|
|
 |
| | | Calanoida Sars, 1902 ( Neocopepoda, Gymnoplea ) ( Classification and Phylogeny  ) ) | | Syn.: | Gymnoplea Giesbrecht, 1892 (p.41); Giesbrecht & Schmeil, 1898 (p.6, 7); Sars, 1901 a (1903) (p.2); Esterly, 1905 (p.113); van Breemen, 1908 a (p.5); Rose, 1933 a (p.17); Mori, 1937 (1964) (p.8); Kabata, 1979 (p.47, 49); Dudley, 1986 (p.10); Staborogatov, 1986 (p.1778); Huys & Boxshall, 1991 (p.316, 403, 419) | | Ref.: | Sars, 1902 (p.4); Gurney, 1931 a (p.82); Wilson, 1932 a (p.18, 540, clé G.); Rose, 1933 a (p.17); Brodsky, 1950 (1967) (p.79); Andronov, 1974 a (p.1002 & suiv.); Kabata, 1979 (p.48, 49); Bowman & Abele, 1982 (p.9); Marcotte, 1982 (p.188); 1986 (p.187); Brodsky & al., 1983 (p.85,139); Dudley, 1986 (p.7); Park,1986 (p.191); Boxshall, 1986 (p.204); Björnberg, 1986 (p.53, 232, Rem.N); Mauchline, 1988 (p.698); J.-S. Ho, 1990 (p.528); Huys & Boxshall, 1991 (p.48, 403, 406, 418, 419, Rem.); Mauchline, 1998 (p.51); Bradford-Grieve & al., 1999 (p.891, 901, Families Key); Boxshall & Halsey, 2004 (p.3; 37; 49; 12: families Key; p.38: Super-families Key); Vives & Shmeleva, 2007 (p.138, Families key.); Vives & Shmeleva, 2010 (p.27, 138 : Rem., families key); Bradford-Grieve & al., 2010 (p.291, phylogeny, families cladistic analysis)
For Bradford-Grieve & al., 2014 ( p.507, p.527: fig.12 phylogram of families); Renz & al., 2018 (p.24, Fig.15: Phylogeny); Laakmann & al., 2019 (p.330, figs. 2, 3, phylogenetic relationships); Hirai & al., 2020 (p.1, Fig.4: metabarcoding, Fig.8: OTUs distribution patterns) | | Rem.: | Andronov (1974 a) established 9 Superfamilies including the Platycopioidea. Park (1986) estimates that the Spinocalanidae and the Epacteriscidae should be erected to the rank of superfamily. The Platycopiidae being isolated in a different Order, we count 9 superfamilies of which certain have changed denomination for the reason of priority rule (Andronov, 1991, p.133): Arietelloidea [= Augaptiloidea (part.)], Bathypontioidea , Calanoidea [= Megacalanoidea], Clausocalanoidea [= Pseudocalanoidea (part.)], Diaptomoidea [= Centropagoidea],, Eucalanoidea , Pseudocyclopoidea (Epacteriscoidea included in this family by Bradford-Grieve & al., 2014), Ryocalanoidea , Spinocalanoidea.
Fosshagen & Iliffe, 2004 a (p.353) and Boxshall & Hasley (2004) do not recognize the superfamily of the Fosshagenioidea. These superfamilies are defined in Boxshall & Hasley (2004, p.38).
Ferrari & Ueda (2005, p.333) following the International Code for Zoological Nomenclature (ICZN, 1985) restore the precedence of the Centropagoidea , just as the structure of the urosome of the female copepodite 5 in Fosshagenia ferrarii excludes this form from the Centropagidea and should restore the superfamily of the Fosshagenoidea .
43 pelagic or brackish water families (the Calocalanidae and the Mecynoceridae are integrated with the Paracalanidae) are defined by Boxshall & Hasley (2004), being by alphabetical order:
1 - Acartiidae, 2 - Aetideidae, 3 - Arctokonstantinidae, 4 - Arietellidae, 5 - Augaptilidae, 6 - Bathypontiidae, 7 - Boholinidae, 8 - Calanidae, 9 - Candaciidae, 10 - Centropagidae, 11 - Clausocalanidae, 12 - Diaixidae, 13 - Discoidae, 14 - Epacteriscidae, 15 - Eucalanidae, 16 - Euchaetidae, 17 - Fosshageniidae, 18 - Heterorhabdidae, 19 - Hyperbionychidae, 20 - Kyphocalanidae, 21 - Lucicutiidae, 22 - Megacalanidae, 23 - Mesaiokeratidae, 24 - Metridinidae, 25 - Nullosetigeridae, 26 - Paracalanidae, 27 - Parapontellidae, 28 - Phaennidae, 29 - Pontellidae, 30 - Pseudocyclopidae, 31 - Pseudocyclopiidae, 32 – Peudodiaptomidae, 33 – Rhincalanidae, 34 - Ridgewayiidae, 35 - Rostrocalanidae, 36 - Ryocalanidae, 37 - Scolecitrichidae, 38 - Spinocalanidae, 39 - Stephidae, 40 - Sulcanidae, 41 - Temoridae, 42 - Tharybidae, 43 - Tortanidae.
