|
|
 |
Fiche d'espèce de Copépode |
|
|
Calanoida ( Ordre ) |
|
|
|
Diaptomoidea ( Superfamille ) |
|
|
|
Acartiidae ( Famille ) |
|
|
|
Acartia ( Genre ) |
|
|
|
Odontacartia ( Sous-Genre ) |
|
|
| |
Acartia (Odontacartia) spinicauda Giesbrecht, 1889 (F,M) | |
| | | | | | | Syn.: | no Acartia spinicauda : Wellershaus, 1969 (p.275, figs.M); ? Mori, 1937 (1964) (p.103, figs.F); ? Chen & Zhang, 1965 (p.114, figs.F, Syn. part.)
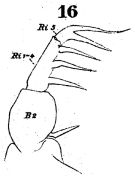
? Acartia (O.) pacifica female : Wellershaus, 1969 (p.275, figs.F) | | | | Ref.: | | | Giesbrecht, 1892 (p.508, 523, 770, figs.F,M); Giesbrecht & Schmeil, 1898 (p.155); A. Scott, 1909 (p.188, Rem.); Sewell, 1912 (p.354, 377); 1914 a (p.241); Steuer, 1923 (p.27, figs.F,M); Sewell, 1924 (p.789); 1932 (p.397); 1933 (p.28); 1934 (p.81); Kasturirangan, 1963 (p.61, 63, figs.F,M); Kos, 1972 (Vol.I, figs.F,M, Rem.); Goswami & Goswami, 1973 (p.242, fig.1, karyotype); Abraham, 1976 (p.77, 79, fig.M); Yoo & Hue, 1983? (p. 11, figs. F,M); Zheng Zhong & al., 1984 (1989) (p.258, figs.F,M; Srinui & al., 2019 (p.89, 90, Rem.: F,M); Lee S. & al., 2019 (p.86, Table 3). |  issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.43, Fig.11]. Male: 11, thoracic segment 5 and urosome (dorsal).
|
 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.30, Fig.16] Male: 16, Mxp (distal portion).
|
 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.30, Fig.21]. Female: 21, P5 (posterior view).
|
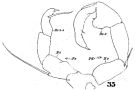 issued from : W. Giesbrecht in Fauna Flora Golf. Neapel, 1892, 19. [Taf.30, Fig.35]. Male: 35, P5.
|
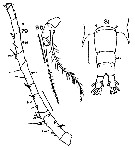
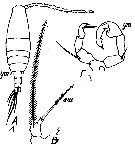
 issued from : T. Mori in The pelagic Copepoda from the neighbouring waters of Japan, 1937 (2nd edit., 1964). [Pl.50, Figs.5-7]. With doubt. Female: 5, habitus (dorsal); 6, P5; 7, urosome (dorsal). Nota: Rostal filaments present. lateral angles of the last thoracic segment produced into the pointed processes. Genital segment with 2 spines which are smaller than those of the following segment. Caudal rami about 3 times as long as wide. A1 with spinules on the proximal segments. Terminal segment of P5 filamentous, and swelled at the base
|
 issued from : S. Abraham in Crustaceana, 1976, 30 (1). [p.75, Fig.17]. Male: 17, right A1 (a portion showing spines on segment distal to geniculation)).
|
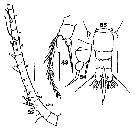
 Issued from : S. Wellershaus in Veröf. Inst. Meeresf. Bremerhaven, 1969, XI. [p. 274, Fig. 79-81]. Female (from Cochin Backwater and outlet, W Thevara): 79, right A1 (dorsal; hook like spine on the back, spines black, an additional small spine lirs on the distal posterior margin of segment 19, c = spine common in many species); 80, P5 (dorsal); 81, urosome (dorsal). Nota: - Ratio prosome : urosome 3.3. - Urosomal segment 1-3 bears son the ventral side groups of long hairs; urosomal segment 5 (= anal somite) shorter hairs. - A1 bears a small spine on the distal end of the posterior side of segment 19 (closely related species A. pacifica bears no such spines on A1. Remarks: The spines on urosomal;segment 4 are of different size in various specimens and can be considerably longer than in fig. 81. Also, A1 differs slightly from the specimens from China (Steuer, 1923).
