|
|
 |
Fiche d'espèce de Copépode |
|
|
Calanoida ( Ordre ) |
|
|
|
Diaptomoidea ( Superfamille ) |
|
|
|
Pontellidae ( Famille ) |
|
|
|
Labidocera ( Genre ) |
|
|
| |
Labidocera euchaeta Giesbrecht, 1889 (F,M) | |
| | | | | | | Syn.: | Labidocera euchäta Giesbrecht, 1889 (p.27, Descr. F); 1892 (p.446, 459, 773, figs.F);
Labidocera euchaeta Dimorph 2 Sewell, 1912 (p.341, figs.M);
Labidocera euchaeta minor Sewell, 1932 (p.362); Silas, 1972 (p.649) | | | | Ref.: | | | Giesbrecht & Schmeil, 1898 (p.135, Rem. F); Sewell, 1912 (p.328, 339, 353, 369, Rem., figs.F,M); 1933 (p.28); Mori, 1937 (1964) (p.93, figs.F); Shen & Bai, 1956 (p.221, figs.F,M); Chen & Zhang, 1965 (p.98, figs.F,M); Fleminger, 1965 (p.125: Rem.); Nyan Taw, 1974 (p.270, Rem.); Silas & Pillai, 1973 (1976) (p.800, Rem.F, figs.F, Rem.); Goswami & Goswami, 1979 a (p.259, figs. caryotypes); Li & Fang, 1983 a (p.375, figs.juv.); Zheng Zhong & al., 1984 (1989) (p.254, figs.F,M); Chihara & Murano, 1997 (p.867, Pl.147,150: F,M); Sanu & al., 2016 (p.99, Table 1, 2, 3, molecular database); Abo-Taleb, 2019 (p.367, 369: Key F, M), 372: Rem., abundance, Table 2, figs.F, M). | 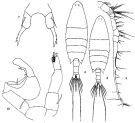 issued from: Q.-c. Chen & S.-z. Zhang in Studia Marina Sinica, 1965, 7. [Pl.40, 6-10]. Female (from E China Sea): 6, habitus (dorsal); 7, P5 (posterior). Male: 8, habitus (dorsal); 9, right A1; 10, P5 (posterior).
|
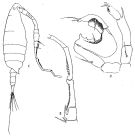 Issued from : R.B.S. Sewell in Rec. Indian Mus., 1912, 7. [Pl.XIX, Figs.1-3]. Male (from Bay of Bengal): 1, habitus (lateral right side); 2, A1 (grasping segments); 3, P5. Nota: Abdomen 5-segmented, 3rd is the longest and the 5th very short; roportional lengths of urosomites and furca as 22:20:30:15:7:22. Grasping organ of A1 has the knee-joint between the 18 th and 19 th segments; the 17th segment bears a spine and the toothed-plate of the 1th is longer than the segment itself and is produced proximally over the 17th; the 19th segment bears a row of 6 angular teeth which increase in size distally.
|
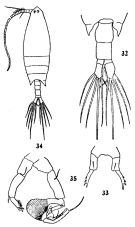 issued from : C.-j. Shen & S.-o. Bai in Acta Zool. sin., 1956, 8 (2). [Pl. V, Figs.32-35]. Female (from Yellow and Bai Rivers estuarie): urosome (dorsal); 33, P5. P5 uniramous, asymmetrical. Caudal rami asymmetruical, the right one being bigger than the left. Male: 34, habitus (dorsal); 35, P5. Caudal rami almost symmetrical. Distal segment of left leg with 3 finger-like processes and is hairy at the distal portion of its inner border.
|
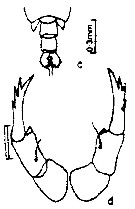 issued from : E.G. Silas & P.P. Pillai in J. mar. biol. Ass. India, 1973 (1976), 15 (2). [p.799, Fig.11, c-d]. Female (frommouth of Rangoon River): c, urosome (dorsal); d, P5. Nota: Anterior margin of cephalon imperfectly rounded. Dorsal eye lenses small and placed apart. Cephalic hooks absent. Urosome 2-segmented, segments more or less of equal size. Caudal rami asymmetrical, right ramus broader, 2nd caudal seta much longer than others. A1 23-segmented. P5 symmetrical.
