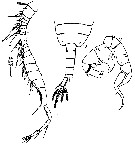|
|
 |
Fiche d'espèce de Copépode |
|
|
Calanoida ( Ordre ) |
|
|
|
Diaptomoidea ( Superfamille ) |
|
|
|
Pseudodiaptomidae ( Famille ) |
|
|
|
Pseudodiaptomus ( Genre ) |
|
|
| |
Pseudodiaptomus annandalei Sewell, 1919 (F,M) | |
| | | | | | | Syn.: | Pseudodiaptomus nostradamus Brehm,1933; Kiefer,1938;
P. dubius Kiefer, 1936 (syn. after Wellershaus, 1969); Grindley, 1981; 1984 (p.219, fig.M);
Schmackeria annandalei : Marsh, 1933 (p.42, figs.F,M); Krishnaswamy, 1953 (p.124); Saraswathy, 1966 (1967) (p.79);
Schmackeria dubia : Chen & Zhang, 1965 (p.82, figs.F,M); Shen,1979
Pseudodiaptomus dubia : C. Li & al., 2009 (p.866, Development) | | | | Ref.: | | | Sewell, 1919 (p.5, Rem.F, M, fig.M); 1924 (p.787, figs.F,M); 1932 (p.240); 1948 (p.323, 367); Wellershaus, 1969 (p.263, figs.F,M): Silas, 1972 (p.648); Pillai, 1976 (1980) (p.248, figs.F,M); Ranga Reddy & Radhakrishna, 1982 (p.268, Redescr.F,M, figs.F,M); Dussart & Defaye, 1983 (p.32); Walter, 1986 (p.132, figs.F,M, Rem.); 1986 a (p.503); 1987 (p.367, 389, Descr.F, M, figs.F,M, Rem.); Walter & al., 2002 (p.657, figs.F,M); Mulyadi, 2004 (p.152, figs.F,M, Rem.); Walter & al., 2006 (p.203, 211, Rem.); Phukham, 2008 (p.102, fig.F; Altaff, 2018 (p.387, figs.: oviducal glands) | 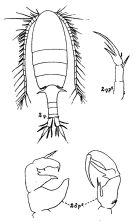 issued from : R.B.S. Sewell in Fauna of the Chilka Lake, 1924, 12. [Pl. XLIV, Fig.2]. Female and Male.
|
 issued from : R.B.S. Sewell in Rec. Indian Mus., (1918) 1919, XYI. [Pl.X, Fig.9]. Male (from Chilka Lake): 9, P5.
|
 issued from : T.C. Walter in J. Plankton Res., 1986, 8 (1). [p.160, Fig.14]. Female: A, habitus (dorsal); B, portion of prosome and urosome (lateral right side); C, P5 (postrior view). Nota: Urosme segments and caudal rami with proportions 37:15:19:10:19 = 100. A1 22-segmented.
Male: D, habitus (dorsal); E, last thoracic segment and urosome (lateral left side); F, A1 (segment 1, with straight spine); G, A1 (segment 17 dorsal flange); H, P5 (posterior view); I, idem (anterior view).
Nota: Urosomal segments and caudal rami with proportions 13:20:20:20:7:20 = 100. Right A1 20-segmented. Caudal rami 2.5 times longer than wide.
|
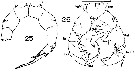 Issued from : S. Wellershaus in Veröff. Inst. Meeresforsch. Bremerh., 1969, 11 (2). [p.255, Figs.25-26]. Female: 25, P5. Male: 26, P5.
Scale : single line = 0.1 mm ; double line = 0.05 mm.
|
 issued from : T.C. Walter, S. Ohtsuka, S. Putchakarn, K. Pinkaew & S. Chullasorn in Proc. Biol. Soc. Washington, 2002, 115 (3). [p.664, Fig.9, C-D]. Female: C, genital double somite (ventral view); D, magnification of same view. Scale bars: 0.1 mm.
|
 issued from : N. Phukham in Species diversity of calanoid copepods in Thai waters, Andaman Sea (Master of Science, Univ. Bangkok). 2008. [p.186, Fig.60]. Female (from W Malay Peninsula): a, habitus (dorsal); b, urosome (dorsal); c, P5. Body length after the drawing: F = 1.274 mm.