|
 Issued from : S. Wellershaus in Veröf. Inst. Meeresf. Bremerhaven, 1969, XI. [p. 274, Fig. 82-85]. As Acartia (Odontacartia) pacifica Female. Doubtful. Female (from Cochin Backwater and outlet, W Thevara): 82, right A1 (c = as in A. spinicauda); 83-84, P5 (two aspects); 85, posterior part of thoracic segment 5 and urosome (dorsal) Nota: - Ratio prosome : urosome 3.3 (or more in other specimens). - Proportions of the urosomal segments + caudal rami 36 : 16 : 14 : 34 = 100. - Proportions of the caudal setae (in % of the urosome length: Si = 60; St1 = 111; St2 = 178; St3 = 109; St4 = 76; Se = 40. - A1 is stronger in the proximal portion yjan in other Acartia species; segment 19 is barev of spines; segment 2-6 to 13 seem not completely separated. - Rostral horns dagger-like in profile (as in A. centrura female).
|
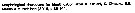 Remark: Erratum concerning the name: read Srinui and not Siruani. Female: 1 - Genital double-somite having paired posterodorsal processes. 2 - 2nd segment of A1 with strong curved processes posteriorly. 3 - 1st Segment of A1 lacking processes. Male: 1 - Urosomite 3 with large spine-like peocesses dorsally. 2 - Dorsal processes of urosomite 3 long, reaching half-length of anal somite. 3 - Urosomite 4 with 4 spine-like processes between pair of dorsal processes.
|
 Issued from : M.C. Kos in Field guide for plankton. Zool Institute USSR Acad., Vol. I, 1972. After Stueur, 1923. Female: 1, habitus (dorsal); 2, P5. Male: 3, P5.
|
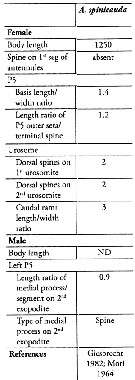 Issued from : S. Lee, H.Y. Soh & W. Lee in ZooKeys, 2019, 893. [p.84, Table 3]. Acartia (Odontacartia) spinicauda: Morphological characters. Compare with other Odontacartia species. Nota: 1 - Absence of spine on 1st to 2nd segments of female A1 .....2. 2 - Spine present on dorsal surface of female genital double-somite ..... 3. 3 - Dorsal surface of female genital bouble-somite and 2nd urosomite with 2 strong spines .....4. 4 - Female P5 outer seta longer than terminal spine ..... 5. 5 - Length-width ratio of female caudal rami as 3; medial process on 2nd exopodite male left P5 as spine.
| | | | | Ref. compl.: | | | Carl, 1907 (p.17); Sewell, 1948 (p.324); Yamazi, 1958 (p.153, Rem.); Itoh, 1970 (tab.1); Subbaraju & Krishnamurphy, 1972 (p.25, 26 ); Patel, 1975 (p.660); Chen Q-c, 1980 (p.794); Stephen, 1984 (p.161, Distribution vs thermocline & geographic); Guangshan & Honglin, 1984 (p.118, tab.); Madhupratap & Haridas, 1986 (p.105, tab.2); Sarkar & al., 1986 (p.178); Mitra & al., 1990 (fig.3); Dai & al., 1991 (tab.1); Yoo & al., 1991 (p.261); Gajbhiye & al., 1991 (p.188); Gajbhiye & Abidi, 1993 (p.137); Godhantaraman, 1994 (tab.5, 6, 7); Shih & Young, 1995 (p.66); Ramaiah & al., 1996 (p.3); Marcus, 1996 (p.143, as spinacauda); Ramaiah & Nair, 1997 (tab.1); Park & Choi, 1997 (Appendix); Mauchline, 1998 (tab.8, 40); Nair & Ramaiah, 1998 (p.272, fig.4); Achuthankutty & al., 1998 (p.1, Table 2, fig.5, 6, seasonal abundance vs monsoon); Dalal & Goswami, 2001 (p.22, fig.2); Rainbow & Wang, 2001 (p.240); Rezai & al., 2004 (p.489, tab.2, p.495, tab.8); ? Zuo & al., 2006 (p.163: tab.1); Hwang & al., 2006 (p.943, tab.I); Dur & al., 2007 (p.197, Table IV); Madhu & al., 2007 (p.54, Table 4, abundance vs monsoon); Rakhesh & al., 2008 (p.154, abundance vs stations); Jerling, 2008 (p.55, Tabl.1); Fernandes, 2008 (p.465, Tabl.2); Perumal & al., 2008 (p.149, abundance vs hydrographic parameters); Tseng L.