The Diaptomidae with freshwater genera are not taken into account above.
The esentially freshwater and brackish (sometimes coastal) Pseudodiaptomidae are listed in an exhaustive way and indicated in the geographic distribution matrix.
The Calanoida incertae sedis are reunited in an arbitrary "group" (inc. sed-calanoida).
Boxshall & Hasley (2004, p.188) consider the family Parkiidae as synonym of the Scolecitrichidae in the absence of a true phylogenetic analysis. Vyshkvartzeva (2004, p.176 & foll.) contests the position of the former authors concerning the genus Parkius and restores the family status of the Parkiidae. br>
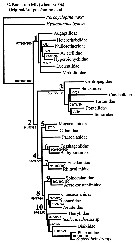
Ohtsuka & Huys (2001, p.461) draw up the phylogenetic tree of the Superfamilies. | |
 issued from : S. Ohtsuka & R. Huys in Hydrobiologia, 2001, 453/454. [Fig. 15, p.461] Phylogenetic tree of calanoid superfamilies, after Andronov (1974) and Park (1986). Each superfamily is defined by sexually dimorphic characters (apomorphies). Arrows indicate synapomorphies |
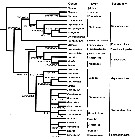
 issued from : L. Blanco-Bercial, J. Bradford-Grieve & A. Bucklin in Molecular Phylogenetics and Evolution, 2011. [p.103, Fig.2]. Simplified. Phylogram of families of the copepod Order Calanoida and outgroups. RAxML Maximum Likelihood tree, with nodes indicating percentage bootstrap recovery under three analyses: exclusion of all third codon positions in the protein-codifying genes (3rd pos.); and translation of protein-codifying genes to amino acid sequences (amono acid). The tree topology and the branch lengths shown correspond to the original sequence analysis.
See full text and fig.2: doi:10.1016/j.ympev.2011.01.008 The phylogeny agrees with previously-published morphological phylogenies (Andronov, 1974; Park, 1986), including e.g., enlargement of the Bathypontioidea to include the Fosshageniidae. |
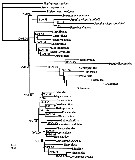
 issued from : D.F. Figueroa in J. Crustacean Biol., 2011, 31 (1). [p.161, Fig.6]. Calanoida phylogenetic tree based on the 18S ribosomal RNA gene. Branch values correspond to bootstrap support for maximum parsimony, maximum likehood, neighbor joining, and Bayesian posterior probabilities ( -, indicates taht the branch failed the 50% bootstrap support. Nota: After the author, this particular genetic analysis suggests: 1- Centropagoidea is the sister branch to all other Calanoida; 2- Ridgewayiidae and Pseudocyclopidae likely share a common ancestor with Augaptiloidea; 3- Ridgewaiidae and Pseudocyclopidae should be included in the same superfamily, Pseudocyclopoidea; 4- Spinocalanoidea likely needs to be included in Clausocalanoidea to recover the monophyly of the latter superfamily. |
 Issued from : J.M. Bradford-Grieve, G.A. Boxshall & L. Blanco-Bercial in Zool. J. Linnean Soc., 2014, 171. [p.527, Fig.12]. Phylogram of taxa of the copepod order Calanoida and out-groups. The topology and branch length correspond with the RAxML maximum-likelihood tree. Numbers at nodes indicate percentage of bootstrap recovery (when > 50%)/Bayesian posterior probability (when > 0.95, except for the Augaptiloidea/Centropagoidea node). For the super-family groupings, see Appendix S1 on line at the publisher's web-site. |
 Issued from : J.M. Bradford-Grieve, G.A. Boxshall, S.T. Ahyong & S. Ohtsuka in Invert. Syst., 2010, 24. [p.310, Fig.14]. Strict consensus of 66 trees, length 428, consistency index (CI) = 0.28, retention index (RI) = 0.72. The outgroup is a hypothetical calanoid ancestor. Calanoid copepod taxa used as exemplars in the cladistic analysis of calanoid families indicated in Table 1 (p.294). |
 Issued from : J.M. Bradford-Grieve, G.A. Boxshall, S.T. Ahyong & S. Ohtsuka in Invert. Syst., 2010, 24. [p.311, Fig.15]. Strict consensus of three trees after one round of successive weighting. Clade numbers above the line, jackknife supprt below. Outgroup is a hypothetical calanoid ancestor. Calanoid copepod taxa used as exemplars in the cladistic analysis of calanoid families indicated in Table 1 (p.294). |
 Issued from : J.M. Bradford-Grieve, G.A. Boxshall, S.T. Ahyong & S. Ohtsuka in Invert. Syst., 2010, 24. [p.294, Table 1]. Calanoid copepod taxa used as exemplars in the cladistic analysis of calanoid families. |
