|
|
 |
Fiche d'espèce de Copépode |
|
|
Calanoida ( Ordre ) |
|
|
|
Calanoidea ( Superfamille ) |
|
|
|
Calanidae ( Famille ) |
|
|
|
Neocalanus ( Genre ) |
|
|
| |
Neocalanus flemingeri Miller, 1988 (F,M) | |
| | | | | | | Syn.: | Calanus sp. Sato, 1913;
Calanus tonsus : Campbell, 1934 (figs.7 b,c); Brodsky, 1938; 1948; 1950 (1967) (figs.F); Tanaka, 1954 (figs.1,4); 1956 (part., fig.22:F);
Calanus plumchrus : Yamada, 1938; Mori, 1937 (1964) (part., p.15, Pl.3: figs.5, 7: Juv.F); Brodsky, 1972 (1975) (part., figs.28 f, 57 Dt., 73 a,b,f); Kos, 1972 (1975) (part., figs.24 A, C, 25 A, C, 27 A, C); Brodsky & al., 1983 (part., fig.79); Calanus tonsus typica : Brodsky, 1950 (1967) (part., p.91);
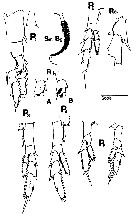
Calanus tonsus : Tanaka, 1954 (p.30) | | | | Ref.: | | | Miller, 1988 (1989) (p.232, figs.F,M, Rem.); Chihara & Murano, 1997 (p.740, Pl.68: F,M); Bucklin & al., 1999 (p.239, molecular systematic); Goldblatt & al., 1999 (p.2619, tabl.1, 2); Heinrich, 2000 (p.1020, figs. juv.); Bucklin & al., 2003 (p.335, tab.2, fig. 2, Biomol.); Machida & al., 2006 (p.1071, tab.1,3, figs.3, Rem/ large form and short form, molecular phylogeny); Ohtsuka & Nishida, 2017 (p.575, 577, fig.22.2, Rem.) |  issued from : C.B. Miller in Prog. Oceanog., 1988 (1989), 20. [p.233, Fig.2]. Female (from Subarctic Pacific Ocean): 1-2, habitus (dorsal and lateral, respectively); 3, head (lateral); 4-5, urosome (ventral aspect; showing seminal receptacles and antral opening muscles internally); 6-7, habitus from Bering Sea (dorsal and lateral, respectively); 8, head (enlargement; from the same specimen); 9, urosome (same specimen). Scale bar 2.00 mm in 1, 2, 3, 6, 7; 0.45 mm in 4, 5, 9; 0.90 mm in 3 and 8. 1, 2, 3 are three different specimens.
|
 issued from : C.B. Miller in Prog. Oceanog., 1988 (1989), 20. [p.236, Fig.4]. Female: A1 (left ); A2 (right, medial aspect); Md A, B from two specimens, lateral (palp) and anterior (gnathobase) aspects; Mxi (left); Mx2 (right) and Mxp (medial aspects); P1 (anterior); Sm B2, detail of medial seta on basipodal segment 2 of P1; P2-P5 (right legs, anterior aspect). Scale bar: 0.4 mm in A1, P2-P5; 0.2 mm in A2, Md, P1; 0.1 mm in Sm-B2.
|
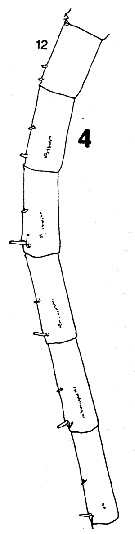 issued from : C.B. Miller in Prog. Oceanog., 1988 (1989), 20. [p.237, Fig.5]. Female: 4, right A1 (segments 12 to 17 (ventral side, showing acuminate denticles in a single row). Nota: Compare with A1 in Neocalanus plumchrus.
|
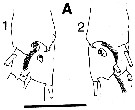 issued from : C.B. Miller in Prog. Oceanog., 1988 (1989), 20. [p.240, Fig.7]. Saccular organ near the base of exopodite segment 1 of P1 (the roughly circular patches at the base of the organ are clearly the termination of a nerve. 1:Female; 2: Male. Nota: Compare with exopodite segment 1 of P1 in Neocalanus plumchrus.
|
 issued from : C.B. Miller in Prog. Oceanog., 1988 (1989), 20. [p.241, Fig.8]. Female: Seminal receptacles and spermatophores; 1 A (ventral); 1 B (lateral); 2, left and rigbt of six specimens (A-F); 3, ventral (A) and antero-lateral (B) views of spermatophore; 4, as (3) for another specimen. Scale bar 0.4 mm.