Scale as in Calanopia minor.
|
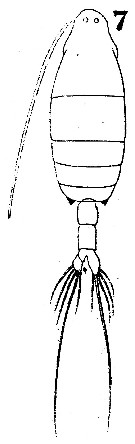 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf.41, Fig.7]. As Labidocera euchäta. Female: 7, habitus (dorsal).
|
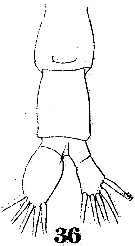 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf.41, Fig.36]. As Labidocera euchäta. Female: 36, urosome (ventral).
|
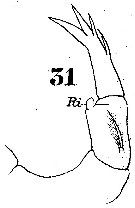 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892. Atlas von 54 Tafeln. [Taf.23, Fig.31]. As Labidocera euchäta. Female: 31, P5 (posterior view). Ri = endopod.
|
 issued from : T. Mori in The pelagic Copepoda from the neighbouring waters of Japan, 1937 (2nd edit., 1964). [Pl.42, Figs.11-13]. Female: 11, A2; 12, P5; 13, habitus (dorsal).
|
 issued from : Nyan Taw in Austr. J. mar. Freshwat. Res., 1974, 25. [p.270, Table 1]. Male: Characteristics.
|
 issued from : N.G. Das & S. Das in Chittagong Univ. St., 1980, II, 4. [p.46, Figs.7-12]. Female (from Karnafully River estuary): 7, habitus (dorso-lateral); 8, A1; 9, P5. Male: 10, corners of the last thoracic segment and urosome; 11, right A1; 12, P5. Nota: The morphological charaters coincide with those descibed by Sewell (1912) except that a long seta which is found on the 2nd segment of the exopod of P5 female of Sewell's paper is absent in specimens of Karnafully River estuary.
|
 Issued from : H.A. Abo-Taleb in Egyptian J. Aqua. Res., 2019, 45. [p.369]. Key to species of Labidocera in the Red Sea. Female: 1 - Anterior end of cephalosome rounded without median crest. 2 - Lateral hooks on cephalosome absent. 3 - Urosome 2-segmented. 4 - Caudal rami asymmetrica, right is broader than left with 2nd inner caudal seta much longer. male: 1 - Anterior end of cephalosome rounded without a median crest. 2 - Last posterolateral end of metasome asymmetrical.3 - cephalosome without lateral hooks. 4 - Right P5 chela with exopodal segment 1 has very short thumb, 1 long seta on the terminal marginof the claw.
| | | | | Ref. compl.: | | | Sewell, 1948 (p.324); Chiba & al., 1957 a (p.11); Ganapati & Shanthakumari, 1962 (p.9, 15); Chen Q-c, 1980 (p.794); Sarkar & al., 1986 (p.178); Madhupratap & Haridas, 1986 (p.105, tab.1); Gao & Li, 1988 (p.684, feeding rhythm); 1989 (p.299, size, weight); Hernandez-Trujillo, 1989 (tab.1); Cervantes-Duarte & Hernandez-Trujillo, 1989 (tab.3); Lin & Li, 1989 (p.203, egg production); Yoo, 1991 (tab.1); Dai & al., 1991 (p.45, tab.1); Hwang & Choi, 1993 (tab.3); Hernandez-Trujillo, 1994 (tab.1); Myung & al., 1994 (tab.1); Shih & Young, 1995 (p.71); Yang, 1997 (p.299); Park & Choi, 1997 (Appendix); Hsieh & Chiu, 1998 (tab.2); Suarez-Morales & Gasca, 1998 a (p.110); Wong & al, 1998 (tab.2); Mauchline, 1998 (tab.8); Dolganova & al., 1999 (p.13, tab.1); Rezai & al., 2004 (p.489, tab.2, Rem.); Chang & Fang, 2004 (p.456, tab.1); Wang & Zuo, 2004 (p.1, Table 2, dominance, origin); Lan & al., 2004 (p.332, tab.1); Yin & al., 2004 (p.3); Choi & al., 2005 (p.710: Tab.III); Zuo & al., 2006 (p.159, tab.1, 3, fig.9, spatial abundance, fig.8: stations group); Hwang & al., 2006 (p.943, tabl. I); Lavaniegos & Jiménez-Pérez, 2006 (p.146, tab.2, 4, Rem.); Dur & al., 2007 (p.197, Table IV); Wang M.-h & al., 2007 (p.679, trophic transfer); Tseng L.-C. & al., 2008 (p.46, table 2, abundance vs moonsons, fig.8, table 3: indicator species); Ohtsuka & al., 2008 (p.115, Table 5); Lan & al., 2009 (p.1, Table 2); Jiang Z.-B. & al., 2009 (p.196, Table 1, 2); Hwang & al., 2009 (p.49, fig.4, 5); Zhang G.-T. & al., 2010 (p.492, Table 1, 2); Sun & al., 2010 (p.1006, Table 2); W.-B. Chang & al., 2010 (p.735, Table 2, abundance); Zhaoli & al., 2011 (p.84, abundance %); Gao X. & al., 2011 (p.591, p.597: occurrence); Zengling MA & al., 96, mortality rate, respiration vs Chlorine concentration); DiBacco & al., 2012 (p.483, Table S1, ballast water transport); Johan & al., 2012 (p.647, Table 1, fig.2, salinity range); Huo & al., 2012 (p.1, Table 1: dominance); Lee D.B. & al., 2012 (p.88, co-occurrence); Moon & al., 2012 (p.1, Table 1); Seo & al., 2013 (p.448, Table 1, occurrence) | | | | NZ: | 6 | | |