|
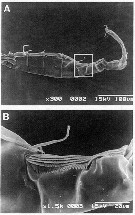 issued from : G. Dur, S. Souissi, Schmitt F.G., Beyrend-Dur D. & Hwang J.-S. in J. Exp Mar. Biol. Ecol., 2011, 402. [p.8, Fig.5, A, B]. Male (25°05'02''N, 121°30'10''E): A, SEM of the right geniculate A1; B, detail of the toothed ''comb'' located on the geniculate A1
|
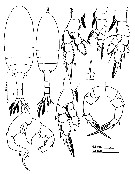 issued from : Mulyadi in Published by Res. Center Biol., Indonesia Inst. Sci. Bogor, 2004. [p.153, Fig.86]. Female (from W Java): a, habitus (dorsal); b-f, P1 to P5, espectively. Male: g, habitus (dorsal); h, P5; i, segment 17 (with elongate straight spine).
|
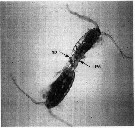 Issued from : G. Dur, Souissi S., Schmitt F.G., Cheng S.^H. & J.-S. Hwang in Zool. Stud., 2012, 51 (5). [p.592, Fig.1]. Individuals in the copulation position, in which the male (top) clasps the female (bottom) urosome by its modified P5 (LP5). A spermatophore (sp) is also visible in the picture. Courtesy of D. Beyrend-Dur.
|
 Issued from : Y. Ranga Reddy & Y. Radhakrishna in Hydrobiologia, 1982, 87. [p.269, Plate VI, Figs. 1-9]. Female (from 16°32'-16°47'N, 81°4'-81°22'E): 1, posterior part of prosome and urosome (dorsal); 2, postrior prosome and genital segment (lateral); 3, genital segment (ventral); 4, A1; 5, P1; 6, P2; 7, P3; 8, P4; 9, P5.
|
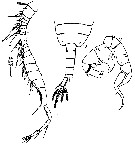 Issued from : Y. Ranga Reddy & Y. Radhakrishna in Hydrobiologia, 1982, 87. [p.269, Plate VI, Figs. 10-12]. Male: 10, posterior part of prosome and urosome (dorsal); 11, right A1; 12, P5 (posterior view).
|
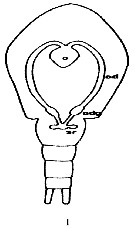 Issued from : K. Altaff in International Journal Zoology and Applied Biosciences, 2018, 3 (3). [p.389, Fig.1]. Female reproductive system of Pseudodiaptomus annandalei. 0: ovary; odg: oviducal gland; sr: seminal receptacle. Nota: As all calanoids, the female reproductive system consists odf a median ovary, a pair of oviducts antrum, seminal receptacle and reproductive pore. Ovary is an elobgate organ lying on the dorsal side of the anterior midgut. It has a wider anterior end and a narrow as well as rounded posterior end. A pair of oviducts originates from the anterior end of the ovary and takes a posterior course and then proceeds laterally through the perivisceral cavity towards the end of the prosome. The oviducts enlarge terminally to give rise to oviducal gland which extends into the genital segment and opens into the antrum. The antrum in turn opens to the exterior through female reproductive pore. A pair of spherical seminal receptacle occurs in the genital segment. In this species fertilized eggs are held in the ovisac where embryonic development up to naupliar stage takes place. The ovisac contains 23 ± 3 eggs.