|
 issued from : C.B. Miller in Prog. Oceanog., 1988 (1989), 20. [p.243, Fig.9]. Male: 1-2, habitus (lateral); 3 A-D, forehead from 4 specimens; 4, comparison of forehead of N. femingeri (dashed line for 3 specimens) to that of N. plumchrus (solid line for 3 specimens); 5, urosome (dorsal); 6, dorso-lateral and dorsal views of male reproductive system; 7, lateral outline of seminal vesicle and spermatophore-forming organ in 5 specimens (A-E); 8, P5 (anterior aspect from 2 specimens A-B); 9, left exopodite segments 2 and 3 (from 5 specimens A-E); 10, left P5(in usual curled position).
Scale bar: 2.4 mm for 7; 2.0 mm for 1 and 2; 1.0 mm for 3 and 4; 0.8 mm for 6; 0.5 mm for 5 and 10; 0.25 mm for 8 and 9.
|
 issued from : C.B. Miller in Prog. Oceanog., 1988 (1989), 20. [p.245, Fig.10]. Male: left A1 (ventral); right A2, Md, Mx1, Mx2, Mxp (medial aspects); left P1 (anterior aspect with detail of exopodite segment 1 (Ri 1) and medial seta of basipodite 2 (Sm B2); left P2-P4 (anterior aspects). Scale bar: 0.4 mm for A1 and P2-P4; o.2 mm for other figures.;
|
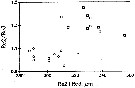 issued from : C.B. Miller in Prog. Oceanog., 1988 (1989), 20. [p.246, Fig.11]. Comparison of proportions (exopodite segment 2/ exopodite segment 3 versus exopodite segments 2 + 3) of the left exopodite of P5 in males of N. femingeri (open circle) and N. plumchrus (square).
|
 issued from : C.B. Miller in Prog. Oceanog., 1988 (1989), 20. [p.263, Fig.21]. Copepodite 5: right A2 (medial aspect); right Md, lateral (palp) and anterior (gnathobase) aspects; right Mx1 and left Mx2 (medial aspects); right Mxp ( medial with detail of lateral side of 2nd segment). Scale bar: 0.2 mm for all figures.
|
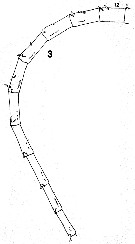 issued from : C.B. Miller in Prog. Oceanog., 1988 (1989), 20. [p.237, Fig.5 (3)]. Copepodite 5: 3, right A1 (segments 12 to 21).
|
 issued from : C.B. Miller in Prog. Oceanog., 1988 (1989), 20. [p.263, Fig.21]. Copepodite 5: P1 (anterior aspect with details showing variants of hair patch on endopodite segment 1 and medial seta on basipodite segment 2 showing setulation); P2 and P4 (anterior aspects of left leg); P3 (anterior aspect of right leg); P5 (anterior and posterior aspects of right leg. Detail of exopodite segment 1 on P2 shows posteriorly curved projection. Scale bar: 0.2 mm for P1 and detail on P2; 0.4 mm for P2 to P4; 0.1 mm for Sm-B2;
|
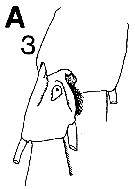 issued from : C.B. Miller in Prog. Oceanog., 1988 (1989), 20. [p.240, Fig.7 (A3)]. Copepodite 5: A3, saccular organ near the base of exopodite 1 of P1.
|
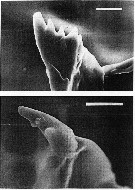 issued from : C.B. Miller in Prog. Oceanog., 1988 (1989), 20. [p.266, Fig.24]. Copepodite 5: scanning electon micrographs of the ventral tooth on the mandibular gnathobase. Above: N. flemingeri, below N. plumchrus. Scale bars are 0.010 mm.
|
 issued from : C.B. Miller in Prog. Oceanog., 1988 (1989), 20. [p.269, Table 10]. Copepodite 5: Comparison of data length and setule spacing of N. flemingeri and N. plumchrus. Values are means for sevenn specimens from Station P (50°N, 145°W).
|
 issued from : C.B. Miller in Prog. Oceanog., 1988 (1989), 20. [p.258, Fig.17]. Copepodite 5: Shape comparison of N. flemingeri (1-4) and N. plumchrus.
