|
|
 |
|
Calanoida ( Order ) |
|
|
|
Clausocalanoidea ( Superfamily ) |
|
|
| |
| | | |
| Clausocalanidae Giesbrecht, 1892 ( Clausocalanoidea ) | | Syn.: | Clausocalanina Giesbrecht, 1892 (p.49); Clausocalaninae : Esterly, 1905 (p.141); Pseudocalanidae Sars, 1900 (p.69); 1901 a (1903) (p.19); Esterly, 1924 (p.88); Gurney, 1931 a (p.84); Rose, 1933 a (p.79); Brodsky, 1950 (1967) (p.83, 110, clé des G.); Farran & Vervoort, 1951 e (n°37, p.3); Tanaka, 1956 c (p.381); Gonzalez & Bowman, 1965 (p.246); Björnberg, 1972 (p.24); Razouls, 1972 (p. Annexe: p.28); Andronov, 1974 a (p.1005); Björnberg & al., 1981 (p.626); Brodsky & al., 1983 (p.144, 147, 218, Genera Key); Sazhina, 1985 (p.113, Nauplius); | | Ref.: | Bowman & Abele, 1982 (p.9); Razouls, 1982 (p.123); 1993 (p.310); Bayly, 1982 (p.162); Andronov & Vyshkvartzeva, 1986 (B.Z.N., 43, 3, p.297); Bowman, 1987 (B.Z.N, 44, 2, p.129); Mauchline, 1988 (p.726: cuticular pores); Huys & Boxshall, 1991 (p.461); Razouls, 1993 (p.310); Vyshkvartzeva, 1994 (p.119); Bradford-Grieve, 1994 (p.106, Def.); Madhupratap & al., 1996 (p.863, Table 5: %/copepods); Chihara & Murano, 1997 (p.775); Bradford-Grieve & al., 1999 (p.878, 902, 904, 914, 915: Genera Key); Ohtsuka & Huys, 2001 (p.461); Boxshall & Halsey, 2004 (p.15, 16; 49; 91: Def.; p.92: Genera Key); Vives & Shmeleva, 2007 (p.611, part. Genera Key); Blanco-Bercial & al., 2011 (p.103, Table 1, Fig. 2, 3, 4, Biol. mol, phylogeny); Laakmann & al., 2019 (p.330, fig.1, 2, 3, phylogenetic relationships); Hirai & al., 2020 (p.1, Fig.4: metabarcoding, Fig.8: OTUs distribution patterns, Fig.9: phylogenetic analysis)
Bradford-Grieve J.M., (2002 onwards). Key to calanoid copepod families. Version 1 : 2 oct 2002. http://www.crustacea.net/crustace/calanoida/index.htm  | | Rem.: | Type-genus: Clausocalanus Giesbrecht, 1888. Total: 8 G.: Clausocalanus, Ctenocalanus, Drepanopus, Farrania, Microcalanus, Peniculoides, Pseudocalanus, Spicipes.
For Marhkaseva & Renz (2015, p.1044) attribution of the new genus Peniculoides Markhaseva & Renz (2015) to the Clausocalanidae is provisional and awaits the description of undamaged female antennules and the discovery of the male.
Key to genera from Boxshall & Halsey (2004, p.92) (Peniculoides not included) :
1 – P1 with 4 setae on 1-segmented endopod ………. 2.
1’ – P1 with 5 setae on 1-segmented endopod …….. 3.
2 – P1 w-ith 1-segmented exopod ; P2 to P4 with 1-segmented endopods …….. Spicipes.
2’ – P1 with 3-segmented exopod ; P2 to P4 with 2 or 3-segmented endopods …….. Microcalanus.
3 – P5 absent or rudimentary in female ; male P5 4-segmented on left side, 5-segmented on right side, both terminating in spines ……. Pseudocalanus.
3’ – P5 female 2 or 3-segmented ; male P5 strongly asymmetrical ……. 4.
4 – female P5 asymmetrical, uniramous and 3-segmented on left side only ; outer margin spines on 3rd exopodal segment of P3 and P4 with cristate inner margins ……. Ctenocalanus.
4’ – Female P5 symmetrical, 2 or 3-segmented on both sides ; outer margin spines on these segments not cristate …….. 5.
5 – Distal margin of basis of P3 ornamented with elaborate spinous processes ………. Clausocalanus.
5’ – Distal margin of basis of P3 without processs …….. 6.
