|
|
 |
Fiche d'espèce de Copépode |
|
|
Calanoida ( Ordre ) |
|
|
|
Clausocalanoidea ( Superfamille ) |
|
|
|
Stephidae ( Famille ) |
|
|
|
Stephos ( Genre ) |
|
|
| |
Stephos longipes Giesbrecht, 1902 (F,M) | |
| | | | | | | Syn.: | Stephus longipes Giesbrecht, 1902 (p.20, figs.F,M); Wolfenden, 1908 (p.23, figs.F,M); 1911 (p.205, Rem.); Farran, 1929 (p.208, 226); Sewell, 1948 (p.574); Fukuchi & Tanimura, 1981 (p.37); Hoshiai & Tanimura, 1981 (p.44); Kawaguchi & al., 1986 (tab.2); Zmijewska, 1987 (tab.2a); Knox & al., 1996 (tab.1) | | | | Ref.: | | | Brady, 1918 (p.24, figs.F, juv.M); Tanaka, 1960 (p.37, figs.M, Juv.F ); 1964 (p.7); Zvereva, 1972 (1975) (p.254, figs.F,M); Bradford-Grieve, 1999 a (p.25, Table1: Rem.; Costanzo & al., 2002 (p.855, figs. Nauplii); Michels & Schnack-Schiel, 2005 (p.483, fig.6: Md); Park & Ferrari, 2009 (p.143, Table 1, Appendix 1, biogeography from Southern Ocean); Kos, 2016 (p.25, figs.F, M, Rem.) | 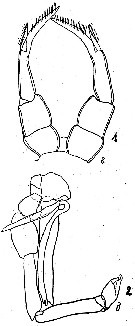 issued from : J.A. Zvereva in Issled. Fauny Moreï, 1972, 12 (20). [p.219, Fig.2, 1-2]. As Stephus longipes. Female (Antarctic coast): 1, P5. Male: 2, P5.
|
 issued from : J. Michels & S.B. Schnack-Schiel in Mar. Biol., 2005, 146. [p.488, Fig.6]. Mandibular gnathobase. a, b: Female (from Weddell and Bellingshausen Seas); c, Male. a, b: left gnathobase from cranial; c: right gnathobase from caudal. V: ventral tooth, C1-C4: central teeth, D1-D3: dorsal teeth, B: dorsal bristle, arrow: the hole-like cut-out.. Scale bars 0.020 mm. Nota: The life cycle is partly associated with the sea ice (Schnack-schiel & al., 1995, 1998, 2001). Feeding experiments and analyses of faecal pellets have shown that the species feeds on ice algae (Kurbjeweit & al., 1993; Schnack-Schiel & al., 1995). The late cipepodite stages (especially the stage 4) settle down in deep water layers, perhaps even to the sediment, and overwinter there. Since the species uses triglycerols to store energy, it is probable that this species feeds during the whole year (Schnack-Schiel & al., 1995). The authors suggest that the copepodite stage 4 lives partly associated with the sediment in the benthal during winter and feeds on phyto- and zooplankton and detritus.
|
 issued from : W. Giesbrecht in Copepoden. Res. voyage du S. Y. Belgica. Rapports scientifiques, Zoologie, 1902. [Taf. II, Figs. 6-14]. As Stephus longipes. Female: 7, urosome (dorsal); 8, last thoracic segment and genital segment (lateral); 9, A1; 10, Md (cutting edge of gnathobase); 11, P4; 12, P5. Male: 6, habitus (dorsal); 13, P5. Copepodid Male: 14, P5. sin = left; dex = right; B1 = basipodite 1; B2 = basipodite 2.
|
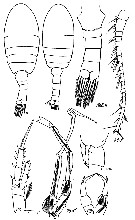
 Issued from : O. Tanaka in Spec. Publs. Seto mar. biol. Lab., 10, 1960 [Pl. XIV, 1-6]. Male (from 66°51'S, 41°19'E): 1-2, habitus (lateral and dorsal, respectively); 3, anal segment and caudal rami (dorsal); 4, P2; 5, right P5; 6, left P5. Nota: Cephalothorax and abdomen in the proportional lengths 71 to 29. Head and 1st thoracic segment separate, 4th and 5th thoracic segments fused. Rostrum represented by a blunt process. Posterior thoracic margin emarginate in lateral view. Abdomen 5-segmented; segments and caudal rami in proportional lengths 16 : 21 : 17 : 14 : 18 : 14 = 100. Genital segment swollen laterally in dorsal view.3rd and 4th abdominal segments each with a small spine on the lateral distal corner of the segment. caudal rami rounded in shape, and with characteristic setae. P5 5-segmented on each side; long and slender. Left leg has a process furnished with a tubercle on the 2nd segment. The 3rd and 4th segments of both right and left legs very long. The terminal segment of right leg tapers gradually into a fine point, and the distal half denticulated. The terminal segment of the left leg is bill-shaped, and looks like a head of a bird.