1, 5: habitus (lateral view); 2, 6: enlargement of cephalosome; 3, 7: dorsal aspect of urosome; 4, 8: dorsal aspect of urosome showing setation (ribbon setules begin at the small arrow in 8).
Scale bar: 2.0 mm in 1 and 5; 0.67 mm in 2 and 6; 0.38 mm in 3 and 7; 0,67 mm in 4 and 8.
|
 issued from : R.J. Machida, M.U. Miya, M. Nishida & S. Nishida in Mar. Biol., 2006, 148. [p.1077, Table 5]. Comparisons of selected biological characteristics of 6 species plus 1 subspecies of Neocalanus.
|
 issued from : A. Yamaguchi, K. Ohgi, T. Kobari, G. Padmavati & T. Ikeda in Bull. Fish. Sci. Hokkaido Univ., 2011, 61 (1). [p.18, Fig.5 (part.)] Schematic diagram showing life cycle timing (mating, spawning, quiescence or diapause) of Neocalanus flemingeri pacifica, one of five dominant copepods in the Oyashio region, western subarctic Pacific (Site H: 41°30'N-42°30'N, 145°00'E-146°00'E). Compare with Metridia pacifica, Eucalanus bungii, Neocalanus cristatus, and Neocalanus plumchrus.
|
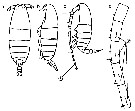 Issued from : M. Chihara & M. Murano in An Illustrated Guide to Marine Plankton in Japan, 1997. [p.747, Pl. 68, fig.85 a-d]. After Miller, 1988. Female: a-b, habitus (dorsal and lateral, respectively); cd, Segments 13-17 of A1. Male: c, habitus (lateral). Numbers show species characteristics.
| | | | | Ref. compl.: | | | Miller & Clemons, 1988 (p.293); Hattori, 1991 (tab.1, Appendix); Miller & al., 1992 (p.389, size variation/long-term serie); Dagg, 1993 (p.163, Table 1, 2, 3, figs., abundance, gut pigment); Kotani & al., 1996 (tab.2); Mauchline, 1998 (tab.58); Heinrich, 1998 b (p.805); Dolganova & al., 1999 (p.13, tab.1); Mackas & Tsuda, 1999 (p.335, Table 1); Saito & Tsuda, 2000 (p.2141, egg production, development, figs. e-f); Yamaguchi & al., 2002 (p.1007, tab.1); Heinrich, 2002 (p.77, life history); Yamaguchi & al., 2004 (p.479, tab.2); Park, W & al., 2004 (p.464, tab.1); Miller C.B., 2005 (p.219, Rem.); Coyle, 2005 (p.77, fig.7, 9, tab.3); Mackas & al., 2007 (p.223, climatic change index); Wilson S.E. & al., 2008 (p.1636, Rem.: p.1645); Kobari & al., 2008 (p.1648, coppodids I-VI, depth distribution); Takahashi & al., 2008 (p.222, Table 2, grazing impact); Galbraith, 2009 (pers. comm.); Chiba & al., 2009 (p.1846, Table 1, occurrence vs temperature change); Hopcroft & al., 2009 (p.9, Table 3); Dagg & al., 2009 (p.716, feeding rates); Mackas & Beaugrand, 2010 (p.300, figs.13); Tatebe & al., 2010 (p.409, Table 1, 2, horizontal transport); Kobari & al., 2010 (p.1703: feeding); Kobari & al., 2010 (p.1703: feeding); Kobari & al., 2010 (p.1715, figs., Table 2, 3, development, growth); Yamaguchi & al., 2010 (p.1679, figs.5, 10, Table 1, population structure); Yamaguchi & al., 2010 (p.1691, figs.2, 7, 10, vertical distribution); Machida & Tsuda, 2010 (p.1: gene sequences, large and small forms); Homma & Yamaguchi, 2010 (p.965, Table 2); Doi & al., 2010 (p.1733, trophic niche); Hopcroft & al., 2010 (p.27, Table 1, 2, fig.9); Matsuno & Yamaguchi, 2010 (p.123, Rem. p.129); Saito & al., 2011 (p.145, abundance, size vs. temperature); Yamaguchi & al., 2011 (p.13, seasonal variation, life history); Homma & al., 2011 (p.29, Table 2, 3, 4, 5, abundance, feeding pattern: suspension feeders); Sato & al., 2011 (p.1230, vertical distribution); Batten & Walne, 2011 (p.1643, Table I, abundance vs. temperature interannual variability); Yoshiki & al., 2011 (p.63, egg