|
Carte de distribution de Labidocera euchaeta par zones géographiques
|
| | | | | | | | | 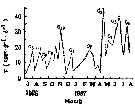 issued from : S. Lin & S. Li in J. Xiamen Unv. (Nat. Sc.), 1989, 28 (2). [p.205, Fig.4]. Seasonal fluctuation in the average rate egg production of the population (F) based on which 10 generations a year (G1-G10) are proposed. issued from : S. Lin & S. Li in J. Xiamen Unv. (Nat. Sc.), 1989, 28 (2). [p.205, Fig.4]. Seasonal fluctuation in the average rate egg production of the population (F) based on which 10 generations a year (G1-G10) are proposed.
Nota: The reproductive rate was investigated between April 1986 and August 1987 in Xiamen Harbor (24°26'N, 118°10'E).
Results show that at a constant temperature (20°C), specific rate of egg production is positively correlated with food (Artemia nauplius) density, with a gross efficiency of egg production of ca. 62% in carbon which was independent of food density nand ingestion rate. The percentage of egg-lying females fluctuated seasonally similarly to in situ rate of egg production., but maintened quite high levels all year round, suggesting that this species in Xiamen reproduces continuously, with summer as high reproductive season and winter a low one.
Ten gegnerations a year are suggested based only on the number of the peaks of average population egg production rate. |
 issued from : S. Lin & S. Li in J. Xiamen Unv. (Nat. Sc.), 1988, 27 (6). [p.691, Table 1, Fig.4]. issued from : S. Lin & S. Li in J. Xiamen Unv. (Nat. Sc.), 1988, 27 (6). [p.691, Table 1, Fig.4].
At sufficient food supply, specific rate of egg production, unrelated to the body length of adults females, between April 1986 and August 1987 at Xiamen Harbor, has a positive correlation (r) with temperature, with seasonal variations occurring in the correlation pattern.
The correlation is observed between rate of egg production (F) and temperature as well as body weight of adults females.
Total egg production is also positively correlated with body size and more completexy with temperature.
Hatching rate of eggs, in independent of temperature within the normal temperature range, maintainely high levels all year round. |
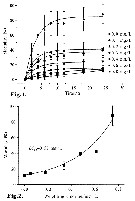 Issued from : Zengling MA, Hongping LIN, Xiaolian GU & Zhaoli XU in Acta Oceanol. Sin., 2011, 30 (2). [p.98, Figs.1, 2]. Issued from : Zengling MA, Hongping LIN, Xiaolian GU & Zhaoli XU in Acta Oceanol. Sin., 2011, 30 (2). [p.98, Figs.1, 2].
Fig.1: Effects of different conce,tration residual chlorine on the mortality of L. eucharta (from Xiamen Bay: 24°26.778' N, 118°02.363' E; in September 2009).
Fig.2: mortality of L. euchaeta treated for 24 h with different residual chlorine concentration.
The means and standard errors were based on triplicate incubations in all experiments.
Nota: The mortality increased with enhanced chlorine concentration and prolonged exposure time. Furthermore, the mortality increased faster at the beginning, reached the maximal value in 8 h and leveled off from then on. |
 Issued from : Zengling MA, Hongping LIN, Xiaolian GU & Zhaoli XU in Acta Oceanol. Sin., 2011, 30 (2). [p.99, Figs.3, 4, 5]. Issued from : Zengling MA, Hongping LIN, Xiaolian GU & Zhaoli XU in Acta Oceanol. Sin., 2011, 30 (2). [p.99, Figs.3, 4, 5].
Fig.3: Changes in Chlorella sp. cell concentration due to grazing of L. euchaeta with residual chlorine concentration at 0 and at 0.2 mg per L.
The means and standard errors were based triplicate incubations.
Fig.4: Effects of 0.2 mg per L residual chlorine on filtering and grazing rates of L. euchaeta.