Nota: Compare with P. serricaudatus. Nota: The ovary is oval in shape while that of P. serricaudatus has broader center and tapering anterior and posterior ends. The oviducal glands show higher elongation than those of P. serricaudatus. Occurrence of oviducal glands in marine copepod is reported for the first time. The oviducal glands show resemblance with the oviducal glands of freshwater diaptomids, that suggest that a closer phylogenetic affinity of the Pseudodiaptomus genus with freshwater diaptomids (See Altaff & Chandran, 1994; Dharani, 1998). For Altaff & Chandran (1994), histochemical tests indicated the direct involvement of the elastic sac secretion in the formation of the ovisac.
| | | | | Ref. compl.: | | | Subbaraju & Krishnamurphy, 1972 (p.25, 26, Table 1: abundance vs India localities); );Sarkar & al., 1986 (p.178); Madhupratap & Haridas, 1986 (p.105, tab.2); Mitra & al., 1990 (fig.3); Tiwari & Nair, 1993 (p.67); Ramaiah & al., 1996 (p.3); Ramaiah & Nair, 1997 (tab.1); Achuthankutty & al., 1998 (p.1, Table 2, seasonal abundance vs monsoon); Chen Q. & al., 2006 (p.575, reproduction & survival vs salinity); Madhu & al., 2007 (p.54, Table 4, abundance vs monsoon); Cheng S.-H. & al., 2008 (p.580, spermatophore transfer between males); Ohtsuka & al., 2008 (p.115, Table 5); Lee C.-H. & al., 2010 (p.17, behavior); Dur & al., 2010 (p.423, Table 5, mobility pattern); Maiphae & Sa-ardrit, 2011 (p.641, Table 2, 3, Rem.); Lee & al., 2011 (p.1085, figs., Rem.: mating); Dur & al., 2011 (p.197, mating behavior); Beyrend-Dur & al., 2011 (p.1, dynamics vs. environmental factors); Michalec & al., 2012 (p.24, swimming behavior); Mulyadi & Rumengan, 2012 (p.202, Rem.: p.204); Dur & al., 2012 (p.589, sex ratio vs. mating behavior); Drillet & al., 2012 (p.155, Table 1, culture); Dhanker & al., 2012 (p.44, feeding); Beyrend-Dur & al., 2013 (p.771, population dynamics); Jiang J.-L. & al., 2013 (p.203, gene expresion vs nickel exposure); Sabia & al., 2014 (p.8, Table 5: comparative data swimming behaviour): Sabia & al., 2015 (p.460, Table 3, Rem.); Lehette & al., 2016 (p.456, respiration rates vs dry weight and temperature, females, males, acclimated) | | | | NZ: | 3 | | |
|
Carte de distribution de Pseudodiaptomus annandalei par zones géographiques
|
| | | | | | | | | 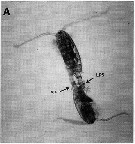 issued from : G. Dur, S. Souissi, Schmitt F.G., Beyrend-Dur D. & Hwang J.-S. in J. Exp Mar. Biol. Ecol., 2011, 402. [p.8, Fig.6, A]. issued from : G. Dur, S. Souissi, Schmitt F.G., Beyrend-Dur D. & Hwang J.-S. in J. Exp Mar. Biol. Ecol., 2011, 402. [p.8, Fig.6, A].
A: Individuals in copulation, position in which the male (bottom) clasps the female (top) urosome by its modified left P5 (LP5). A spermatophore is visible on the photo (sp). |
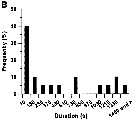 issued from : G. Dur, S. Souissi, Schmitt F.G., Beyrend-Dur D. & Hwang J.-S. in J. Exp Mar. Biol. Ecol., 2011, 402. [p.8, Fig.6, B]. issued from : G. Dur, S. Souissi, Schmitt F.G., Beyrend-Dur D. & Hwang J.-S. in J. Exp Mar. Biol. Ecol., 2011, 402. [p.8, Fig.6, B].
B: Frequency distribution of copulation duration (in second). |
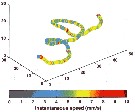 issued from : F.-G. Michalec, M. Holzner, J.-S. Hwang & S. Souissi in J. Exp. Mar. Biol. Ecol., 2012, 438. [p.28, Fig.4]. issued from : F.-G. Michalec, M. Holzner, J.-S. Hwang & S. Souissi in J. Exp. Mar. Biol. Ecol., 2012, 438. [p.28, Fig.4].
