|
|
 |
|
Calanoida ( Ordre ) |
|
|
|
Calanoidea ( Superfamille ) |
|
|
| |
| | | |
| Calanidae Dana, 1846 ( Calanoidea ) | | Ref.: | Giesbrecht, 1892 (p.41); Giesbrecht & Schmeil, 1898 (p.12); Sars, 1901 a (1903) (p.8); Esterly, 1905 (p.122); van Breemen, 1908 a (p.5, Genera Key); Wilson, 1932 a (p.19); Rose, 1933 a (p.55); Mori, 1937 (1964) (p.2, Genera Key); Brodsky, 1950 (1967) (p.81, 84); Vervoort, 1951 (p.55); Farran & Vervoort, 1951 (n°32, p.3); Chen & Zhang, 1965 (p.25); Björnberg, 1972 (p.24); Brosdsky, 1972 (1975) (p.1, 115, Rev.); Bradford & Jillett, 1974 (p.5, Rev.); Andronov, 1974 a (p.1005); Brodsky, 1976 (p.5); Vyshkvartzeva, 1976 (p.11); Björnberg & al., 1981 (p.616); Bowman & Abele, 1982 (p.10); Razouls, 1982 (p.57); Brodsky & al., 1983 (p.147); Sazhina, 1985 (p.107, 109, N); Fleminger, 1985 (p.273, 277, Table 2, Rem. A1); Mauchline, 1988 (p.719); Bradford, 1988 (p.73, Rev.); Zheng Zhong & al., 1984 (1989) (p.224, Genera Key); Nishida, 1989 (p.174, Rem.); Huys & Boxshall,1991 (p.461); Razouls, 1993 (p.308); Bradford-Grieve, 1994 (p.22, Def.); Madhupratap & al., 1996 (p.863, Table 5: %/copepods); Chihara & Murano, 1997 (p.737); Barthélémy, 1999 a (p.30); Bradford-Grieve & al., 1999 (p.877, 902, 904, 905, 906, Genera Key); Ohtsuka & Huys, 2001 (p.445, 461); Boxshall & Halsey, 2004 (p.13; 49; 78: Def.; p.80: Genera Key); Mulyadi, 2004 (p.10, Genera Key for Indonesian Seas); Vives & Shmeleva, 2007 (p.889, Genera Key); Bradford-Grieve & Ahyong, 2010 (p.279, phylogeny, figs.4, 5); Blanco-Bercial & al., 2011 (p.103, Table 1, Fig.2, 3, 4, molecular biology, phylogeny); Laakmann & al., 2019 (p.330, fig. 2, 3, phylogenetic relationships); Hirai & al., 2020 (p.1, Fig.4: metabarcoding, Fig.8: OTUs distribution patterns, Fig.9: phylogenetic analysis)
Bradford-Grieve J.M., (2002 onwards). Key to calanoid copepod families. Version 1 : 2 oct 2002. http://www.crustacea.net/crustace/calanoida/index.htm  | | Rem.: | Fleminger (1985) étudie le nombre et la disposition des soies et aesthetes sur les A1 de 25 espèces de la famille des Calanidae. L' auteur définit 4 types de soies et 3 formes d'aesthetes. Outre le dimorphisme sexuel des A1, qui se traduit toujours chez le (M) par une fusion des 2 premiers articles proximaux, et, selon les genres, la fusion de certains autres articles, on observe chez certaines (F) de nombreuses espèces, une disposition de groupes de soies et d'aesthetes homologue à celle des (M) en proportion faible et variable dans le temps. Ces (F) à phénotype (M) posent deux problèmes : l'un d'intérêt systématique et phylogénétique, l'autre concerne l'hypothèse d'un changement de sexe au cours du développement et de son déterminisme. Pour l'auteur les vues de Brodsky (1972) comme celles exprimées par Bradford et Jillett (1974) ne sont pas satisfaisantes.
Ainsi Neocalanus est limité aux 2 espèces N. gracilis et N. robustior de même Calanus , sensu lato , renferme les taxons dont le statut générique est encore incertain (C. cristatus , C. hyperboreus , C. plumchrus , C. propinquus , C. simillimus , C. tonsus ). Peuvent être considérés comme Calanus sensu stricto les espèces regroupées dans le complexe ' Calanus finmarchicus ' (C. chilensis , C. finmarchicus , C. glacialis , C. pacificus C. orientalis , C. sinicus , C. australis , C. helgolandicus , C. marshallae ). Le genre Nannocalanus est maintenu ainsi que ceux proposés par Bradford & Jillett (1974): Calanoides, Canthocalanus, Cosmocalanus, Mesocalanus, Undinula.
Bradford (1988) conteste le schéma établi par Brodsky (1972). Elle s'appuie sur des relations morphologiques qui permettent des groupements d'espèces plus stricts et plus neutres concernant les relations phylogénétiques entre les genres.
Aux seuls critères morphologiques peuvent maintenant être envisagés divers aspects de leur biologie et de leur génome (Bucklin, Frost & Kocher, 1992, 1995, Bucklin & Lajeunesse, 1994; Bucklin, Thomas & Kocher, 1996; Bucklin, Lajeunesse, Curry, Wallinga & Garrison, 1996).