production, development vs. pressure); Matsuno & al., 2011 (p.1349, Table 1, abundance vs years); Matsuno & al., 2012 (p.105, Table 2); Takenaka & al., 2012 (p.1669, fig.2, 3, Table 1, bioluminescence); Chiba & al., 2012 (p.63, temporal variation vs regions); Volkov, 2012 (p.474, Table 6, fig.7, 14, abundance, distribution, interannual variation); Kobari & al., 2013 (p.78, Table 2); Liu & al., 2013 (p.271, growth models); Ohashi & al., 2013 (p.44, Table 1, Rem.); Questel & al., 2013 (p.23, Table 3, interannual abundance & biomass, 2008-2010); Yoshiki & al., 2013 (p.993, interannual variations 2001-2009); Eisner & al., 2013 (p.87, Table 3: abundance vs water structure); Chiba S. & al., 2015 (p.968, Table 1: length vs climate); Ershova & al., 2015 (p.100, Rem.: interannual abundance, 1946-2015 as Neocalanus spp. [see in N. plumchrus]); Ohtsuka & Nishida, 2017 (p.565, Table 22.1); Baumgartner & Tarrant, 2017 (p.387, Table 1, fig.4, diapause); Record & al., 2018 (p.2238, Table 1: diapause); | | | | NZ: | 4 | | |
|
Carte de distribution de Neocalanus flemingeri par zones géographiques
|
| | |  issued from : R.J. Machida, M.U. Miya, M. Nishida & S. Nishida in Mar. Biol., 2006, 148. [p.1077, Fig.4]. issued from : R.J. Machida, M.U. Miya, M. Nishida & S. Nishida in Mar. Biol., 2006, 148. [p.1077, Fig.4].
Relationship between the phylogeny and distribution pattern of the Neocalanus species.
Shaded portions of the maps represent distributions based on the litterature. Clades A-E correspond to those in Fig.3, p.1075. |
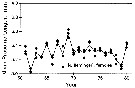 issued from : C.B. Miller, J. Fulton & B.W. Frost in Can. J. Fish. Aquat. Sci., 1992, 49. [p.394, Fig.2]. issued from : C.B. Miller, J. Fulton & B.W. Frost in Can. J. Fish. Aquat. Sci., 1992, 49. [p.394, Fig.2].
Time series of mean lenths for Neocalanus flemingeri in resting phase, in the Gulf of Alaska; at Station "P" (50°N, 145°W.
Each symbol is the mean for a single sample. The overall means for the years (unweighted mean of the sample means) are connected by lines.
Nota: Between-year variation of prosome length is significantly greater than the within-year variation, implying similarity of year-to-year variation in growth conditions over an indeterminate, subregional scale. |
 Issued from : A. Yamaguchi, K. Ohgi, T. Kobari, G. Padmavati & T. Ikeda in Bull. Fish. Sci. Hokkaido Univ., 2011, 61 (1). [p.20, Fig.6 (part.)]. Issued from : A. Yamaguchi, K. Ohgi, T. Kobari, G. Padmavati & T. Ikeda in Bull. Fish. Sci. Hokkaido Univ., 2011, 61 (1). [p.20, Fig.6 (part.)].
Schematic diagram showing life cycle timing (mating, spawning, quiescence or diapause) of Neocalanus flemingeri, one of five dominant copepods in the subarctic Pacific (except Oyashio region, western subarctic Pacific (Site H: See Fig.5).
Compare with Metridia pacifica, Eucalanus bungii, Neocalanus cristatus and Neocalanus plumchrus. |
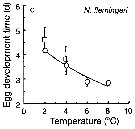 Issued from : T. Yoshiki, T. Ono, A. Shimizu & T. Toda in Mar. Ecol. Prog. Ser., 2011, 430. [p.66, Fig. 1c]. Issued from : T. Yoshiki, T. Ono, A. Shimizu & T. Toda in Mar. Ecol. Prog. Ser., 2011, 430. [p.66, Fig. 1c].
Average (±SEM) egg development times from the Neocalanus cristatus in the Oyashio region of the Western Subarctic Pacific in January 2006 and January 2007 (o) and from Saito & Tsuda (2000) (suare) under atmospheric pressure and different temperatures.