6 – Female P5 2-segmented, terminating in long curved spinous process ……… Drepanopus.
6’ – Female P5 3-segmented, terminating in 3 curved spines …….. Farrania. |  issued from : E.L. Markhaseva & F.D. Ferrari in Invert. Zool., 2005, 2 (2). [p.162, Table 4] Setation of oral parts in females Clausocalanidae (Clausocalanoidea) and ancestral condition of setation. |
 Issued from : G.A. Boxshall & S.H. Halsey in An Introduction to Copepod Diversity. The Ray Society, 2004, No 166, Part. I. [p.91]. Armature formula of swimming legs P1 to P4. Setation of both rami sometimes reduced. Nota: Males typically lacking outer margin spines on 1st and 2nd exopodal segments of P1. 3rd exopodal segment of P2 to P4 with only 2 spines in Spicipes and Drepanopus bispinosus Bayly. Surface of basis of P2 and P3, and of rami of P1 to P4 with or without ornamentation of spinules. - Female P5 uniramous and 3-segmented: comprising fused coxae and intercoxal sclerite, and 2 free segments: 1st free segment (basis) unarmed, 2nd free segment (exopod) with 2 spinous processes apicallyt. P5 sometimes rudimentary, absent in female Pseudocalanus and Microcalanus. - Male P5 rarely biramous with rudimentary endopod, typically uniramous and asymmetrical; short leg, 1 to 3-segmented with minute spinules on apex: long leg elongate, with short coxa fused to body, basis and 3 exopodal segments each elongate; armed with 2 apical setae. Short leg typically on right side and long leg on left, occasionally reversed with long leg on right side and short on left. - Eggs released into water or retained in single ventral mass, as in Pseudocalanus and some species of Clausocalanus. |
 Issued from : E.L. Markhaseva & J. Renz in Crustaceana, 2015, 88 (9). [p.1042, Table I]. Differences in A1 and Mx2 setation between taxa of Clausocalanidae and Aetididae. The data obtained provided the basis for a primary revision of the clausocalanid characters through the re-examination of the antennule and oral parts of Microcalanus Sars, 1901, Ctenocalanus Giesbrecht, 1888, Farrania Sars, 1920 and a few species of Aetideidae. As a result of the study a combination of characters is proposed to distinguish between the families Clausocalanidae and Aetideidae. The genera Clausocalanus, Drepanopus, Ctenocalanus, Microcalanus and Pseudocalanus of the family Clausocalanidae have been found to share the following combination of characters that distinguish them from the aetideid genera : 1 - Male A1 ancestral segments I-II fused (vs male A1 ancestral segments I-II separate in Aetideiae (Bradford-Grieve & al., 2010). 2 - Female A1 ancestral segments XIII, XVII, XIX, XXI armed as ''1s + 1ae'' or only ''1s'' (vs XIII, XVII, XIX armed as ''2s"", or ''2s + 1ae'' in Aetideidae). 3 - Mx2 praecoxal endite armed with 4 or 5 long setae (vs 3 long setae, or 3 long setae plus 1 very small seta in Aetideidae) ( see in Aetideopsis armata fig.6, C). A further more detailed study of the clausocalanid morphology can add to the list of their diagnostic characters in order to compile the differential diagnosis of the family Clausocalanidae among the other members of the superfamily. |
 Issued from : G.A. Boxshall & S.H. Halsey inThe Ray Society, 2004, N° 166. [p.93, Fig.15]. Clausocalanidae. A, Pseudocalanus elongatus, habitus female; B, habitus male; C, female P2; D, male P5; E, Microcalanus pusillus male P5; F, Drepanopus pectinatus female P5; G, Clausocalanus arcuicornis basis of female P2; H, female P5. Sars, 1901: A-D; Sars 1903a: E; Giesbrecht, 1893a: F-H. | | | | | | (1) Clausocalanus Giesbrecht, 1888 | |