8 G.: Calanoides, Calanus, Canthocalanus, Cosmocalanus, Mesocalanus, Nannocalanus, Neocalanus, Undinula.
After Madhupratap & al. (1996), la famille des Calanidae représente de 4 à 22 % des copepodes selon la saison dans la couche de mélange des eaux océaniques de la région ouest de l'Inde (Mer Arabe) en usant un filet type Multiple Plankton Closing Net à 200 µm de vide de maille (mesh aperture). |  Issued from : A. Fleminger in Mar. Biol., 1985, 88. [p.277]. Morphological types of setae and aesthetascs. Nota: Four types of antennal setae may be recognized among the Calanidae: Type 1: smooth-surfaced, acuminate filaments varying from shorter than the bearing segment to more than 10 times its length (D), and located anteriorly on the segment. Type 2: short, coniform processes (E), always found on the anterior, distal end of Segment 8 and in many species on Segment 12. Type 3: pseudo-annulated setae bearing spike-like setules (F), located on Segments 22, 23 and 24. Type 4: short and with a more or less capitate apex, usually filled with lipid-like globules (G); they occur only in adults males on one or more of the following segments depending upon the species (fused Segment 1-2, Segment 7 and Segment 9. The capitate setae occupy the distal position that is filled by a Type 1 seta in the female and in the copepodite stage V. Aesthetascs:appear in 3 forms: Category 1: slender, vermiform processes (A) located on most segments of the female antenna. Category 2: digitiform, irregularly swollen or pinched along their length, and rounded at the apex (B); they occur on Segments 1 thriugh 9 in males. Category 3: occurring in males on Segments 10 to 23, vary structurally in fine detail among the genera, but all have a central cuticular keel articulating proximally with the antero-distal corner of the segment and extending to the succeeding antennal segment (C). A 'fleshy' layer covers the keel and extends as dorsal and ventral lamellae over the length of the Category 3 aesthetasc. These lamellae may be fused over the entire length of the aesthetasc or may separate at the distal end. The 'fleshy' layer is fragile and often is partially to totally eroded by capturing nets or during storage in formaldehyde. |
 Issued from : A. Fleminger in Mar. Biol., 1985, 88. [p.278, Table 2]. Calanidae Number of setae (Set) and aesthetascs (Aes) per antennule segment in the Calanidae genera examined. Individual segments with similar numbers of Set and Aes are grouped together. Arrangement of setae and aesthetascs: In all species studied, most or all of the adult females had a trithek arrangement (i.e. 1 proximal seta, 1 distal seta, and 1 distal aesthetasc) on each segment of A1 except Segments 1 and 20 to 25 (see Calanus pacificus californicus Fleminger, 1985). Segment 1 bears 3 setae and 1 aesthetasc. Segment 2, apparently a fusion of 3 segments, bears 3 such tritheks in positions 2a, 2b, and 2c. Segments 20 and 21 each lack 1 seta and the latter usually lacks the aesthetasc as well. Segments 22 and 23 have only 1 seta and 1 aesthetasc anteriorly and 1 posteriorly positioned, plumose, pseudoannulated seta (Type 3). Segment 24 has 2 setae similar to those of Segment 23, but no aesthetasc. Segment 25 bears 4 relatively short setae, i minute seta and 2 aesthetascs (see fig. A1 of calanus pacificus californicus and Calanoides philippinensis from Fleminger, 1985) ; it may be derived from the fusion of two or more segments. The A1 of adult males exhibits alternation of trithek and quadrithek combinations, the latter consisting of 2 setae and 2 aesthetascs and occurring on Segments 2b, 3, 5, 7 and 9. Segments 2a, 2c, 4, 6, 8 and 10 through 19 bear tritheks (as in the female).Segment 21 bears 1 seta and 1 aesthetasc in the male. The remaining segments carry the same numbers and types of setae and aesthetascs as found in the female. All the setae and th escale-like male aesthetascs are articulated and rotate through about 90° from normal to the antenna surface to flat against it. |
 Issued from : A. Fleminger in Mar. Biol., 1985, 88. [p.279, Table 2 (continued)]. Calanidae Number of setae (Set) and aesthetascs (Aes) per antennule segment in the Calanidae genera examined. Bracketed segments are completely fused; Segments 8 and 9 are incompletely fused. Individual segments with similar numbers of Set and Aes are grouped together. |
 Issued from : A. Fleminger in Mar. Biol., 1985, 88. [p.279, Table 3]. Calanus s.s. Antennule segmentation, setation and aesthetasc. Individual segments with similar numbers of Set and Aes are grouped together. Setae: Set = setiform; Spi = spiniform; Plu = plumose and pseudoannulated; Cap = capitate. Aesthetascs: Ver = vermiform; Dig = digitiform; Sca = scale-like. |