Egg development times significantly descreased with increasing temperature. |
 Issued from : T. Yoshiki, T. Ono, A. Shimizu & T. Toda in Mar. Ecol. Prog. Ser., 2011, 430. [p.65, Table 1]. Issued from : T. Yoshiki, T. Ono, A. Shimizu & T. Toda in Mar. Ecol. Prog. Ser., 2011, 430. [p.65, Table 1].
Neocalanus flemingeri: Spawning interval, clutch size, and egg hatching success in the Oyashio region of the Western Subarctic Pacific in January 2006 and January 2007, and from Saito & Tsuda (2000).
d: day; na: data not available.
Compare with N. cristatus, N. plumchrus. |
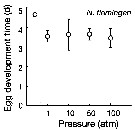 Issued from : T. Yoshiki, T. Ono, A. Shimizu & T. Toda in Mar. Ecol. Prog. Ser., 2011, 430. [p.66, Fig. 2c]. Issued from : T. Yoshiki, T. Ono, A. Shimizu & T. Toda in Mar. Ecol. Prog. Ser., 2011, 430. [p.66, Fig. 2c].
Average (±SEM) egg development time under pressures from 1 to 100 atm at 4°C to Neocalanus flemingeri collected in the Oyashio region of the Western Subarctic Pacific in January 2006 and January 2007.
Differences in egg development time between pressure conditions were not statistically significant. |
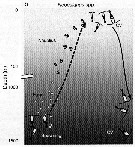 Issued from : T. Yoshiki, T. Ono, A. Shimizu & T. Toda in Mar. Ecol. Prog. Ser., 2011, 430. [p.69, Fig.5b]. Issued from : T. Yoshiki, T. Ono, A. Shimizu & T. Toda in Mar. Ecol. Prog. Ser., 2011, 430. [p.69, Fig.5b].
Neocalanus spp. (N. cristaus, N. plumchrus, N. flemingeri) collected in the Oyashio region of the Western Subarctic Pacific in January 2006 and January 2007. Depth ranges experienced during early life stages (from egg to nauplius; dotted line). |
 Issued from : H. Saito & A. Tsuda in Deep-Sea Res. I, 47. [p.2146, Table I]. Issued from : H. Saito & A. Tsuda in Deep-Sea Res. I, 47. [p.2146, Table I].
Egg diameter, carbon and nitrogen contents and prosome length (PL) of females of Neocalanus flemingeri from 42°00' N, 145°00' E. (Oyashio region, western subarctic Pacific). In incubators at 2°C and 4°C, also 6°C; salinity: 32.0-33.5 psu.
Nota: Compare with N. cristatus and N. plumchrus and others species in genus Calanus. |
 Issued from : H. Saito & A. Tsuda in Deep-Sea Res. I, 47. [p.2146, Table 2]. Neocalanus flemingeri: Issued from : H. Saito & A. Tsuda in Deep-Sea Res. I, 47. [p.2146, Table 2]. Neocalanus flemingeri:
Mean ± 1 S.E. of prosome length (mm), carbon and nitrogen contents (µg), and C:N ratio. Values in parenthesis give the range.
Compare with N. plumchrus and N. cristatus. |
 Issued from : H. Saito & A. Tsuda in Deep-Sea Res. I, 47. [p.2149, Table 4]. Neocalanus flemingeri: Issued from : H. Saito & A. Tsuda in Deep-Sea Res. I, 47. [p.2149, Table 4]. Neocalanus flemingeri:
Egg hatching time (days: mean ± 1 S.E.) at different temperature and egg hatching success (%) of Neocalanus spp.
ND: mean no data.
Compare with N. plumchrus anxd N. cristatus.
Nota: Developmental times of hatching -C1 and CI-CV were estimated to be 38 and 49 days at 2°C. In the Oyashio region , many CII and CIII stages were observed in December, but the CV stage were observed until April (Tsuda & al., 1999). This suggests that N. flemingeri has different relative developmental time compared to others species, or the development does not follow the equiproportional rule of development (see Corkett & al., 1986 showed that the equiproportional rule of development was appropriate for Calanus spp., and relative times of reaching various stages between hatchung and molting to CI were similar among species). |
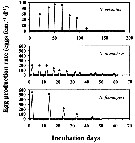 Issued from : H. Saito & A. Tsuda in Deep-Sea Res. I, 47. [p.2147, Fig. 3]. Issued from : H. Saito & A. Tsuda in Deep-Sea Res. I, 47. [p.2147, Fig. 3].
Typical egg laying pattern comparison between Neocalanus cristatus (top), N. plumchrus (middle) and N. flemingeri (bottom).
