived state; 3 - the plesiomorphic state (used of ancestral or primitive character) for the Mxp praecoxal armament in ''Bradfordians'' is assured to be 1, 2 and 3 sclerotized setae on the proximal, middle and distal praecoxal endites, respectively. The first transformation of the distal praecoxal endite is assumed to be loss of 1 sclerotized seta (1, 2, 2), the second transformation is assumed to be the loss of a second sclerotized seta (1, 2, 1). In all corresponding groups - group A, B and C genera without transformed setae on the praecoxal endites of Mxp were observed and within each of these groups further transformation from a sclerotized into a worm-like and brush-like seta is assumed to be the derived state. 4 - the exopod of A2 of the primitive ''Bradfordian'' calanoid is assumed to have 9 medial and 3 terminal setae (the 2nd segment is apparently a long complex of 3 fused segments, each represented by a medial seta). A proximal-to-distal loss of setae on the segments of A2 exopod, followed by loss of the arthrodial membrane between the long segment (2nd ptoximal) and the proximal of the 4 small segments is assumed to be a derived setae. 5 - Mx2 endite lobe and endopod of the primitive ''Bradfordian'' calanoid is assumed to have 9 setae because no more than 9 sclerotized setae are present on any species inn the superfamily Clausocalanoidea (Vaupel Klein & Rijerkerk, 1997). The loss of either a sclerotized seta or worm-like seta, resulting in 8 setae, is assumed to have occurred early in the evolution of the group (Ferrari & Markhaseva, 1996, and other authors). 6 - transformations of sclerotized setae on the praecoxal endites of Mxp apparently determine an early branching and are assumed to precede changes in the exopod of A2 in all cases. Changes in Mx2 endite lobe and endopod are assumed to be the last to occur during the evolutionary history of ''Bradfordian'' species. 7 - the most plesiomorphic character state is considerd for each of the genus included into the figure, described since that time from the deep near-bottom were included into this preliminary phylogenetic analysis, based on the compilation of the taxonomy of ''Bradfordian'' in Markhaseva & al. (2014). |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.70, Fig.5]. Schematic view of right A1 in males of ''Bradfordian'' families. Roman numerals: ancestral segments; black arrows: fused ancestral segments; black dotted arrows: position of geniculation or morphologically transformed segments; dotted lines:between A1 segments: incomplete fusion. A1 of Sensiava and Procenognatha schematized after Markhaseva & Schulz (2006, 2010), remaining genera after Markhaseva (pers. obs.). |
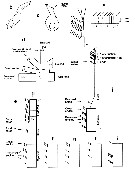 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.71, Fig.6]. Schematic oral limb stucture. a, A2 exopod, schematized, ancestral condition of setation in ''Bradfordians''; b, Phaenna spinifera Claus, 1863 (Phaennidae), Md gnathovase (lateral view); c, Sxolecithrix bradyi Giesbrecht, 1888 (Scolecitrichidae), Md gnathobase (lateral view); d, schematic view of Mx1, arrow shows setation of endites in sequence as given in setal formula for Scolecitrichidae; e, ''Bradfordian'' Mxp, schematized, ancestral condition of setation in Diaixidae and Tharybidae; f, Mxp, schematized, ancestral condition of setation in Phaennidae; g, Mxp, schematized, ancestral condition of setation in Scolecitrichidae; h, Mxp, schematized, setation in Rostrocalanidae; i, Mxp, schematized, setation in Kyphocalanidae; j, Mxp, schematized, setation in Parkiidae e-j, simple line represents; stippled enclosure a worm-like seta; dark enclosure a brush-like seta. a sclerotized seta |
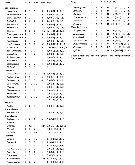 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.73, Table 1]. A2 armament (number of seta) in different ''Bradfoprdian'' genera (females). |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.74, Table 2 (part)]. Md armament (number of seta) in different ''Bradfoedian'' genera (females) See following table. b basis; End1 endopodal segment 1; End 2 endopodal segment 2; Exp exopod; gn gnathobase. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.75, Table 2 continued]. Md armament (number of seta) in different ''Bradfoedian'' genera (females) |