 Issued from : J.M. Bradford & J.B. Jillett in Crustaceana, 1974, 27 (1). [p.13, 14 fig.2]. Key to genera in Calanidae. 1 - Coxa of P5 inner edge with small teeth (fig.2i, j) ......... 2. 1 - Coxa of P5 inner edge naked (fig.2g, m) ......... 3. 2 - Basis of female P2 and P3 with posterior surface spines (fig.2c). Male left P5 prehensile (fig.2j) ........... Cosmocalanus. 2 - Basis of female P2 and P3 naked (fig.2d). Neither male P5 prehensile (fig.2i)....... Calanus. 3 - Exopod segment 2 of P2 in both sexes with outer proximal edge evaginate. Male left P5 prehensile (fig.2e,k) ............... Undinula. 3 - These characters absent ........... 4. 4 - Basis of P1 with distally directed seta on anterior surface modified into proximally thickened spine (fig.2a)................. Canthocalanus. 4 - Basis of P1 with distally directed setae of ordinary plumose type (fig.2b) ........... 5. 5 - Endopod of female P5 with 8 setae (fig.2f). Endopod of male right P5 with 8 setae (fig.2l) .............. Neocalanus. 5 - Endopod of female P5 with 7 setae (fig.2g). Endopod of male right P5 with 7 setae (fig.2m) ............. Mesocalanus. 5 - Endopod of female P5 with 6 setae (fig.2h). Endopod of male right P5 with no more than 6 setae (fig.2n) ......... Calanoides. |
 Issued from : G.A. Boxshall & S.H. Halsey in An Introduction to Copepod Diversity. The Ray Society, 2004, No 166, Part. I. [p.78]. Armature formula of swimming legs P1 to P4. Setation sometimes reduced. First exopodal segment of P2 with recurved spine in Neocalanus; 2nd exopodal segment of P2 with deep invagination in Undinula. Nota : Female P5 with 3-segmented rami as for legs P1 to P4, maximum setation as given in formula, often reduced. Inner margin of coxa P5 with row of teeth in some genera. - Male P5 weakly to strongly asymmetrical ; biramous with both rami typicalmly 3-segmented although in some genera one or both endopods are reduced or unsegmented. Right leg similar to P1 to P4 ; setation usually reduced by loss of inner margin setae from all exopodal segments. Setatio,n formula of endopod 0-1 ; 0-1 ; 2, 2, 2, often reduced. Left leg variable, often much longer than right leg. Exopod 3-segmented , as in P1-P4 in some genera, but lacking inner margin setae, to highly modified in Undinula and Cosmocalanus as complex grasping appendage. Eggs released into water. |
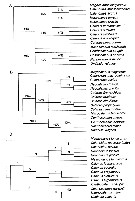 Issued from : J.M. Bradford-Grieve & S.T. Ahyong in J. Nat. Hist., 2010, 44 (5-6). [p.289, Fig.4]. (A) Strict consensus of six most parcimonious trees, length 81, consistency index = 0.58, retention index = 0.66, numbers above the branches indicate Jackknife support; (B) 50% majority-rule consensus of six most parcimonious trees; (C) single most-parcomonious tree derived from single round of successive weighting. Numbers above the branches are clade numbers. Megacalanus longicornis (Sars, 1905) (= Megacalanus princeps Wolfenden, 1904) is the outgroup. For the authors (p.294) the Calanidae probably evolved from a magacalanid-like ancestor. Although direct evidence of the ancestral habitat is lacking, the ancestral megacalanid was bathypelagic as are its modern relatives. It is suggested that the Calanidae radiated during the mid to late Triassic, possibly from the bathypelagic Megacalanidae (which may be adapted to low oxygen conditions) with high-latitude epipelagic locations being the first near-surface environments to be re-colonized. It is in surface environments that the metabolic rates of the Calanidae possibly adjusted to a well-oxygenated environment with a possible loss of their reliance on elevated of the level lactate deshydrogenase activity (LHD) (see Thuesen & al., 1998). Cladistic analysis of Calanidae recovered two major clades: a tropical epipelagic clade composed of species without ontogenetic vertical migration ( Canthocalanus + Cosmocalanus + Nannocalanus + Undinula); and a clade of ontogenetically migrating genera ( Neocalanus + Calanoides + Calanus + Mesocalanus) |
 Issued from : J.M. Bradford-Grieve & S.T. Ahyong in J. Nat. Hist., 2010, 44 (5-6). [p.292, Fig.5]. Clades derived from molecular analysis based on 18s ribosomal RNA. (A) Bucklin & al., 2003; (B) Taniguchi & al., 2004. | | | | | | (1) Calanoides Brady, 1883 | |
| | Syn.: | Carinocalanus Brodsky, 1972 (1975) (p.112) | | Ref.: | Brady, 1883 (p.74); 1914 (p.4); A. Scott, 1909 (p.10); Sewell, 1929 (p.25); Vervoort, 1946 (p.29); 1951 (p.55, Rem.); Carvalho, 1952 a (p.137); Tanaka, 1956 (p.259); Chen & Zhang,1965 (p.28); Ramirez, 1966 (p.6); Brodsky, 1972 (1975) (p.113, 114, 116); Bradford & Jillett, 1974 (p.6, 9, 13, Rev.); Fleminger, 1985 (p.273, 275, 278, 285, Table 2, Rem.: A1); Mauchline, 1988 (p.719); Razouls, 1982 (p.74); Bradford, 1988 (p.73,76, Rev.); Razouls, 1993 (p.308); Bradford-Grieve, 1994 (p.23, Déf.); Chihara & Murano, 1997 (p.741); Mauchline, 1998 (p.68); Bradford-Grieve & al., 1999 (p.907: spp. Key); Boxshall & Halsey, 2004 (p.80) ; Vives & Shmeleva, 2007 (p.890); Sabatini & al., 2007 (p.341, Rem.: Comparison of species) | | Rem.: | type: Calanoides patagoniensis Brady, 1883, non C. carinatus Kröyer,1849.