The means and standard errors were based on triplicate incubations. The symbol ** indicates significant (p<0.01) difference between the treatments.
Fig.5: Effects of residual chlorine on respiration of L. euchaeta.
The means and standard errors were based on triplicate incubations. The symbol ** indicates significant (p<0.05) difference between treatments. |
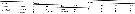 Issued from : H.A. Abo-Taleb in Egyptian J. Aquat. Res., 2019, 45. [p.371, Table 2]. Issued from : H.A. Abo-Taleb in Egyptian J. Aquat. Res., 2019, 45. [p.371, Table 2].
Average values with standard deviation and ranges (individuals/m3) in the different studied habitats (SG: seagrass, CR: coral reef, SSL: sheltered lagoon, and OW: open sea.
See fig.1: sampling sites in the northern Red Sea; and Table 1: measured physical environmental parameters. |
 Issued from : H.A. Abo-Taleb in Egyptian J. Aquat. Res., 2019, 45. [p.368, Fig.1]. Issued from : H.A. Abo-Taleb in Egyptian J. Aquat. Res., 2019, 45. [p.368, Fig.1].
Sampling sites (SG: seagrass; CR: coral reef; SSL: shallow lagoon; ODW: open water) in the northern Red Sea. |
 Issued from : H.A. Abo-Taleb in Egyptian . J. Aquat. Res., 2019, 45. [p.369, Table 1]. Issued from : H.A. Abo-Taleb in Egyptian . J. Aquat. Res., 2019, 45. [p.369, Table 1].
Average values of the measured environmental parameters ± standard deviation (SD) for the different studied habitats in the northern Sea (SG: seagrass; CR: coral reef; SSL: sheltered shallow lagoon; OW: open sea). |
 Issued from : T. Zuo, R. Wang, Ya-qu. Chen, Sh-wu Gao & Ke. Wang in J. Mar. Syst., 2006, 59. [p.169, Fig.9C]. Labidocera euchaeta Issued from : T. Zuo, R. Wang, Ya-qu. Chen, Sh-wu Gao & Ke. Wang in J. Mar. Syst., 2006, 59. [p.169, Fig.9C]. Labidocera euchaeta
Spatial of representative species abundance with isobaths shown in gray.
The circles are proportional in diameter to square root value of abundance.
Copepods collected vertically with plankton net (200 µm mesh aperture) from a depth near the bottom to the surface in autumn 2000 (18 October to 21 November).
Body length (mm): 2.68. |
| | | | Loc: | | | Red Sea, Indian (N & SE), India (Lawson's Bay, Karnafully River estuary, Hooghly estuary), Bay of Bengal, Rangoon River estuary, Perai Ricer estuary (Penang), Straits of Malacca, Indonesia-Malaysia, Philippines, Hainan (Sanya Bay), Hong Kong, Amoy, Xiamen Harbour, Taiwan Strait, Taiwan (S, SW, W, Kaohsiung Harbor, NW, off Danshuei River, N), China Seas (Bohai Sea, Yellow Sea, East China Sea, Changjiang River estuary, Xiamen Bay, South China Sea), S & W Korea, Muan Bay, Asan Bay, Japan Sea, Japan, W Baja California, G. of California | | | | N: | 81 | | | | Lg.: | | | (44) F: 2,4-1,8; (46) F: 2,1-2; (81) M: 1,88; (91) F: 2,4-2; (256) F: 2,31; (290) F: 2,5-2,85; M: 2,25-2,45; (351) F: 1,96-2,18; M: 1,67-1,77; (1113)* F: 4,05; M: 3,76; {F: 1,80-2,85; M: 1,67-2,45}
*: The body lengths measured by Das & Das (1980) appear much more high tha the other authors. | | | | Rem.: | néritique.
La plupart des localisations in C.B. Wilson (1950, p.244) ne sont pas retenues.
Observé dans les ballasts des navires à San Francisco.
Voir aussi les remarques en anglais | | | Dernière mise à jour : 09/12/2020 | |
|
|
 Toute utilisation de ce site pour une publication sera mentionnée avec la référence suivante : Toute utilisation de ce site pour une publication sera mentionnée avec la référence suivante :
Razouls C., Desreumaux N., Kouwenberg J. et de Bovée F., 2005-2026. - Biodiversité des Copépodes planctoniques marins (morphologie, répartition géographique et données biologiques). Sorbonne Université, CNRS. Disponible sur http://copepodes.obs-banyuls.fr [Accédé le 06 février 2026] © copyright 2005-2026 Sorbonne Université, CNRS
|
|
 |
 |



