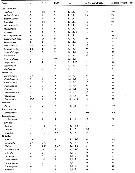
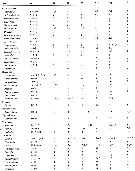 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.76, Table 3 part.]. Mx1 armament (number of seta) in different ''Bradfoedian'' genera (females). pa: praecoxal arthrite (setal formula of praecoxal arthrite: terminal+posterior+anterior setae); ce: coxal endite; bp: proximal basal endite; bd: distal basal endite; End: endopod; Exp: exopod; Epi: epipodite. See table following. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.77, Table 3 continued]. Mdx1 armament (number of seta) in different ''Bradfoedian'' genera (females) |
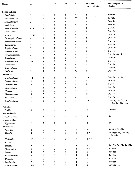 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.78, Table 4 part.]. Mx2 armament (number of seta) in different ''Bradfoedian'' genera (females) at: attenuation; pe: praecoxal endite; ce: coxal endite; bp: proximal basal endite; bd: distal basal endite; el: enditic-like lobe of proximal endopodal segment; End: endopod; w: worm-like seta; br: brush-like seta; se: sclerotized seta |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.79, Table 4 continued]. Mx2 armament (number of seta) in different ''Bradfoedian'' genera (females). |
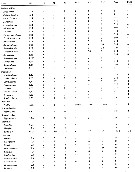 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.80, Table 5 part.]. Mxp armament (number of seta) in different ''Bradfoedian'' genera (females). at: attenuation; pes: praecoxal endites of syncoxa (from proximal to distal; ces: coxal endite of syncoxa; bp: basis proximal; bd: basis distal; End: endopod. For more detailed morphology of the setae on the praecoxal endites of the Mxp syncoxa see Markhaseva & Ferrari (2005) and Markhaseva & al. (2008). |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.81, Table 5 continued]. Mxp armament (number of seta) in different ''Bradfoedian'' genera (females). |
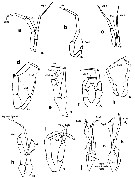 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.82, Fig. 8]. Diaixidae P5 males: (changed and schematised after the authors) a, Xancithrix ohmaniMarkhaseva, 2012. b, Neoscolecithrix caetanovi Alvarez, 1985. c, Cenognatha farrani (Smirnov, 1935). d, ¨rocenognatha semisensata Markhaseva & Schulz, 2010. e, Sznsiava longiseta Markhaseva & Schulz, 2006. f, Xantharus formosus Andronov, 1981. g, Xantharus renatehaassae Schulz, 1998. h, Paraxantharus brittae Schulz, 2006. i, Byrathis arnei Schulz, 2006. j, Diaixis trunovi Andronov, 1981. k, Diaixis helenae Andronov, 1981. Exp: exopod; End: endopod; (R): right leg. |
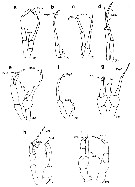 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.72, Fig. 7]. Phaennidae, Parkiidae, Scolecitrichidae, Tharybidae, Kyphocalanidae, P5 males: (changed and schematised after the authors). a, Xanthocalanus borealis Sars, 1900, Phaennidae. b, Xanthocalanus propinquus Sars, 1902, Phaennidae. c, Xanthocalanus kurilensisBrodsky, 1950, Phaennidae. d, Xanthocalanus polarsternae Markhaseva, 1998, Phaennidae. e, Pseudoamallothrix longispina Schulz, 1991, Scolecitrichidae. f, Scolecitrichopsis elenae Schulz, 2005, Scolecitrichidae. g, Parkius sp., Parkiidae. (changed and schematized after Grice & Hulsemann, 1967). h, Tharybis cf. macrophthalma , Tharybidae (Markhaseva (pers. obs.). i, Kyphocalanus sp., Kyphocalanidae, Markhaseva (pers. obs.). Exp: exopod; End: endopod; (R): right leg. | | | | | Diaptomoidea | | Syn.: | Centropagoidea : Blanco-Bercial & al., 2011 (p.1, 3, Fig.2, Table 1, Biol. mol, phylogeny, Rem.: p.7) | | Ref.: | Vives & Shmeleva, 2010 (p.138, Rem.) | | Rem.: | 10 familles marines : Acartiidae, Candaciidae, Centropagidae, Fosshageniidae, Parapontellidae, Pontellidae, Pseudodiaptomidae, Sulcanidae, Temoridae, Tortanidae. | | | | Epacteriscoidea | | Syn.: | Ridgewayioidea : Vives & Shmeleva, 2010 (p.139, Rem.) | | Ref.: | Bradford-Grieve & al., 2014 (p.507, 526, Rem: p.529) | | Rem.: | 2 familles : Epacteriscidae, Ridgewayiidae. | | | | Eucalanoidea | | Ref.: | Vives & Shmeleva, 2010 (p.139, Rem.); Blanco-Bercial & al., 2011 (p.1, 5, Table 1, Fig.2, Biol. mol, phylogeny) | | Rem.: | 2 familles : Eucalanidae, Rhincalanidae.