6 spp. | | Remarques sur les dimensions et le sex-ratio: | | Le rapport des tailles male/femelle est de 0,980 (= 5; SD = 0,0388) si l'on ne prend en compte que les rapports de chacune des espèces considérées |  issued from : M. E. Sabatini, F. C. Ramirez & J. Bradford-Grieve in Invert. Syst., 2007, 21. [p.361, Table 4]. Comparaison des espèces du genre Calanoides: Clef des caractères distinctifs des femelles |
 issued from : M. E. Sabatini, F. C. Ramirez & J. Bradford-Grieve in Invert. Syst., 2007, 21. [p.362, Table 5]. Comparaison des espèces du genre Calanoides: Clef des caractères distinctifs des males. |
 issued from : J.M. Bradford in Hydrobiologia, 1988, 167/168. [p.76, Table 2]. Selected characters of species of the genus Calanoides. |
 Issued from : J.M. Bradford, L. Blanco-Bercial & I. Prusova in J. Nat. Hist., 2017, 51 (13-14). [p. 811, Fig.2]. Location diagram of samples examined or mentioned in the phylogenic test. open square:- C. acutus; open diamond - C. brevicornis; black diamond - C. carinatus s.s.; open circle - C. natalis; black suare - C. patagoniensis; bleck point - C. philippinensis. |
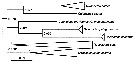 Issued from : J.M. Bradford, L. Blanco-Bercial & I. Prusova in J. Nat. Hist., 2017, 51 (13-14). [p. 815, Fig.4]. Bayensian tree obtained from COI molecular data. Individuals are collapsed to species. Values at nodes indicate posterior probability. Monophyletic Neocalanus spp. and Mesocalanus tenuicornis were included as topology constraints. All species described by the authors in text were recovered as monophyletic. | | | | | | Syn.: | Monoculus Gunnerus,1770; Cyclops (part.) Müller,1776;
Cetochilus Roussel de Vauzème,1834;
Nannocalanus (part.) Sars,1925 | | Ref.: | Kröyer, 1848 (in Damkaer & Damkaer, 1979, p.12); Brady, 1878 (p.37); Giesbrecht, 1892 (p.45, 88); Dahl, 1894 (p.74, spp. Key); Giesbrecht & Schmeil, 1898 (p.12, spp. Key); Wheeler, 1901 (p.164); Sars, 1901 a (1903) (p.8); Esterly, 1905 (p.123, clé spp.); van Breemen, 1908 a (p.6, spp. Key); A. Scott, 1909 (p.7); Wolfenden, 1911 (p.190); Esterly, 1924 (p.83); Sars, 1925 (p.5); Sewell, 1929 (p.19, Rem.); Wilson, 1932 a (p.19, spp. Key); Rose, 1933 a (p.55, spp. key); Mori, 1937 (1964) (p.11, spp. Key); Vervoort, 1946 (p.22); Brodsky, 1950 (1967) (p.85, clé spp.); Tanaka, 1956 (p.251); Brodsky, 1959 a (p.1548, spp. Key, Biogeography); 1961 (p.5, spp. Key); Chen & Zhang, 1965 (p.25); Ramirez, 1966 (p.5); Marshall & Orr, 1972 (p.155 & suiv.); Sheldon & al., 1972 (p.327, fig. 13: size particle vs. production's rate) Brodsky, 1972 (1975) (p.4, 112, 115, Rev., S/G & spp. Keys); Vyshkvartzeva, 1972 (1975) (p.186); 1976 (p.11); Frost, 1974 (p.77); Bradford & Jillett, 1974 (p.5, Rev.); Kabata, 1979 (p.20); Razouls, 1982 (p.62); Gardner & Szabo, 1982 (p.135); Brodsky & al., 1983 (p.149, spp. Key); Van der Spoel & Heyman, 1983 (p.147); Zheng Zhong & al., 1984 (1989) (p.225, spp. Key); Fleminger, 1985 (p.277, Table 2, 3, 4, Rem.: A1); Mauchline,1987 (p.719); Bradford, 1988 ( p.73, Rev.); De Decker & al., 1991 (p.27); Razouls, 1993 (p.308); Bradford-Grieve, 1994 (p.29, Def.); Bucklin & al., 1995 (p.655); Chihara & Murano, 1997 (p.738); Mauchline, 1998 (p.67); Bradford-Grieve & al., 1999 (p.907: spp. Key); Boxshall & Halsey, 2004 (p.80); Vives & Shmeleva, 2007 (p.892, spp. Key) | | Rem.: | type: Monoculus finmarchicus Gunnerus, 1770. 14 spp. Certaines présentant des génomes correspondants à des variantes de l'espèce type.
Calanus finmarchicus est l'une des espèces de copépodes la plus anciennement connue. Décrite par Gunnerus (1770) de Trondheim, à partir d'organismes provenant de Rensholmmen situé au sud du port de Hammerfest (nord Norvège), sous l'apelation initiale comme Monoculus finmarchicus, conformément au genre Monoculo créé par Linnée (1747), transféré par la suite au genre Cyclops in Müller (1776), Cetochilus par Roussel de Vauzème (1834), puis définitivement dans le genre Calanus par Leach (1819). S.M. Marshall & A.P. Orr (1972) donne l'historique des différents synonymes de ce genre depuis les origines, comme l'historique supposé du nom.