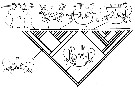 Issued from : J.M. Bradford-Grieve, G.A. Boxshall, S.T. Ahyong & S. Ohtsuka in Invert. Syst., 2010, 24. [p.316, Fig.17]. Diagramatic representation of new hypothesis of evolutionary trends in female genitalia (oviducts and shell ducts omitted). (A) hypothetical ancestor, Ridgewayiidae ( Brattstromia) and Epacteriscidae; (B) Pseudocyclopidae and Boholinidae; (C) Ridgewayiidae, Centropagoidea (except Acartia), Augaptilidae, Lucicutiidae, Heterorhabdidae,and Bathypontioidea?; (D) Augaptiloidea (Arietellidae, Hyperbionychidae, Nullosetigeridae, Metridinidae; (E) Acartiidae ( Acartia) - not in analysis- no operculum, only integumental fold with genital slit opening to outside: (F) Megacalanidae, Calanidae, Paracalanidae, Eucalanidae, Clausocalanoidea, (not Euchaetidae?). Large arrows indicate directions of transformations, small arrows indicate postulated places on tree (Fig. 16) where each type of configuration probably occurred (information from Cioc & al., 1997; Barthélémy & al., 1998; Barthélémy, 1999a, 1999b, and original data). cp = copiulatory pore; ga = genital atrium; go = genital operculum; gp = gonopore; gs = genital slit; ml = opercular muscles; m2 = gonoporal plate muscles; m3 = genital atrial muscles; sp = seminal pore; sr = seminal receptacle. |
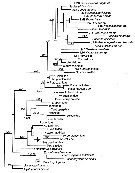 Issued from : S. Laakmann,, E.L. Markhaseva & J. Renz in Mol. Phylog. Evol., 2019, 130. [p.335, Fig. 2]. Phylogenetic relationships of all calanoid superfamilies and ''Bradfordian'' specimens with one individual per genus, respectively. Analyses are based on concatenated 18S (930 bp) - 28S (719 bp) - COI (547 bp) - cyt b (327) gene fragment (total length 2526 bp) and illustrated from maximum Likelihood Analysis Numbers on branches represent bootstrap values > 75% (first number) from Maximum Likelihood and posterior probabilities > 0.95 (second number) from Bayesian Inference Analysis. Grey names are sequences from Bradford-Grieve & al. (2014), black names are sequences for this study. Detailed settings for phylogenetic analyses are given in Appendix Table B (p.339-343) |
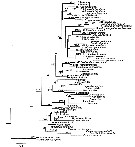 Issued from : S. Laakmann,, E.L. Markhaseva & J. Renz in Mol. Phylog. Evol., 2019, 130. [p.336, Fig. 3]. Phylogenetic relationships of representatives of all calanoid superfamilies and ''Bradfordian'' specimens from this study. Analyses are based on concateneded 18S (931 bp) - 28S (731 bp) - COI (547 bp) - cyt b (327 bp) gene fragment (total length 2536 bp) and illustrated from Maximum Likelihood Analysis. Numbers on branches represent bootstrap values > 75% (first number) from Maximum Likelihood and posterior probabilities > 0.95 (second number from Bayesian Inference Analysis. Grey names are sequences from Bradfoe-Grieve & al. (2014), black names are sequences from this syudy. Detailed settings for phylogenetic analyses are given in Appendix Table B (p.339-343 |
 Issued from : T.S. Jørgensen, B. Petersen, H.C.B. Petersen, P.D. Browne & al. in Genome Biol. Evol., 2019, 11 (5). [p.1442, Fig.1, D]. Within Copepoda, a 100-fold difference in genome size from the smallest Cyclopoid to the largest Calanoid can be seen. Within the order Calanoida, the genome sizes vary > 10-fold between the smallest Diaptomidae and the largest Calanidae. For the authors, DNA sequencing-based analyses suggest is a 14-fold difference in genome size between the six members of copepoda with available genomic information. This finding complements nucleus staining genome size estimations, where 100-fold difference has been reported within 70 species. The information confirms the evolution of genome size in Copepoda and expands the scope for evolutionary inferences in Copepoda by providing several levels of genetic information from a key planktonic crustacean species. See genome study for Acartia tonsa species (Calanoida: Diaptomoidea: Acartiidae) | | | | | Arietelloidea | | Syn.: | Augaptiloidea Blanco-Bercial & al., 2011 (p.1, 5, Table 1, Fig.2, Biol. mol, phylogeny) | | Ref.: | Vives & Shmeleva, 2010 (p.138, Rem.); Ohtsuka & Nishida, 2017 (p.583, Rem. bioluminescence) | | Rem.: | 5 families : Arietellidae, Augaptilidae, Discoidae, Heterorhabdidae, Nullosetigeridae.