| | | | Pseudocyclopoidea | | Syn.: | Epacteriscoidea Fosshagen, 1973. | | Ref.: | Vives & Shmeleva, 2010 (p.139, Rem.); Bradford-Grieve & al., 2014 (p.507, 526, Rem: p.529, phylogeny) | | Rem.: | 1 famille : Pseudocyclopidae, [Syn.: Boholinidae et Ridgewayidae]. | | | | Ryocalanoidea | | Ref.: | Vives & Shmeleva, 2010 (p.139, Rem.); Renz & al., 2018 (p.1, 24, Figs. 15 & 16, p.25, 25, Rem.: phylogeny) | | Rem.: | 1 famille : Ryocalanidae. |  Issued from : J. Renz, E.L. Markhaseva & S. Laakmann in Zool. J. Linn. Soc., 2018, XX [p.26, Fig.16] Relationship of the Ryocalanoidea (red) and Spinocalanoudea (green) based on cocatenated genes of 18S (1767 bp), 28S (842 bp), IT (266 nbp), COI (657 bp) and cytochrome b (327 bp). A, Bayesian Inference with Bayesian posterior probabilities > 0.90 are shown. B, Maximum Likehood analysis with 10 000 bootstrap replicates. Bootstrap values > 50 are shown. First numbers without and second number with consideration of codon positions for mitochondrial genes COI and citochrome b. The illustrated topology of the tree is from the analysis withot consideration of codon positions for COI and cytochrome b. | | | | | Spinocalanoidea | | Ref.: | Blanco-Bercial & al., 2011 (p.1, 5, Table 1, Fig.2, Biol. mol, phylogeny); Renz & al., 2018 (p.24, figs.15, 16, phylogeny relationships) | | Rem.: | 2 familles : Arctokonstantinidae, Spinocalanidae. | | | | Acartiidae Sars, 1903 ( Diaptomoidea ) | |
| | | | Aetideidae Giesbrecht, 1892 ( Clausocalanoidea ) | |
| | | | Arctokonstantinidae Markhaseva & Kosobokova, 2001 ( Spinocalanoidea ) | |
| | | | Arietellidae Sars, 1902 ( Arietelloidea ) | |
| | | | Augaptilidae Sars, 1905 ( Arietelloidea ) | |
| | | | Bathypontiidae Brodsky, 1950 ( Bathypontioidea ) | |
| | | | Boholinidae Fosshagen & Iliffe, 1989 ( Pseudocyclopoidea ) | |
| | | | Calanidae Dana, 1846 ( Calanoidea ) | |
| | | | Candaciidae Giesbrecht, 1892 ( Diaptomoidea ) | |
| | | | Centropagidae Giesbrecht, 1892 ( Diaptomoidea ) | |
| | | | Clausocalanidae Giesbrecht, 1892 ( Clausocalanoidea ) | |
| | | | Diaixidae Sars, 1902 ( Clausocalanoidea ) | |
| | | | Discoidae Gordeeva, 1975 ( Arietelloidea ) | |
| | | | Epacteriscidae Fosshagen,1973; emend.: Fosshagen, Boxshall & Iliffe, 2001 ( Epacteriscoidea ) | |
| | | | Eucalanidae Giesbrecht, 1892 ( Eucalanoidea ) | |
| | | | Euchaetidae Giesbrecht, 1892 ( Clausocalanoidea ) | |