|
 Issued from : O. Tanaka in Spec. Publs. Seto mar. biol. Lab., 10, 1960 [p.37]. Male: Proportional lengths of segmets of A1. Nota: A1 24 segmented, extends about to the posterior margin of the 3rd thoracic segment.
|
 Issued from : C. Razouls in Ann. Inst. océanogr., Paris, 1994, 70 (1). [p.134]. Caractéristiques morphologiques de Stephos longipes femelle et mâle adultes. Terminologie et abbréviations: voir à Calanus propinquus.
|
 Issued from : M.S. Kos in Zoological Institut RAS, St. Petersburg, 2016, 179. [p.26, Fig.9]. Stephos longipes female and male (redrawn in Giesbrecht, 1902).
| | | | | Ref. compl.: | | | Schnack & al., 1985 (p.256, fig.4); Tucker & Burton, 1990 (p.591, tab.1, fig.4, seasonal, spatial variations); Eslake & al., 1991 (p.93, Table 2, temporal changes); Kurbjeweit & al., 1993 (p.255, life cycle); Menshenina & Melnikov, 1995 (p.128); Schnack-Schiel & al., 1995 (p.45, seasonal abundance, vertical distribution, ice/water interface); Swadling & al., 1997 (p.39, grazing); Schnack-Schiel & al., 1998 (p.173, abundance, vertical distribution, sea ice); Atkinson, 1998 (p.289, Table 1, biological data); Elwers & Dahms, 1998 (p151); Razouls & al., 2000 (p.343, tab. 3, 5, Appendix); Fuentes & Schnack-Schiel, 2005 (p.253); Deibel & Daly, 2007 (p.271, Table 6a, Rem.: Antarctic polynyas); Guglielmo & al., 2007 (p.747, Table 4, 5); Schnack-Schiel & al., 2008 (p.1046: Tab.2); Schnack-Schiel & al., 2008 (p.1056, Table 1, 2, 4, fig.7); Kiko & al., 2008 (p.1000, Table 3, fig.4); Loots & al., 2009 (p.599, fig.8); Swadling & al., 2010 (p.887, Table A1, abundance); Kramer & al., 2011 (p.1062, Table 2, occurrence vs meiofauna); Yang & al., 2011 a (p.921, Table 2, inter-annual variation 1999-2006); Michels & al., 2012 (p.369, Table 1, fig.13, occurrence frequency, vertical distribution); Lee D.B. & al., 2013 (p.1215, Table 1, 2: abundance, composition); Record & al., 2018 (p.2238, Table 1: diapause); | | | | NZ: | 2 | | |
|
Carte de distribution de Stephos longipes par zones géographiques
|
| | | | | | | | | 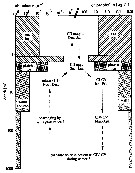 Issued from : F. Kurbjeweit, R. Gradinger & J. Weissenberger in Mar. Ecol. Prog. Ser., 1993, 98. [p.260, Fig.9]. Issued from : F. Kurbjeweit, R. Gradinger & J. Weissenberger in Mar. Ecol. Prog. Ser., 1993, 98. [p.260, Fig.9].
Schematic representation of the abundance of different developmental stages of Stephos longipes and phytoplankton standing stock within and below the sea ice, and in the upper 1000 m of the water column.
Nota: Reproduction experiments showed significantly higher egg production rates with ice algae as food than with planktonic diatoms (Kurbjeweit unpubl.).
Adult females and males occur in a 1:1 ratio from mid-November until mid-December just beneath rhe sea ice for mating on the wester and eastern shelf of the Weddell Sea (Kurbjeweit unpubl.) and females need direct contact with a substrate for egg laying (the eggs are extremely sticky)., they may attach their eggs directely to ice crystals or ice platelets. This act explains the dissemination by drifting ice crtstals.
The sea ice habitats serve as a 'nursery' for the larval copepods. |
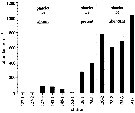 Issued from : F. Kurbjeweit, R. Gradinger & J. Weissenberger in Mar. Ecol. Prog. Ser., 1993, 98. [p.258, Fig.5]. Issued from : F. Kurbjeweit, R. Gradinger & J. Weissenberger in Mar. Ecol. Prog. Ser., 1993, 98. [p.258, Fig.5].
Abundance in the under ice water layer in relation to the relative abundance of platelet ice.
Nota: |
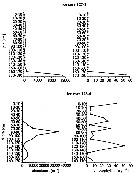 Issued from : F. Kurbjeweit, R. Gradinger & J. Weissenberger in Mar. Ecol. Prog. Ser., 1993, 98. [p.259, Fig.8]. Issued from : F. Kurbjeweit, R. Gradinger & J. Weissenberger in Mar. Ecol. Prog. Ser., 1993, 98. [p.259, Fig.8].