According to Blanco-Bercial & al. (2011, Table 1): 8 families: Arietellidae, Augaptilidae, Discoidae, Heterorhabdidae, Hyperbionychidae, Lucicutiidae, Metridinidae, Nullosetigeridae.
Nota from Ohtsuka & Nishida (2017, p.583): The evolution of genes coding luciferase in the oceanic Arietelloidea and their adaptative significance are seen by Takenaka & al. (2013). | | | | Bathypontioidea | | Ref.: | Vives & Shmeleva, 2010 (p.138, Rem.); Blanco-Bercial & al., 2011 (p.1, 5, Table 1, Fig.2, Biol. mol, phylogeny) | | Rem.: | 1 family : Bathypontiidae. | | | | Calanoidea | | Syn.: | Megacalanoidea Blanco-Bercial & al., 2011 (p.1, 5, Table 1, Fig.2, Biol. mol, phylogeny) | | Ref.: | Vives & Shmeleva, 2010 (p.139, Rem.) | | Rem.: | 4 families : Calanidae, Mecynoceridae, Megacalanidae, Paracalanidae. | | | | Clausocalanoidea | | Ref.: | Vives & Shmeleva, 2010 (p.139, Rem.); Blanco-Bercial & al., 2011 (p.1, 5, Table 1, Fig.2, Biol. mol, phylogeny); Laakman & al., 2019 (p.330: Rem concerning the ''Bradfordian'' families. | | Rem.: | 13 families : Aetideidae, Clausocalanidae, Diaixidae, Euchaetidae, Kyphocalanidae, Mesaiokeratidae, Parkiidae, Phaennidae, Pseudocyclopiidae, Rostrocalanidae, Scolecitrichidae, Stephidae, Tharybidae.
After Laakmann & al. (2019, p.330), among the most derived calanoid copepod in the superfamily Clausocalanoidea about half of the genera belong to the ''Bradfordian'' families These families: Diaixidae, Tharybidae, Rostrocalanidae, Kyphocalanidae, Phaennidae, Parkiidae, Scolecitrichidae, and any incertae sedis genera. The study of molecular multi-gene analysis, using five gene fragments show the monophyly of ''Bradfordian'' as one lineage in the superfamily Clausocalanoidea. Except for the support of species belonging to the same genus and specimens belonging to the same species, no phylogenetic relationships among genera and families were supported. The impossibility of resolving phylogenetic relationships among ''Bradfordian'' genera and families may be due to the young age or fast radiation of ''Bradfordian'' within the mostly derived calanoid superfamily Clausocalanoidea. Therefore, mutation rates might be not sufficient to track phylogenetic relationships.
|  Issued from : S. Laakmann,, E.L. Markhaseva & J. Renz in Mol. Phylog. Evol., 2019, 130. [p.331, Table 1]. Compilation of information on relationships among ''Bradfordian'' genera from Markhaseva & Ferrari (2005) and Markhaseva & al. (2014). Abbreviations: A2, antenna; Md, mandible; Mx1, maxillule; Mx2, maxilla; Mxp, maxilliped; P5, leg 5; w, worm-like sensory seta; b, brush-like sensory seta; s, sclerotized seta. |
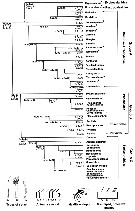 Issued from : S. Laakmann,, E.L. Markhaseva & J. Renz in Mol. Phylog. Evol., 2019, 130. [p.333, Fig. 1]. Relationships among ''Bradfordian'' genera. Nota: Relationships are based on number and type of setae on praecoxal endites of the Mxp; setation of five proximal segments of the exopod of A2; number and types of setae of the Mx2 zndopod. For Mxp, praecoxal endites sequence of 3 numbers separated by commas are number of setae on the proximal, middle and distal praecoxal endites (s: sclerotized seta; w: worm-like seta; b: brush-like seta. For A2 exopod: sequence of the 5 numbers separated by commas (artrodial membrane present) and dashes (arthrodial membrane absent) are segment/seta on the proximal five segments. For Mx2 endopod sequence of 3 numbers separated by commas are the number of sclerotized, worl-like, brush)like setae (compiled from Markhaseva & Ferrari, 2005; Markhaseva & Schulz, 2009; Markhaseva & al., 2008, 2014) Asterisk marks genera described since 2005. For the genera not yet completely or poorly described and not included in the morphological analysis see Table 1. The proposed evolutionary trends in the ''Bradfordian'' relationships consider: 1 - sensory setae arose asz transformation from sclerotized setae and are considered an apomorphic modification (derived from and differing from an ancestral condition); 2 - the loss of setae is a derived state and the transformation of setae from sclerotized into worm-like setae is a derived state, further, a brush-like seta is assumed to be derived from a worm-like seta (Ohtsuka & al., 2003), therefore a brush-like seta represent a more derived state; 3 - the plesiomorphic state (used of