Open circles mean the day of death.
Nota: N. flemingeri clutch size was the largest of the three Neocalanus species studied, but intervals between clutches were intermediate.
The first two clutches were largest with clutch size decreasing as spawning proceeded.
The maximum clutch size observed was 687 eggs and the mean ± 1 S.E. of the largest clutch size of each female that produced more than 4 clutches was 443 ± 133 eggs.
The maximum clutch number was 6, and the maximum fecundity was 1398 eggs per female.
The clutch number and the fecundity of a single female with ''developing'' ovaries (Miller & Clemons, 1988) in the beginning of the experiment were 4 and 1043 eggs per female.
Mean fecundity of females that produced more than 4 clutches was 924 ± 346 eggs per female.
Total carbon and nitrogen production as eggs per female were 456 µg C and 49 µg N.
The intervals between clutches were more or less constant, and the mean ± 1 S.E. were 9.3 ± 1.8 days at 2°C and 8.2 ± 2.1 days at 4°C.
The egg laying period calculated from the mean interval and the maximum clutch number was 47 days ay 2°C and 42 days at 4°C.
Females producing more than 4 clutches laid 65-86% of their eggs in the largest 2 clutches, that is, within 9-11 days.
After the final egg laying, spent females of N. plumchrus and N. flemingeri were alive for 6-35 days. Spent N. cristatus stayed alive up to 115 days. |
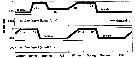 Issued from : S. Ohtsuka & S. Nishida in Species diversity of Annuals in Japan Tokyo , eds MotokawaM. & Kajihara H., 2017, Springer/ [p.584, Fig. 22.6]. Issued from : S. Ohtsuka & S. Nishida in Species diversity of Annuals in Japan Tokyo , eds MotokawaM. & Kajihara H., 2017, Springer/ [p.584, Fig. 22.6].
Ontogenic migration and biennial life cycle of the oceanic calanoid Neocalanus flemingeri (small and large forms) in the Oyashio region.
Phytoplankton bloom occurred in April and May. C1-C5 first to fifth copepodid stages.
After A. Tsuda, H. Saito & H. Kasai, 2001.
Compare with Neocalanus plumchrus for the same region. The two sibling species exhibit different annual life cycles in the North Pacific (Tsuda & al., 1999) and the Sea of Japan (Miller & Terazaki, 1989).
After Ohtsuka & Nishida (2017, p.577), this species exhibit biennial life cycles in the Oyashio region. |
| | | | Loc: | | | Arct. (Chukchi Sea, off NW Alaska), N Pacif. (sub-Arct.), Japan Sea, Japan (E, NE), S, SE Hokkaido (Ishikari Bay), Oyashio Region, Station H (41°31'N, 145°50'E), E Kuril Is., E Kamchatka, Okhotsk Sea, Bering Sea, Station K2 (47°N, 160°E), S Aleutian Basin, S Aleutian Is., Station Knot, Station "P" *; ''R'' *, G. of Alaska (Icy Strait), Vancouver Is.
* Ocean Weather Station "P": 50°N, 145°W; ''R'': 53°N, 145°W. | | | | N: | 61 | | | | Lg.: | | | (125) F: 6,32-4,83; M: 4,82-4,4; (127) F: 4,9-4,18; M: 4,73-3,75; (866) F: 4,2-5,2; M: 4,2-4,6; {F: 4,18-6,32; M: 3,75-4,82}
Chiba S. & al., 2015 (p.971, Table 1: Total length female (June-July) = 4.7 mm [optimal SST (°C) = 4.7]. | | | | Rem.: | Des confusions existent entre cette espèce et N. plumchrus , N. tonsus
Voir aussi les remarques en anglais | | | Dernière mise à jour : 14/06/2022 | |
|
|
 Toute utilisation de ce site pour une publication sera mentionnée avec la référence suivante : Toute utilisation de ce site pour une publication sera mentionnée avec la référence suivante :
Razouls C., Desreumaux N., Kouwenberg J. et de Bovée F., 2005-2025. - Biodiversité des Copépodes planctoniques marins (morphologie, répartition géographique et données biologiques). Sorbonne Université, CNRS. Disponible sur http://copepodes.obs-banyuls.fr [Accédé le 04 décembre 2025] © copyright 2005-2025 Sorbonne Université, CNRS
|
|
 |
 |

