Pour résumer A. Daniélou (dans son Histoire de l'Inde, 1983, édit. Fayard): Lors de la conquête de l'Inde, Alexandre le Grand en 326 av. JC atteint Taxila, centre commercial et culturel important dans lequel trois grandes religions coexistent, brahmanique, Jaïna et boudhique. Alexandre impressionné par l'endurance des yogis et par la vie des ascètes jaïna s'attacha les services du philosophe Calanos qui exigeait que les Grecs soient dénudés pour suivre son enseignement. Calanus devait tomber malade à Suze et refuser les remèdes et les conseils des médecins grecs. il fit préparer un bûcher et avant que les flammes entourent l'ascète immobile, il dit à Alexandre: nous nous retrouverons à Babylone. Alexandre étonné par son courage lui fit rendre les honneurs militaires, Néarque fait sonner les trompettes, l'armée pousse le cri de guerre, tandis qu'en écho les éléphants poussent leur barrissement aigu et que les indiens entonnent leurs chants de deuil. Ayant renoncé à poursuivre la conquête des Indes après consultation démocratique de l'armée et après avoir obtenu des augures un avis défavorable, Alexandre fit retraite par la cote désertique du Bélouchistan et devait mourir en 323 av. JC à Babylone des suites de ses blessures et de dysenterie réalisant la prophétie de Calanos. D'après Marshall et Orr, l'ascète aurait murmuré 'Kalan' (peut-être 'Kalyam', i.e. Dieu vous bénisse, ou peut être bon, soit 'Kalanos' *; l'extension latérale des antennules a pu suggérer à Leach l'attitude pratiquée par les Yogi.
*: Kalanos en grec = Brahmane (in A. Bailly, 26e édit. 1963) | | Remarques sur les dimensions et le sex-ratio: | | Le rapport des tailles male/femelle est de 0,941 (n = 14; SD = 0,0670) si l'on ne prend en compte que les rapports de chacune des espèces considérées |  issued from : J.M. Bradford in Hydrobiologia, 1988, 167/168. [p.76, Table 2]. Selected characters of species of the genus Calanus. |
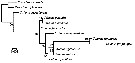 Issued from : R. Kozol, Blanco-Bercial L. & A. Bucklin in PLoS ONE 7(10): e45710. doi:10.1371/Journal.pone.0045710 [p.6, Fig.3]. Maximum Likelihood tree for 10 species of Calanus. Tree is based on a 658 bp region of the 285 rRNA gene under GTR. Numbers at nodes indicate percentage of recovery after 1,000 bootstraps. |
 Issued from : A. Bucklin, B.W. Frost & T.D. Kocher in Mar. Biol., 1995, 121. [p.657, Fig.1]. Morphological phylogeny of calanus species showing the two sibling species groups, C. finmarchicus and C. helgolandicus. Based on Frost (1974) and unpublished work of A. Fleminger (Scripps Institution of Oceanography). |
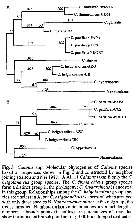 Issued from : A. Bucklin, B.W. Frost & T.D. Kocher in Mar. Biol., 1995, 121. [p.661, Fig.3]. Molecular phylogenies of Calanus specie percent base differences for a 387 base sequence of the 16S rRNA gene for six species of calanus, including two or three populations of C. finmarchicus, C. helgolandicus and C. pacificus. Nannocalanus minor included for comparison. Nota: For the authors, the discrimination of species of the genus Calanus is problematical, especially in regions of sympatry. Although the species of Calanus exhibit morphological similarity, they are quite distinct in genetic character. The DNA base sequences of the mitochondrial large subunit (16S) ribosomal RNA (rRNA) gene unambiguously discriminated the species. Statistical analysis of the sequence data using a variety of tree-building algorithms separated the taxa into one group of species corresponding to the C. finmarchicus group and another ungrouped set of species corresponding to the C. helgolandicus group. The C. helgolandicus group may be older than the C. finmarchicus group, making the tree topology less nreliable in this area. C. hyperboreus was an outlier. nannocalanus minor was the outgroup. The morphological similarity of Calanus species is noteworthy in terms of the previous estimations of the time since divergence of the species. Using identical analysis that Cunningham & al. (1992) of the sequence data for the same mtDNA region in copepods, the genus may be estimated to have 80 million years ago, the sibling species groups may have separated 40 million years ago, and the sibling species may be 20 million years old. This is considerably older than copepod szystematists have suggested. Most students of the calanus assemblage hypothesize that the group experienced a recent radiation (possibly during the late Pleistocene) and that speciation has been associated with palaeoceanographic events which have allowed geographic isolation, genetic divergence, and reproductive isolation (Frost, 1974; Fleminger & Hulsemann, 1977). The lack of morphological divergence of Calanus species may be a consequence of the means by which reproductive isolation is attained. For copepods, reproductive isolation may not require extensive morphological divergence. Rather, small changes in the structure of the secondary sexual characteristics may be sufficient to prevent interbreeding. For example, Fleminger & Hulsemann (1977) found that the positions of integumental organs on the urosome of females differed among some Atlantic Ocean species of Calanus and within C. helgolandicus; they speculated that these structures may produce contact pheromones and that their species specific positioning mat act as a pre-zygotic barrier to hybridization . Alteration of reproductive structures has been proposed as a mechanism ensuring reproductive isolation in copepods by a number or researchers. It is important to note that some taxinomists consider the Calanus species group to be monophyletic, which is an asumption of this molecular analysis.