ancestral or primitive character) for the Mxp praecoxal armament in ''Bradfordians'' is assured to be 1, 2 and 3 sclerotized setae on the proximal, middle and distal praecoxal endites, respectively. The first transformation of the distal praecoxal endite is assumed to be loss of 1 sclerotized seta (1, 2, 2), the second transformation is assumed to be the loss of a second sclerotized seta (1, 2, 1). In all corresponding groups - group A, B and C genera without transformed setae on the praecoxal endites of Mxp were observed and within each of these groups further transformation from a sclerotized into a worm-like and brush-like seta is assumed to be the derived state. 4 - the exopod of A2 of the primitive ''Bradfordian'' calanoid is assumed to have 9 medial and 3 terminal setae (the 2nd segment is apparently a long complex of 3 fused segments, each represented by a medial seta). A proximal-to-distal loss of setae on the segments of A2 exopod, followed by loss of the arthrodial membrane between the long segment (2nd ptoximal) and the proximal of the 4 small segments is assumed to be a derived setae. 5 - Mx2 endite lobe and endopod of the primitive ''Bradfordian'' calanoid is assumed to have 9 setae because no more than 9 sclerotized setae are present on any species inn the superfamily Clausocalanoidea (Vaupel Klein & Rijerkerk, 1997). The loss of either a sclerotized seta or worm-like seta, resulting in 8 setae, is assumed to have occurred early in the evolution of the group (Ferrari & Markhaseva, 1996, and other authors). 6 - transformations of sclerotized setae on the praecoxal endites of Mxp apparently determine an early branching and are assumed to precede changes in the exopod of A2 in all cases. Changes in Mx2 endite lobe and endopod are assumed to be the last to occur during the evolutionary history of ''Bradfordian'' species. 7 - the most plesiomorphic character state is considerd for each of the genus included into the figure, described since that time from the deep near-bottom were included into this preliminary phylogenetic analysis, based on the compilation of the taxonomy of ''Bradfordian'' in Markhaseva & al. (2014). |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.70, Fig.5]. Schematic view of right A1 in males of ''Bradfordian'' families. Roman numerals: ancestral segments; black arrows: fused ancestral segments; black dotted arrows: position of geniculation or morphologically transformed segments; dotted lines:between A1 segments: incomplete fusion. A1 of Sensiava and Procenognatha schematized after Markhaseva & Schulz (2006, 2010), remaining genera after Markhaseva (pers. obs.). |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.71, Fig.6]. Schematic oral limb stucture. a, A2 exopod, schematized, ancestral condition of setation in ''Bradfordians''; b, Phaenna spinifera Claus, 1863 (Phaennidae), Md gnathovase (lateral view); c, Sxolecithrix bradyi Giesbrecht, 1888 (Scolecitrichidae), Md gnathobase (lateral view); d, schematic view of Mx1, arrow shows setation of endites in sequence as given in setal formula for Scolecitrichidae; e, ''Bradfordian'' Mxp, schematized, ancestral condition of setation in Diaixidae and Tharybidae; f, Mxp, schematized, ancestral condition of setation in Phaennidae; g, Mxp, schematized, ancestral condition of setation in Scolecitrichidae; h, Mxp, schematized, setation in Rostrocalanidae; i, Mxp, schematized, setation in Kyphocalanidae; j, Mxp, schematized, setation in Parkiidae e-j, simple line represents; stippled enclosure a worm-like seta; dark enclosure a brush-like seta. a sclerotized seta |
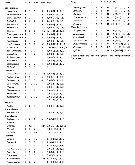 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.73, Table 1]. A2 armament (number of seta) in different ''Bradfoprdian'' genera (females). |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.74, Table 2 (part)]. Md armament (number of seta) in different ''Bradfoedian'' genera (females) See following table. b basis; End1 endopodal segment 1; End 2 endopodal segment 2; Exp exopod; gn gnathobase. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.75, Table 2 continued]. Md armament (number of seta) in different ''Bradfoedian'' genera (females) |
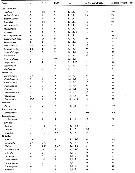