-C. & al., 2008 (p.153, Table 2, fig.5, occurrence vs geographic distribution, indicator species); Jiang Z.-B. & al., 2009 (p.196, Table 1, 2); W.-B. Chang & al., 2010 (p.735, Table 2, abundance); Shanthi & Ramanibai, 2011 (p.132, Table 1); Maiphae & Sa-ardrit, 2011 (p.641, Table 2); Chew & Chong, 2011 (p.127, fig.4, 5, Table 2, 3, abundance vs location); Zhang D. & al., 2011 (p.86, pH effects); Yoshida & al., 2012 (p.644, fig.1, 3, Table 1, egg development time and hatching vs temperature); Johan & al., 2012 (p.647, Table 1, 2, fig.2, salinity range); Jose & al., 2012 (p.20, fig.3 a,b,c: % vs monsoon); Beyrend-Dur, 2013 (p.771, fig.6: sex ratio, composition); Jagadeesan & al., 2013 (p.27, Table 3, 4, 6, fig.11, seasonal abundance); Anjusha & al., 2013 (p.40, Table 3, abundance & feeding behavior); Varadharajan & Soundarapandian, 2013 (p.2: occurrence vs stations); Rakhesh & al., 2013 (p.7, Table 1, 4, abundance vs stations); Trottet & al., 2017 (p. 7, Table 3: resting stage) | | | | NZ: | 6 | | |
|
Carte de distribution de Acartia (Odontacartia) spinicauda par zones géographiques
|
| | | | | | | | |  Carte de 1996 Carte de 1996 | |
 issued from : C.T. Achuthankutty, N. Ramaiah & G. Padmavati in Pelagic biogeography ICoPB II. Proc. 2nd Intern. Conf. Final report of SCOR/IOC working group 93, 9-14 July 1995. Workshop Report No. 142, Unesco, 1998. [p.8, Fig.6]. issued from : C.T. Achuthankutty, N. Ramaiah & G. Padmavati in Pelagic biogeography ICoPB II. Proc. 2nd Intern. Conf. Final report of SCOR/IOC working group 93, 9-14 July 1995. Workshop Report No. 142, Unesco, 1998. [p.8, Fig.6].
Salinity ranges for A. spinicauda in coastal and estuarine waters of Goa (India).
Shaded area indicates the range of higher abundance. |
 Issued from : T. Yoshida, C.-F. Liong, A.M. Majid, T. Toda & B.H.R. Othman in Zoological Studies, 2012, 51 (5). [p.651, Fig.3]. Issued from : T. Yoshida, C.-F. Liong, A.M. Majid, T. Toda & B.H.R. Othman in Zoological Studies, 2012, 51 (5). [p.651, Fig.3].
Geographical records of A. pacifica, A. spinicauda, A. erythraea occurrences throughout the East Asian region. |
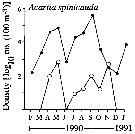 issued from : C.T. Achuthankutty, N. Ramaiah & G. Padmavati in Pelagic biogeography ICoPB II. Proc. 2nd Intern. Conf. Final report of SCOR/IOC working group 93, 9-14 July 1995. Workshop Report No. 142, Unesco, 1998. [p.6, Fig.5]. issued from : C.T. Achuthankutty, N. Ramaiah & G. Padmavati in Pelagic biogeography ICoPB II. Proc. 2nd Intern. Conf. Final report of SCOR/IOC working group 93, 9-14 July 1995. Workshop Report No. 142, Unesco, 1998. [p.6, Fig.5].
Monthly occurrence of Acartia spinicauda in coastal (black circle) in front of Goa and and the Mandovi estuary (clear circle). |
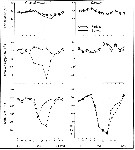 issued from : C.T. Achuthankutty, N. Ramaiah & G. Padmavati in Pelagic biogeography ICoPB II. Proc. 2nd Intern. Conf. Final report of SCOR/IOC working group 93, 9-14 July 1995. Workshop Report No. 142, Unesco, 1998. [p.3, Fig.2]. issued from : C.T. Achuthankutty, N. Ramaiah & G. Padmavati in Pelagic biogeography ICoPB II. Proc. 2nd Intern. Conf. Final report of SCOR/IOC working group 93, 9-14 July 1995. Workshop Report No. 142, Unesco, 1998. [p.3, Fig.2].
Hydrography of the coastal station in front of Goa and Mandovi estuarine station (W India).