Speciation has been more extensive in the northern hemisphere (Brodsky, 1965); tropical and arctic groups are presumably derived from the temperate, northern hemisphere forms (Brodsky, 1967 a) |
 Issued from : A. Bucklin, B.W. Frost & T.D. Kocher in Mar. Biol., 1995, 121. [p.658, Table 1]. Calanus spp. List of species studied, regions sampled, coordinates for collection sites, and collectors. | | | | | (3) Canthocalanus A. Scott, 1909 | |
| | Ref.: | A. Scott, 1909 (p.8); Vervoort, 1946 (p.36); Tanaka, 1956 (p.259); Chen & Zhang, 1965 (p.29); Brodsky, 1972 (1975) (p.114, 116, Rev.); Bradford, 1972 (p.14); Bradford & Jillett, 1974 (p.6, 10); Razouls, 1982 (p.76); Zheng Zhong & al., 1984 (1989) (p.227); Fleminger, 1985 (p.273, 278, Table 2, Rem.: A1); Mauchline, 1987 (p.719); Bradford, 1988 (p.73, 76, 79, Rem.); Razouls, 1993 (p.308); Bradford-Grieve, 1994 (p.33, Déf.); Chihara & Murano, 1997 (p.739); Mauchline, 1998 (p.68); Boxshall & Halsey, 2004 (p.80) | | Rem.: | type: Calanus pauper Giesbrecht, 1888. 1 sp. | | Remarques sur les dimensions et le sex-ratio: | | For one species only in the genus, concerning 21 specimens measured in the world: The mean female size is 1.526 mm (n = 30; SD = 0.1872), and the mean male size is 1.388 mm (n = 30; SD = 0.1843). The size ratio (male : female) is 0.90. The sex ratio (female : male) is possibly = 1 (17 females vs 19 males noted) |  issued from : J.M. Bradford in Hydrobiologia, 1988, 167/168. [p.76, Table 2]. Selected characters of species of the genus Canthocalanus. | | | | | (4) Cosmocalanus Bradford & Jillett, 1974 | |
| | Ref.: | Bradford & Jillett, 1974 (p.6, 12); Razouls, 1982 (p.83); Fleminger, 1985 (p.273, 278, Table 2, Rem.: A1); Mauchline, 1987 (p.719); Bradford,1988 (p.74, 75, 79); Razouls, 1993 (p.308); Bradford-Grieve, 1994 (p.35, Def.); Chihara & Murano, 1997 (p.737); Mauchline, 1998 (p.67: F, M); Barthélémy, 1999 a (p.26); Boxshall & Halsey, 2004 (p.80) | | Rem.: | type: Undina darwini Lubbock,1860. 2 spp. | | Remarques sur les dimensions et le sex-ratio: | | Le rapport des tailles male/femelle est de 0,882, si l'on ne prend en compte que les rapports de chacune des espèces considérées. |  issued from : J.M. Bradford in Hydrobiologia, 1988, 167/168. [p.76, Table 2]. Selected characters of species of the genus Cosmocalanus. | | | | | (5) Mesocalanus Bradford & Jillett, 1974 | |
| | Syn.: | Neocalanus (part.): Brodsky & al., 1983 (p.184, clé spp.) | | Ref.: | Bradford & Jillett, 1974 (p.6, 12); Razouls, 1982 (p.79); Gardner & Szabo, 1982 (p.147); Fleminger, 1985 (p.273, 278, Table 2, Rem.: A1); Bradford, 1988 (p.74-76); Razouls, 1993 (p.308); Bradford-Grieve, 1994 (p.38, Def.); Chihara & Murano, 1997 (p.741); Mauchline, 1998 (p.68); Boxshall & Halsey, 2004 (p.80); Vives & Shmeleva, 2007 (p.897) | | Rem.: | Pour Brodsky (1980: comm. pers.) la création de ce genre paraît mal assuré. Type : Calanus tenuicornis Dana,1849. Total: 2 spp. | | Remarques sur les dimensions et le sex-ratio: | | Le rapport des tailles male/femelle est de 0,820, si l'on ne prend en compte que les rapports de chacune des espèces considérées |  issued from : J.M. Bradford in Hydrobiologia, 1988, 167/168. [p.76, Table 2]. Selected characters of species of the genus Mesocalanus. | | | | | (6) Nannocalanus Sars, 1925 | |
| | Syn.: | Canthocalanus (part.) : Brodsky, 1972 (1975) (p.116);
Calanus (part.) : Bradford & Jillett, 1974 (p.6, 9) | | Ref.: | Sars, 1925 (p.9); Vervoort, 1946 (p.25); Chen & Zhang, 1965 (p.30); Razouls, 1982 (p.79); Zheng Zhong & al., 1984 (1989) (p.227); Fleminger, 1985 (p.273, 278, Table 2, Rem.: A1); Bradford, 1988 (p.76, 79, Rem.); Razouls, 1993 (p.308); Bradford-Grieve, 1994 (p.39, Def.); Chihara & Murano, 1997 (p.737); Barthélémy, 1999 a (p.26); Boxshall & Halsey, 2004 (p.80); Vives & Shmeleva, 2007 (p.899) | | Rem.: | type: Cetochilus minor Claus,1863. Total: 2 spp. | | Remarques sur les dimensions et le sex-ratio: | | Le rapport des tailles male/femelle est de 0,836, si l'on ne prend en compte que les rapports de chacune des espèces considérées présentant des mesures pour les deux sexes. |  issued from : J.M. Bradford in Hydrobiologia, 1988, 167/168. [p.76, Table 2]. Selected characters of species of the genus Nannocalanus. | | | | | (7) Neocalanus Sars, 1925 | |