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.76, Table 3 part.]. Mx1 armament (number of seta) in different ''Bradfoedian'' genera (females). pa: praecoxal arthrite (setal formula of praecoxal arthrite: terminal+posterior+anterior setae); ce: coxal endite; bp: proximal basal endite; bd: distal basal endite; End: endopod; Exp: exopod; Epi: epipodite. See table following. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.77, Table 3 continued]. Mdx1 armament (number of seta) in different ''Bradfoedian'' genera (females) |
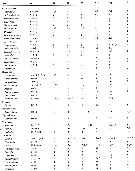
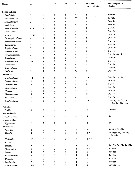 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.78, Table 4 part.]. Mx2 armament (number of seta) in different ''Bradfoedian'' genera (females) at: attenuation; pe: praecoxal endite; ce: coxal endite; bp: proximal basal endite; bd: distal basal endite; el: enditic-like lobe of proximal endopodal segment; End: endopod; w: worm-like seta; br: brush-like seta; se: sclerotized seta |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.79, Table 4 continued]. Mx2 armament (number of seta) in different ''Bradfoedian'' genera (females). |
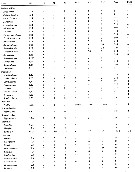 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.80, Table 5 part.]. Mxp armament (number of seta) in different ''Bradfoedian'' genera (females). at: attenuation; pes: praecoxal endites of syncoxa (from proximal to distal; ces: coxal endite of syncoxa; bp: basis proximal; bd: basis distal; End: endopod. For more detailed morphology of the setae on the praecoxal endites of the Mxp syncoxa see Markhaseva & Ferrari (2005) and Markhaseva & al. (2008). |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.81, Table 5 continued]. Mxp armament (number of seta) in different ''Bradfoedian'' genera (females). |
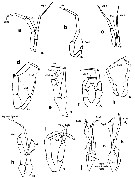 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.82, Fig. 8]. Diaixidae P5 males: (changed and schematised after the authors) a, Xancithrix ohmaniMarkhaseva, 2012. b, Neoscolecithrix caetanovi Alvarez, 1985. c, Cenognatha farrani (Smirnov, 1935). d, ¨rocenognatha semisensata Markhaseva & Schulz, 2010. e, Sznsiava longiseta Markhaseva & Schulz, 2006. f, Xantharus formosus Andronov, 1981. g, Xantharus renatehaassae Schulz, 1998. h, Paraxantharus brittae Schulz, 2006. i, Byrathis arnei Schulz, 2006. j, Diaixis trunovi Andronov, 1981. k, Diaixis helenae Andronov, 1981. Exp: exopod; End: endopod; (R): right leg. |
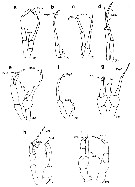 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44. [p.72, Fig. 7]. Phaennidae, Parkiidae, Scolecitrichidae, Tharybidae, Kyphocalanidae, P5 males: (changed and schematised after the authors). a, Xanthocalanus borealis Sars, 1900, Phaennidae. b, Xanthocalanus propinquus Sars, 1902, Phaennidae. c, Xanthocalanus kurilensisBrodsky, 1950, Phaennidae. d, Xanthocalanus polarsternae Markhaseva, 1998, Phaennidae. e, Pseudoamallothrix longispina Schulz, 1991, Scolecitrichidae. f, Scolecitrichopsis elenae Schulz, 2005, Scolecitrichidae. g, Parkius sp., Parkiidae. (changed and schematized after Grice & Hulsemann, 1967). h, Tharybis cf. macrophthalma , Tharybidae (Markhaseva (pers. obs.). i, Kyphocalanus sp., Kyphocalanidae, Markhaseva (pers. obs.). Exp: exopod; End: endopod; (R): right leg. | | | | | Diaptomoidea | | Syn.: | Centropagoidea : Blanco-Bercial & al., 2011 (p.1, 3, Fig.2, Table 1, Biol. mol, phylogeny, Rem.: p.7) | | Ref.: | Vives & Shmeleva, 2010 (p.138, Rem.) | | Rem.: | 10 marine families : Acartiidae, Candaciidae, Centropagidae, Fosshageniidae, Parapontellidae, Pontellidae, Pseudodiaptomidae, Sulcanidae, Temoridae, Tortanidae. | | | | Epacteriscoidea | | Syn.: | Ridgewayioidea : Vives & Shmeleva, 2010 (p.139, Rem.) | | Ref.: | Bradford-Grieve & al., 2014 (p.507, 526, Rem: p.529) | | Rem.: | 2 families : Epacteriscidae, Ridgewayiidae.
For Bradford-Grieve & al. ( 2014, p.507, 526) this Superfamily is a synonym of Pseudocyclopoidea. | | | | Eucalanoidea | | Ref.: | Vives & Shmeleva, 2010 (p.139, Rem.); Blanco-Bercial & al., 2011 (p.1, 5, Table 1, Fig.2, Biol. mol, phylogeny) | | Rem.: | 2 families : Eucalanidae, Rhincalanidae.
| | | | Pseudocyclopoidea | | Syn.: | Epacteriscoidea Fosshagen, 1973. | | Ref.: | Vives & Shmeleva, 2010 (p.139, Rem.); Bradford-Grieve & al., 2014 (p.507, 526, Rem: p.529, phylogeny) | | Rem.: | 1 family : Pseudocyclopidae, [synonymized: Bohalinidae and Ridgewayidae].