Nota: The seasons are arbitrarily classified into the southwest monsoon (June-September), postmonsoon (October-January) and premonsoon (February-May). |
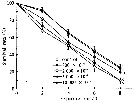 Issued from : D. Zhang, S. Li, G. Wang & D. Guo in Acta Oceanol. Sin., 2011, 30 (6). [p.89, Fig. 1 c]. Issued from : D. Zhang, S. Li, G. Wang & D. Guo in Acta Oceanol. Sin., 2011, 30 (6). [p.89, Fig. 1 c].
Influence of seawater acidification on survival rate in Acartia spinicauda.
Average pH of each pCO2 seawater culture. Control (380) 8.16-8.17; (800) : 7.84-7.85; (2000): 7.39-7.37; (5000): 7.19-7.24; (10 000): 6.92-6.94. |
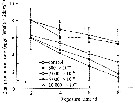 Issued from : D. Zhang, S. Li, G. Wang & D. Guo in Acta Oceanol. Sin., 2011, 30 (6). [p.90, Fig. 2 c]. Issued from : D. Zhang, S. Li, G. Wang & D. Guo in Acta Oceanol. Sin., 2011, 30 (6). [p.90, Fig. 2 c].
Influence of seawater acidification on egg producton rate in Acartia spinicauda.
Average pH of each pCO2 seawater culture. Control (380) 8.16-8.17; (800) : 7.84-7.85; (2000): 7.39-7.37; (5000): 7.19-7.24; (10 000): 6.92-6.94. |
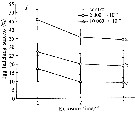 Issued from : D. Zhang, S. Li, G. Wang & D. Guo in Acta Oceanol. Sin., 2011, 30 (6). [p.91, Fig. 3 a]. Issued from : D. Zhang, S. Li, G. Wang & D. Guo in Acta Oceanol. Sin., 2011, 30 (6). [p.91, Fig. 3 a].
Influence of seawater acidification on egg hatching success in Acartia spinicauda.
Average pH of each pCO2 seawater culture. Control (380) 8.16-8.17; (2000): 7.39-7.37; (10 000): 6.92-6.94.
Data are described as mean ± SD (n = 3). Different letters indicate a significant difference among elevated pCO2 levels at p < 0.05. |
| | | | Loc: | | | S Korea, Japan, Tanabe Bay, Hong-Kong, Taiwan (N), Taiwan Strait (Amoy), Taiwan (S, Danshuei Estuary), Viet-Nam ( Cauda Bay), China Seas (Yellow Sea, East China Sea, South China Sea, Xiamen Harbour], Arabian Sea, Sri Lanka, India (Mangalore coast, G. of Manaar, Palk Bay, Pointcalimere-Manamelkudi, Saurashtra coast, W, Bombay, Mandovi-Zuari estuary, Kerala, Goa, S, Burhabalanga estuary,Porto Novo, Godavari region, Kakinada Bay, Chilka Lake, Hooghly estuary, Mandarmani, Parangipettai coast), S South Africa (Richard's Bay Harbour and Mhlathuze Estuary), Oman Sea, Nicobar Is., Bay of Bengal, Burma, Kurau Riv., Straits of Malacca, Sangga estuary, Singapore, Perai River Estuary, Indonesia-Malaysia, Ambon Bay (Baie d'Amboine), Pacif. (W equatorial) | | | | N: | 65 | | | | Lg.: | | | (46) F: 1,25; M: 1,17; ? (91) F: 1,25; (164) F: 1,25; M: 1,17; {F: 1,25; M: 1,17} | | | | Rem.: | ± saumâtre, estuaire-néritique.
Les localisations dans les mers de Chine et la Corée semblent confirmées.
Voir aussi les remarques en anglais | | | Dernière mise à jour : 03/12/2020 | |
|
|
 Toute utilisation de ce site pour une publication sera mentionnée avec la référence suivante : Toute utilisation de ce site pour une publication sera mentionnée avec la référence suivante :
Razouls C., Desreumaux N., Kouwenberg J. et de Bovée F., 2005-2026. - Biodiversité des Copépodes planctoniques marins (morphologie, répartition géographique et données biologiques). Sorbonne Université, CNRS. Disponible sur http://copepodes.obs-banyuls.fr [Accédé le 07 février 2026] © copyright 2005-2026 Sorbonne Université, CNRS
|
|
 |
 |


