| | Syn.: | Tropocalanus & Neocalanus (part.) Brodsky, 1972 (1975) (p.116); Brodsky & al., 1983 (p.184) | | Ref.: | Sars, 1925 (p.7); Sewell, 1929 (p.26, Rem.); Wilson, 1932 a (p.27); Vervoort, 1946 (p.38, Rem.); Oliveira, 1946 (p.454); Tanaka, 1956 (p.261); Chen & Zhang, 1965 (p.26); Bradford & Jillett, 1974 (p.10, Rev.); Razouls, 1982 (p.77); Gardner & Szabo, 1982 (p.141); Zheng Zhong & al., 1984 (1989) (p.227); Fleminger, 1985 (p.273, 278, Table 2, Rem.: A1); Mauchline, 1987 (p.719); Bradford, 1988 (p.74, 76, 79); Miller, 1988 (p.226, 270); Razouls, 1993 (p.308); Bradford-Grieve, 1994 (p.41, Def.); Chihara & Murano, 1997 (p.739); Mauchline, 1998 (p.68); Bradford-Grieve & al., 1999 (p.908: spp. Key); Miller, 2002 (p.139); Boxshall & Halsey, 2004 (p.80); Vives & Shmeleva, 2007 (p.901, spp. Key); Ohtsuka & Nishida, 2019 (p.575, Fig.22.2, biogeography ) | | Rem.: | type: Calanus gracilis Dana,1849. Total: 6 spp. | | Remarques sur les dimensions et le sex-ratio: | | Le rapport des tailles male/femelle est de 0,865 (n = 6; SD = 0,0583) si l'on ne prend en compte que les rapports de chacune des espèces. |  issued from : R.J. Machida, M.U. Miya, M. Nishida & S. Nishida in Mar. Biol., 2006, 148. [p.1077, Fig.4]. Relationship between the phylogeny and distribution pattern of the Neocalanus species. Shaded portions of the maps represent distributions based on the litterature. Clades A-E correspond to those in Fig.3, p.1075. By comparing the habitat localities and the phylogenetic relationship, it has been demonstrated that major linkeages of Neocalanus species diverged into different habitat localities. ''Groups'' classified by Bradford & Jillett (1974) is the first bifurcation among Neocalanus species (clade A), those separated by the temperature gradient between tropical to subtropical waters ( ''gracilis\" group: N.gracilis and N. robustior) and subarctic waters ( ''tonsus'' group: N. tonsus, N. cristatus, N.plumchrus, N. flemingeri). Second bifurcation is between N. tonsus and the rest of the species in the ''tonsus'' group ( N. cristatus, N. flemingeri, N. plumchrus), those separated by the subantarctic and subarctic Pacific (clade B). Although these subantarctic and subarctic Pacific species constitute the sister clade, their distribution areas are widely separated. The occurrence of closely related animals to the north and south of the tropical zone but not within the tropics, known as bipolar or antitropical distribution, among zooplankton assemblage is not an exceptional phenomenon (Reid, 1978). Several mechanisms have been proposed for the development of antitropical distributions, divisible into two classes: dispersal and vicariance (Burridge, 2002). The dispersal mechanism assumes that a taxon was originally represented on one side of the tropics, and subsequently moved across the equator to colonize the opposite hemisphere. On the other hand, the vicariance mechanism assumes that taxa that once occurred in the tropics but were later expatriated, resulting in osolated Northern and Southern Hemisphere populations. The four species that occur in subarctic Pacific and subantarctic clustered monophyletically and all of the species are known to perform ontogenetic vertical migration (Miller, 1988). Therefore, the dispersal mechanism is much more likely than the vicariance mechanism for the explanation of the antitropicality of thes four Neocalanus species. Two explanations have been proposed for the transequatoril exchange of populations in the plankton community (Dunbar, 1979). One is through the deep water link between populations, a low-temperature route. Another explanation is transequatorial dispersal during glaciations, implicating cooler surface water temperature, only applicable to carnivorous or omnivorous zooplankton that complete its life cycle in mesopelagic environment, and not for Neocalanus species, which mainly depend on primary production. |