Diagnosis after Bradford-Grieve & al. (2014, p.526):
Plesiomorphic calanoid copepods with underlying pattern of full development of arthropodial membranes between body somites and limb segments, with some exceptions; A1 female up to 27-segmented, ancestral segments XIX, XX, and XXIII usually without aesthetascs; male A1 always geniculate on right and tendency for ancestral segment XXV to have distoanterior process.
Male and female mouthparts identical.
A2 exopod 9-segmented, segments I-VIII with 1 seta each.
Mxp endopod segment V nearly always with outer border seta (except Edaxiella).
P1-P5 with both rami usually 3-segmented.
P1 and P2 exopodal segment 3 with 2 or 3 outer border spines.
P3 exopodal segment 3 with 2 or 3 outer border spines, P4 and P5 (female) with 2 or 3 outer border spines. | | | | Ryocalanoidea | | Ref.: | Vives & Shmeleva, 2010 (p.139, Rem.); Renz & al., 2018 (p.1, 24, Figs. 15 & 16, p.25, 25, Rem.: phylogeny) | | Rem.: | 1 family : Ryocalanidae.
For Renz & al. (2018, p.26) defined on a particular suite of characteristics: - Armature of the 3rd exopodal segment of P1 as II, I, 4 (2 lateral and 1 terminal spine plus 4 medial setae) in combination with the armature of the 3rd exopodal segment of P2-P4 as III, I, 5,
- Right A1 male which strongly modified for grasping
The differently formed grasping right A1 male, with the main hinges between segments XXII/XXIII and XXIV in Ryocalanus and between XXII and XXIII/XXIV in Yrocalanus clearly separates these genera.
It appears for the first time the close relationship between Spinocalanoidea and Ryocalanoidea based on molecular evidence.
A monophyly of Ryocalanidae was not supported by molecular multi-gene analyses using different fragment lengths, different partitions and analyses as well as single-gene analysis of 288 rDNA.Compared with ryocalanoidean copepods, Spinocalanoidea are characterised by an armature of I, I, 4 on the 3rd exopodal segment of P1.
Andronov (2014) points out rhe close relationship between Spinocalanoidea and Ryocalanoidea, based on the structure of the rostrum, the setation of all oral limbs and the structure and segmentation of P1-P4. The latter includes the rudimentary outer lobe on P1 endopod, the presence of 5 setae at the inner border of P2-P4 exopodal segment 3 as well as 6 setae at the endopodal segment 3 of P3-P4, absence of P5 in females and the very simple structure of the male P5. Andronov considered all these characters to be typical of the representatives of the Spinocalanidae. This author remarked that the grasping right A1 in males is the only character truly distinguishing ryocalanid from spinocalanid copepods. He proposed the status of Ryocalanidae into the family Spinocalanidae.
In at least two members of the family Ryocalanidae (Yrocalanus asymmetricus, Y. bicornis), the armature of the 3rd exopod of P1 was I, I, 4 (see in Markhaseva & Ferrari, 1996). Additionally, while spinocalanoidean copepods are considered to lack any geniculation or hinges of the male right A1, the males of many spinocalanoidean species have an asymmetrical A1 with fusions (i.E between segments XXII-XXIII on the right side, and differences in the form of the segments. However, this fusion of segments was only observed in Ryocalanus.
In fine Renz, Markhaseva & Laakmann (2018, p.31) conclude that an unambiguous interpretation of the status of Ryocalanoidea withi Calanoida is currently not possible and the morphological and molecular taxonomic approaches lead to different results. Multi-gene analyses do not resolve and support the monophyly of each of the two superfamilies Ryocalanoidea and Spinocalanoidea. This might either be caused by a previously unrecognised close morphological relationship between these taxa or by methodological impediments in yje molecular analyses.
The morphology of the male A1 seems to be very important for any interpretation of relationships between different genera within the currently accepted ryocalanoidean and spinocalanoidean calanoids. Despite the ambiguous results, the authors propose to keep the currently accepted system until further data on the male A1 morphology and molecular data of more genera avalable. |  Issued from : J. Renz, E.L. Markhaseva & S. Laakmann in Zool. J. Linn. Soc., 2018, XX [p.26, Fig.16] Relationship of the Ryocalanoidea (red) and Spinocalanoudea (green) based on cocatenated genes of 18S (1767 bp), 28S (842 bp), IT (266 nbp), COI (657 bp) and cytochrome b (327 bp). A, Bayesian Inference with Bayesian posterior probabilities > 0.90 are shown. B, Maximum Likehood analysis with 10 000 bootstrap replicates. Bootstrap values > 50 are shown. First numbers without and second number with consideration of codon positions for mitochondrial genes COI and citochrome b. The illustrated topology of the tree is from the analysis withot consideration of codon positions for COI and cytochrome b. | | | | | Spinocalanoidea | | Ref.: | Blanco-Bercial & al., 2011 (p.1, 5, Table 1, Fig.2, Biol. mol, phylogeny); Renz & al., 2018 (p.24, figs.15, 16, phylogeny relationships) | | Rem.: | 2 families : Arctokonstantinidae, Spinocalanidae.