Trajectory displayed by Pseudodiaptomus annandalei (from Danshuei estuary, Taiwan) ovigerous female at salinity 10, totalizing 863 frames and illustrating variations in the magnitude of instantaneous velocities in three dimensional observation
Coordinates are in mm. |
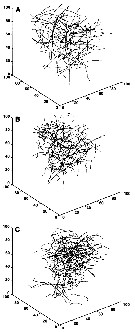 issued from : F.-G. Michalec, M. Holzner, J.-S. Hwang & S. Souissi in J. Exp. Mar. Biol. Ecol., 2012, 438. [p.30, Fig.7]. issued from : F.-G. Michalec, M. Holzner, J.-S. Hwang & S. Souissi in J. Exp. Mar. Biol. Ecol., 2012, 438. [p.30, Fig.7].
Representative trajectories ofv P. annadalei males (A), females (B) and ovigerous females (C), showing in the degree of convolution.
Female and ovigerous female trajectories are globally more complex and males swim in a more rectilinear way. However, both straight and convoluted trajectories can be observed in all three adult stattes.
Sinking behavior, when copepods are not swimming but sink slowly due to gravity, is visible in some trajectories. |
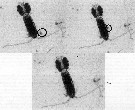 Issued from : R. Dhanker, R. Kumar & J.-S. Hwang in J. Exp. Mar. Biol. Ecol., 2012, 414-415. [p.47, Fig.1]. Issued from : R. Dhanker, R. Kumar & J.-S. Hwang in J. Exp. Mar. Biol. Ecol., 2012, 414-415. [p.47, Fig.1].
Images of P. annandalei female pursuing and ingesting the rotifer Brachionus rotundiformis.
Nota: Both males and females of P. annandalei were able to successfully ingest the neonates and adults of Brachionus rotundiformis. After prey detection the copepod swam slowly towards the rotifer, and after reaching to the close proximity, it usually redirected the rotifer through its feeding current and captured the prey entrained in the feeding appendages.
For both males and females, the hightest probability of ingestion was recorded for the neonates (newborn, recently hatched) followed by non-ovigerous and ovigerous B. rotundiformis respectively.
Females and males spent respectively 1.6 to 8.2 and 4.0 to 11.0 s in handling a rotifer prey before ingestion. Regardless of prey size and reproduction state of prey, the prey handling time was significantly longer for males than for females. Futhermore, in both females and males of P. annandalei, the prey handling time was the longest for ovigerous followed by non-ovigerous and neonates of B. rotundiformis. |
 Issued from : S.-H., C.-H. Lee, H.-U. Dahms & J.-S. Hwang in J. Crust. Biol., 2008, 28 (3). [p.581, Fig. 1, A, B]. Issued from : S.-H., C.-H. Lee, H.-U. Dahms & J.-S. Hwang in J. Crust. Biol., 2008, 28 (3). [p.581, Fig. 1, A, B].
Homosexual mating in Pseudodiaptomus annandalei. A, male using its P5 to transfer a spermatophore to another conspecific male; B, spermatophore attached to male P5. |
 Issued from : P. Lehette, S.M. Ting, L;-L. Chew L.-L. & V.C. Chong inJ. Plankton Res., 2016, 38 (3). [p.459, Fig.1]. Issued from : P. Lehette, S.M. Ting, L;-L. Chew L.-L. & V.C. Chong inJ. Plankton Res., 2016, 38 (3). [p.459, Fig.1].
Ln-transformed Rind (µl O2per ind. per h) versus mean body mass ( µg DW: dry weiht) for P. annandalei (West Malysia Peninsula) reared at 28¨C (filled circles) and acclimated at 24-36°C (open circles) excluding 28°C.
Each point represents the mean individual body mass.
Lines represent the fitted geometric regression model between Rind and DW for each treatment to obtain slope, i.e. b1 and b2 used as a proxy for the standardization of Rsp to a mean individual body mass of 10 µg.
Dw was estimated from the prosome length.
To obtain the weight-specific respiration rate (Rsp) from the respiration rate per individual (Rind), the dry weight (DW, µg) was estimated from the PL measurements-, based on the regression equation provided by Rayner & al (2015). As the body size is of prime importance factor that could affect respiration rates, the authors investigated its possible effect on Rsp by relating the variables using allometric equation expressed as Rind = aDW power b, where a and b are constants calculred from the double Ln-transformed regression model.. All the weight-specific rates (Rsp) were standardized to an equivalent individual biomass of 10 µg based on the aforementioned allometric equation relating metabolic rate of body size (Ivleva, 1980à.