| | | | Fosshageniidae Suarez-Morales & Iliffe, 1996 ( Diaptomoidea ) | |
| | | | Heterorhabdidae Sars, 1902 ( Arietelloidea ) | |
| | | | Hyperbionychidae Ohtsuka, Roe & Boxshall, 1993 ( Arietelloidea ) | |
| | | | Kyphocalanidae Markhaseva & Schulz, 2009 ( Clausocalanoidea ) | |
| | | | Lucicutiidae Sars, 1902 ( Arietelloidea ) | |
| | | | Mecynoceridae Andronov, 1973 ( Calanoidea ) | |
| | | | Megacalanidae Sewell, 1947 ( Calanoidea ) | |
| | | | Mesaiokeratidae Matthews, 1961 ( Clausocalanoidea ) | |
| | | | Metridinidae Sars, 1902 ( Arietelloidea ) | |
| | | | Nullosetigeridae Soh, Ohtsuka, Imabayashi & Suh, 1999 ( Arietelloidea ) | |
| | | | Paracalanidae Giesbrecht, 1892 ( Calanoidea ) | |
| | | | Parapontellidae Giesbrecht, 1894 ( Diaptomoidea ) | |
| | | | Parkiidae Ferrari & Markhaseva, 1996 ( Clausocalanoidea ) | |
| | | | Phaennidae Sars, 1902 ( Clausocalanoidea ) | |
| | | | Pontellidae Dana, 1853 ( Diaptomoidea ) | |
| | | | Pseudocyclopidae Giesbrecht, 1893 ( Pseudocyclopoidea ) | |
| | | | Pseudocyclopiidae T. Scott,1894 ; emend. Jaume, Fosshagen & Iliffe, 1999 ( Clausocalanoidea ) | |
| | | | Pseudodiaptomidae Sars, 1902 ( Diaptomoidea ) | |
| | | | Rhincalanidae Geletin, 1976 ( Eucalanoidea ) | |
| | | | Ridgewayiidae M.S. Wilson, 1958 ( Epacteriscoidea ) | |
| | | | Rostrocalanidae Markhaseva, Schulz & Martinez Arbizu, 2008 ( Clausocalanoidea ) | |
| | | | Ryocalanidae Andronov, 1974 ( Ryocalanoidea ) | |
| | | | Scolecitrichidae Giesbrecht, 1892 ( Clausocalanoidea ) | |
| | | | Spinocalanidae Vervoort, 1951 ( Spinocalanoidea ) | |
| | | | Stephidae Sars, 1902 ( Clausocalanoidea ) | |
| | | | Sulcanidae Nicholls, 1945 ( Diaptomoidea ) | |
| | | | Temoridae Giesbrecht, 1893 ( Diaptomoidea ) | |
| | | | Tharybidae Sars, 1902 ( Clausocalanoidea ) | |
| | | | Tortanidae Sars, 1902 ( Diaptomoidea ) | |
| | | | incertae sedis (Calanoida) | |
| | | | | |
|
|
 Toute utilisation de ce site pour une publication sera mentionnée avec la référence suivante : Toute utilisation de ce site pour une publication sera mentionnée avec la référence suivante :
Razouls C., Desreumaux N., Kouwenberg J. et de Bovée F., 2005-2026. - Biodiversité des Copépodes planctoniques marins (morphologie, répartition géographique et données biologiques). Sorbonne Université, CNRS. Disponible sur http://copepodes.obs-banyuls.fr [Accédé le 07 février 2026] © copyright 2005-2026 Sorbonne Université, CNRS
|
|
 |
 |