See: Renz & al. (2018, p.26-31, figs 15, 16, Table 6) the relationships between the two superfamilies Spinocalanoidea and Ryocalanoidea in remark for Ryocalanoidea | | | | Acartiidae Sars, 1903 ( Diaptomoidea ) | |
| | | | Aetideidae Giesbrecht, 1892 ( Clausocalanoidea ) | |
| | | | Arctokonstantinidae Markhaseva & Kosobokova, 2001 ( Spinocalanoidea ) | |
| | | | Arietellidae Sars, 1902 ( Arietelloidea ) | |
| | | | Augaptilidae Sars, 1905 ( Arietelloidea ) | |
| | | | Bathypontiidae Brodsky, 1950 ( Bathypontioidea ) | |
| | | | Boholinidae Fosshagen & Iliffe, 1989 ( Pseudocyclopoidea ) | |
| | | | Calanidae Dana, 1846 ( Calanoidea ) | |
| | | | Candaciidae Giesbrecht, 1892 ( Diaptomoidea ) | |
| | | | Centropagidae Giesbrecht, 1892 ( Diaptomoidea ) | |
| | | | Clausocalanidae Giesbrecht, 1892 ( Clausocalanoidea ) | |
| | | | Diaixidae Sars, 1902 ( Clausocalanoidea ) | |
| | | | Discoidae Gordeeva, 1975 ( Arietelloidea ) | |
| | | | Epacteriscidae Fosshagen,1973; emend.: Fosshagen, Boxshall & Iliffe, 2001 ( Epacteriscoidea ) | |
| | | | Eucalanidae Giesbrecht, 1892 ( Eucalanoidea ) | |
| | | | Euchaetidae Giesbrecht, 1892 ( Clausocalanoidea ) | |
| | | | Fosshageniidae Suarez-Morales & Iliffe, 1996 ( Diaptomoidea ) | |
| | | | Heterorhabdidae Sars, 1902 ( Arietelloidea ) | |
| | | | Hyperbionychidae Ohtsuka, Roe & Boxshall, 1993 ( Arietelloidea ) | |
| | | | Kyphocalanidae Markhaseva & Schulz, 2009 ( Clausocalanoidea ) | |
| | | | Lucicutiidae Sars, 1902 ( Arietelloidea ) | |
| | | | Mecynoceridae Andronov, 1973 ( Calanoidea ) | |
| | | | Megacalanidae Sewell, 1947 ( Calanoidea ) | |
| | | | Mesaiokeratidae Matthews, 1961 ( Clausocalanoidea ) | |
| | | | Metridinidae Sars, 1902 ( Arietelloidea ) | |
| | | | Nullosetigeridae Soh, Ohtsuka, Imabayashi & Suh, 1999 ( Arietelloidea ) | |
| | | | Paracalanidae Giesbrecht, 1892 ( Calanoidea ) | |
| | | | Parapontellidae Giesbrecht, 1894 ( Diaptomoidea ) | |
| | | | Parkiidae Ferrari & Markhaseva, 1996 ( Clausocalanoidea ) | |
| | | | Phaennidae Sars, 1902 ( Clausocalanoidea ) | |
| | | | Pontellidae Dana, 1853 ( Diaptomoidea ) | |
| | | | Pseudocyclopidae Giesbrecht, 1893 ( Pseudocyclopoidea ) | |
| | | | Pseudocyclopiidae T. Scott,1894 ; emend. Jaume, Fosshagen & Iliffe, 1999 ( Clausocalanoidea ) | |
| | | | Pseudodiaptomidae Sars, 1902 ( Diaptomoidea ) | |
| | | | Rhincalanidae Geletin, 1976 ( Eucalanoidea ) | |
| | | | Ridgewayiidae M.S. Wilson, 1958 ( Epacteriscoidea ) | |
| | | | Rostrocalanidae Markhaseva, Schulz & Martinez Arbizu, 2008 ( Clausocalanoidea ) | |
| | | | Ryocalanidae Andronov, 1974 ( Ryocalanoidea ) | |
| | | | Scolecitrichidae Giesbrecht, 1892 ( Clausocalanoidea ) | |
| | | | Spinocalanidae Vervoort, 1951 ( Spinocalanoidea ) | |
| | | | Stephidae Sars, 1902 ( Clausocalanoidea ) | |
| | | | Sulcanidae Nicholls, 1945 ( Diaptomoidea ) | |
| | | | Temoridae Giesbrecht, 1893 ( Diaptomoidea ) | |
| | | | Tharybidae Sars, 1902 ( Clausocalanoidea ) | |
| | | | Tortanidae Sars, 1902 ( Diaptomoidea ) | |
| | | | incertae sedis (Calanoida) | |
| | | | | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2024. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed April 27, 2024] © copyright 2005-2024 Sorbonne University, CNRS
|
|
 |
 |