For each experiment, the temperature coefficient was determined following Van't Hoff's Q10 (Ikeda, 1985). |
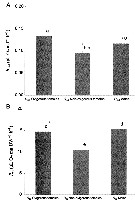 Issued from : P. Lehette, S.M. Ting, L;-L. Chew L.-L. & V.C. Chong inJ. Plankton Res., 2016, 38 (3). [p.460, Fig.2]. Issued from : P. Lehette, S.M. Ting, L;-L. Chew L.-L. & V.C. Chong inJ. Plankton Res., 2016, 38 (3). [p.460, Fig.2].
Rind (A) and Rsp (B) of P. annandalei for ovigerous females, non-ovigerous females and males and dashed lines represent SE.
Individual DW (dry weight) was, respectively 4.46 ± 0.15, 7.56 ±0.30 and 7.67 ± 0.32 mg for males, non-ovigerous frmales and females with ovisacs.
Rsp are standardized to an individual DW of 10 µg.
Homogeneous groups are indicated by same letters above histograms (Duncan's multiple range test, P>0.05).
Copepods originally collected from the Terusan channel, W Malaysia (4/881°N, 100.567°E), Copepods reared with almost pure cultures of Skeletonema costatum at temperature regime 28-31°C. |
 Issued from : P. Lehette, S.M. Ting, L;-L. Chew L.-L. & V.C. Chong inJ. Plankton Res., 2016, 38 (3). [p.460, Table I]. Issued from : P. Lehette, S.M. Ting, L;-L. Chew L.-L. & V.C. Chong inJ. Plankton Res., 2016, 38 (3). [p.460, Table I].
Summary of the respiration rates Rind by Pseudodiaptomus sp, measured by a Clark-type oxygen electrode during laboratory experiments with no fasting period prior to experiments. |
| | | | Loc: | | | India ( Trivandrum coast, Mandapam, Bombay, off Muttukadu & Ennore Station, Kerala, Goa, Madras, Burhabalanga estuary, Chilka Lake, Mandarmani creek, Hooghly estuary, Salt Lakes, Lake Kolleru, Porto Novo), W Malaysia (Terusan Channel: 4.9°N, 100.57E), W Malay Peninsula (Andaman Sea), G. of Thailand, Indonesia, Java (Cilacap Bay, off Tegal), Philippines, China Seas (South China Sea , Zhanjiang, East China Sea), Taiwan, Tungkang coastal pond, Danshuei estuary, Australia | | | | N: | 40 | | | | Lg.: | | | (83) F: 1,18; M: 1,09; (290) F: 1,05-1,2; M: 1-1,1; (530) F: 1,2; (795) F: 1,2; M: 1; (863) F: 1,22-1,26; M: 1,05-1,13; (1005) F: 1,20-1,38; M: 1,02-1,04; (1058) F: 1,13-1,46; M: 1,05-1,11; (1301) F: 1,05-1,28; M: 1,0-1,1; (1122) F: 1,15; M: 1,05; (1193) F: 1,21-1,31; M: 1,04-1,15; {F: 1,05-1,46; M: 1,00-1,15} | | | | Rem.: | dulçaquicole & saumâtre, estuaire-néritique. Salinity: 10-31 p.1000.
Incomplete data.
Voir aussi les remarques en anglais | | | Dernière mise à jour : 07/02/2020 | |
|
|
 Toute utilisation de ce site pour une publication sera mentionnée avec la référence suivante : Toute utilisation de ce site pour une publication sera mentionnée avec la référence suivante :
Razouls C., Desreumaux N., Kouwenberg J. et de Bovée F., 2005-2026. - Biodiversité des Copépodes planctoniques marins (morphologie, répartition géographique et données biologiques). Sorbonne Université, CNRS. Disponible sur http://copepodes.obs-banyuls.fr [Accédé le 13 février 2026] © copyright 2005-2026 Sorbonne Université, CNRS
|
|
 |
 |