Distribution patterns of S. longipes and chlorophyll a within ice cores at two stations on shelf off south Halley Bay.
Nota: No clear correlation was found berween the vertical distribution of S. longipes and chlorophyll a inside the ice floes. In about 50% of all ice cores highest chlorophyll a concentrations were found in the lowermost centimeters of the ice floes, where S. longipes was also most abundant. Other ice core samples showed strong vertical variations of both Chl a and abundance of S. longipes.
In all ice cores the phytoplankton stock mainly consisted of typical ice algae like Nitzschia cylindricus, N/ curta and other pennate diatoms; the faecal pellets of S. longipes contained only frustules of pennate diatoms. |
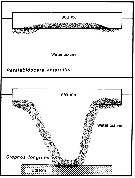 Issued from : A. Tanimura, T. Hoshiai & M. Fukuchi in Antactic Science, 1996, 8 (3). [p.264, Fig.10]. Issued from : A. Tanimura, T. Hoshiai & M. Fukuchi in Antactic Science, 1996, 8 (3). [p.264, Fig.10].
Schematic comparison of the relations with sea ice among Paralabidocera antarctica and Stephos longipes (Kurbjeweit & al., 1993).
Stippled area indicates the main habitat throughout the life cycle in each copepod.
Nota: The two species depend entirely on the ice algae rather phytoplakton in the water as food (Kurbjeweit & al., 1993). However, there are differences in their relationship with sea ice through their life cycles. The relationship with sea ice is much weaker for Stephos longipes than for Paralabidocera antarctica. According to Kurbjeweit & al. (1993), S. longipes is associated with sea ice only during short summer period in naupliar stages and spends the greater part of its life span in the water column away from the sea ice. They postulated that S. longipes spends winter as resting stages of CIV and CV on the bottom sediments. |
 Issued from : J. Michels, S.B. Schnack-Schiel, A. Pasternak, E. Mizdalski, E. Isla & D. Gerdes in Polar Biol., 2012, 35. [p.382, Fig.13 (part., copepodid stages omitted.)]. Issued from : J. Michels, S.B. Schnack-Schiel, A. Pasternak, E. Mizdalski, E. Isla & D. Gerdes in Polar Biol., 2012, 35. [p.382, Fig.13 (part., copepodid stages omitted.)].
Vertical distribution of the adult females and males of Stephos longipes from East Weddell Sea shelf (70°48.558'S, 10°43.698'W) between 9 and 28 December 2003. The water depth at the sampling spots varied between 438 and 484 m.
The white dots represent the weighted mean depths. |
| | | | Loc: | | | Antarct. (Amundsen Sea, Ellsworth Land coast, King George Is., Peninsula, Croker Passage, Syowa station, Weddell Sea, off Halley Bay, Kapp Norvegia, Halley Bay, S Indian Ocean, Davis station-Gardner Is., Fletcher Lake, SE Pacif., Ross Sea, Prydz Bay, McMurdo Sound, Station Davis, Terra Nova Bay, Dumont d'Urville ), off Southern South Africa. Antarctic localities: 69°48' S to 74°18' S and 81°19' W to 92°22' W (Giesbrecht, 1902; Wolfenden, 1911). 66°02' S, 93°01' E (Kos, 2016, p.25) | | | | N: | 48 | | | | Lg.: | | | (10) F: 0,8-0,75; (32) F: 0,8-0,75; M: 0,7-0,65; (33) F: 0,85-0,90; M: 0,75-0,80; (35) F: 0,96-0,72; M: 0,9-0,78; (66) M: 0,87; (114) F: 0,85; {F: 0,72-0,96; M: 0,65-0,90}
The mean female size is 0.820 mm (n = 9; SD = 0.0778), and the mean male size is 0.779 mm (n = 7; SD = 0.0886). The size ratio (male : female) is approximately 0.94. | | | | Rem.: | Sampling depth (Antarct.) : 0-1000 m.
Voir aussi les remarques en anglais | | | Dernière mise à jour : 17/06/2021 | |
|
|
 Toute utilisation de ce site pour une publication sera mentionnée avec la référence suivante : Toute utilisation de ce site pour une publication sera mentionnée avec la référence suivante :
Razouls C., Desreumaux N., Kouwenberg J. et de Bovée F., 2005-2026. - Biodiversité des Copépodes planctoniques marins (morphologie, répartition géographique et données biologiques). Sorbonne Université, CNRS. Disponible sur http://copepodes.obs-banyuls.fr [Accédé le 20 février 2026] © copyright 2005-2026 Sorbonne Université, CNRS
|
|
 |
 |