 issued from : J.M. Bradford in Hydrobiologia, 1988, 167/168. [p.76, Table 2]. Selected characters of species of the genus Neocalanus. | | | | | (8) Undinula A. Scott, 1909 | |
| | Syn.: | Undina (part.) Dana,1847 | | Ref.: | A. Scott, 1909 (p.16); Sars, 1925 (p.10); Wilson, 1932 a (p.29); Dakin & Colefax, 1940 (p.87, clé spp.); Vervoort, 1946 (p.72); Carvalho, 1952 a (p.138); Tanaka, 1956 (p.264); Chen & Zhang, 1965 (p.30); Brodsky, 1972 (1975) (p.114, 116, 117, 120); Bradford & Jillett, 1974 (p.11, Redef.); Razouls, 1982 (p.80); Zheng Zhong & al., 1984 (1989) (p.228); Fleminger, 1985 (p.273, 278, Rem.: A1); Mauchline, 1988 (p.719); Bradford, 1988 (p.74, 77); Razouls, 1993 (p.308); Bradford-Grieve, 1994 (p.44); Chihara & Murano, 1997 (p.739); Mauchline, 1998 (p.68); Boxshall & Halsey, 2004 (p.80); Vives & Shmeleva, 2007 (p.905) | | Rem.: | type: Undina vulgaris (Dana,1849). Total: 1 sp. | | Remarques sur les dimensions et le sex-ratio: | | The mean female size is 2.52 mm (n = 2; SD = 1.025), and for male 2.610 mm (n = 2; SD = 0.8697). The ratio size (male: female) is 1.036. The sex ratio (Female : Male) is 1. |  issued from : J.M. Bradford in Hydrobiologia, 1988, 167/168. [p.76, Table 2]. Selected characters of species of the genus Undinula. | | | | | | | Remarques sur les tailles :
Les valeurs minimales et maximales chez les femelles adultes de la famille des Calanidae sont indiquées sur la figure 1 (en ordonnée: longueur totale en mm; en abscisse : Calanoides de 1 à 5, Calanus de 6 à 19, Canthocalanus : 20, Cosmocalanus : 21 et 22, Mesocalanus : 23 et 24, Nannocalanus : 25; Neocalanus de 26 à 31; Undinula : 32
Figure 1 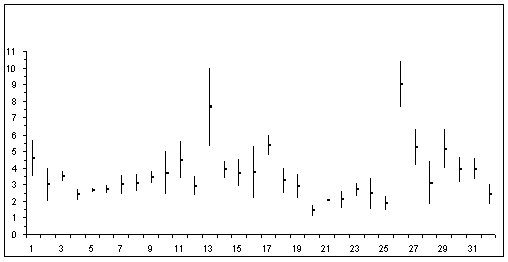
Tableau I: espèces, longueur totale, localisations et profondeurs caractéristiques (E: épipélagique; M: méso-; B: bathy-).
espèces |
Lg (mm) |
|
|
Neocalanus cristatus |
7,50 à 10,50 |
Arct. & sub-arct. (Pacif.) |
E-B |
Calanus hyperboreus |
5,50 à 10 |
Arct. & sub-arct. (Atlant.) |
|
Calanoides acutus |
± 3,50 à ± 6 |
Antarct. & sub-antarct. |
E-M |
Calanus glacialis |
- |
Arct. & sub-arct. |
|
Calanus propinquus |
- |
Antarct. & sub-antarct. |
E-M |
Neocalanus flemingeri |
- |
sub-arct. (Pacif.) |
|
Neocalanus plumchrus |
- |
sub-arct. (Pacif.) |
|
Calanus pacificus |
2,15 à 5 |
sub-arct., tempéré froid |
|
Neocalanus robustior |
- |
cosmop, tropic., subarct. |
E-M |
Neocalanus tonsus |
- |
sub-antarct., tropic. |
|
Calanus finmarchicus |
- |
tempéré froid |
E-M |
Calanoides carinatus |
± 2,50 à ± 4 |
cosmop, sub-antarct, tropic. |
E-B |
Calanoides macrocarinatus |
- |
sub-antarct. |
E-B |
Calanus australis |
- |
sub-antarct. |
E-B |
Calanus chilensis |
- |
tempéré froid |
|
Calanus euxinus |
- |
tempéré |
|
Calanus helgolandicus |
- |
tempéré |
E-M |
Calanus simillimus |
- |
sub-antarct. |
|
Calanus sinicus |
- |
tropic., tempéré froid |
|
Calanus marshallae |
- |
sub-arct. |
|
Calanus jaschnovi |
- |
tempéré |
|
Neocalanus gracilis |
- |
cosmop., topic., tempéré froid |
|
Calanoides patagoniensis |
± 2 à ± 3 |
sub-antarct. |
E-?M |
Calanoides philippinensis |
- |
tropic. |
M |
Calanus agulhensis |
- |
tempéré |
|
Cosmocalanus caroli |
- |
tropic. |
|
Cosmocalanus darwini |
- |
cosmop., topic., tempéré froid |
E-?M |
Mesocalanus lighti |
- |
sub-tropic. |
|
Mesocalanus tenuicornis |
- |
cosmop., topic., tempéré |
E-B |
Undinula vulgaris |
- |
cosmop., tropic. |
|
Nannocalanus minor |
- |
cosmop., tropic., tempéré froid |
E-M |
Canthocalanus pauper |
- |
tropic. (Indo-Pacif.) |
E |
| | | | | | | | | | | |
|
|
 Toute utilisation de ce site pour une publication sera mentionnée avec la référence suivante : Toute utilisation de ce site pour une publication sera mentionnée avec la référence suivante :
Razouls C., Desreumaux N., Kouwenberg J. et de Bovée F., 2005-2025. - Biodiversité des Copépodes planctoniques marins (morphologie, répartition géographique et données biologiques). Sorbonne Université, CNRS. Disponible sur http://copepodes.obs-banyuls.fr [Accédé le 02 octobre 2025] © copyright 2005-2025 Sorbonne Université, CNRS
|
|
 |
 |