| | Ref.: | Giesbrecht, 1892 (p.50, 185); Giesbrecht & Schmeil, 1898 (p.27); Wheeler, 1901(p.171); Esterly, 1905 (p.142); van Breemen, 1908 a (p.22); A. Scott, 1909 (p.31); Esterly, 1924 (p.88); Wilson, 1932 a (p.42); Rose, 1933 a (p.80); Mori, 1937 (1964) (p.34); Vervoort, 1946 (p.140); Davis, 1949 (p.20); Brodsky, 1950 (1967) (p.117); Farran & Vervoort, 1951 f (n°38, p.3); Tanaka, 1956 c (p.382); Gonzalez & Bowman, 1965 (p.246); Frost & Fleminger, 1968 (p.15, Rev., Key spp.); Carli & Crisafi, 1969 (p.277); Razouls, 1972 (Annexe: p.28, Key F); 1982 (p.127); Gardner & Szabo, 1982 (p.179); Brodsky & al., 1983 (p.228); Zheng Zhong & al., 1984 (1989) (p.234, spp. Key); Mauchline, 1988 (p.726); Ferrari, 1992 (p.392, tab.3); Razouls, 1993 (p.310); Bradford-Grieve, 1994 (p.107, Def.); Chihara & Murano, 1997 (p.775, spp. Key); Mauchline, 1998 (p.85); Bradford-Grieve & al., 1999 (p.915, spp. Key); Boxshall & Halsey, 2004 (p.92); Mulyadi, p.181, Def.); Vives & Shmeleva, 2007 (p.612, spp. Key); Bucklin & Frost, 2009 (p.111, morphology vs molecular analysis, phylogeny). | | Rem.: | type: Calanus mastigophorus Claus,1863. The species identification before 1968 is often questionable and the geographical distributions are determined after Frost & Fleminger (1968), completed by later authors.
Total: 13 spp.
Frost & Fleminger (1968, p.17, 29, 44, 66) define 3 groups:
Groupe 1: C. mastigophorus, C. lividus, C. ingens and C. laticeps.
Groupe 2: C. arcuicornis, C. farrani, C. jobei, C. minor, C. paululus.
Groupe 3: C. pergens, C. brevipes, C. parapergens, C. furcatus.
Diagnosis from Frost & Fleminger (1968, p.15) :
- Cephalosome and pedigerous segment 1 fused, pedigerous segments 4 and 5 fused.
- Medial caudal seta short, located on dorsal surface of caudal ramus ; lateral-most caudal seta reduced to a short, lateral spine ; 2 apical and 2 subapical caudal setae long.
A1 segment 25 fused to distal posterior corner of segment 24.
- Exopod of A2 1.5 or more times as long as endopod.
- Exopod of P1-P4 trimemous ; endopod of P1 unimerous, of P2 biremous, of P3 and P4 trimerous.
- Basis (B2) of P2 and P3 broadening distally to about 1.5 or more times their width in region of attacment to coxa (B1) ; distal posterior margin with 3 or more spiniform processes.
Female :
- Urosome of 4 somites.
- Rostrum of 2 short, rigid spiniform processes.
- A1 23-segmented with segments 8-9 and 24-25 fused.
- Right and left P5 present, uniramus, trimerous, essentially symmetrical, distal segment produced distally into a short, pointed, bifid process.
Male :
- Urosome of 5 somites.
- Anal somite very short.
- Rostrum reduced to a single median, ventrally protruding knob or not well developed.
- A1 with segments 1-2, 8-9, 13-14, 15-16, 20-21 , and 24-25 completely fused, and with incomplete fusion of segments 4 to 8-9, 8-9 to 13-14, and 13-14 to 15-16.
- Right and left P5 present, uniramus, rami of very unequal length, longer ramus somewhat styliform, pentamerous, 5th segment short and attached subapically to 4th segment ; 1st segment more than twice as long as uni-, bi-, or trimerous shorter ramus of opposing leg.
Clausocalanus furcatus shows any differences among the other congeners. | | Remarks on dimensions and sex ratio: | | The mean female size is 1.289 mm (n = 26; SD = 0.4019), and the mean male size is 1.0 mm (n = 26; S= 0.2909). The size ratio (Male: Female) is 0.776. The sex ratio is 1. | | | | | (2) Ctenocalanus Giesbrecht, 1888 | |
| | Ref.: | Giesbrecht, 1892 (p.50,194); Giesbrecht & Schmeil, 1898 (p.28); van Breemen, 1908 a (p.24); Esterly, 1924 (p.90); Rose, 1933 a (p.83); Mori, 1937 (1964) (p.37); Davis, 1949 (p.20); Brodsky, 1950 (1967) (p.121); Farran & Vervoort, 1951 f (n°38, p.3); Tanaka, 1956 c (p.384); Ramirez, 1966 (p.12); Razouls, 1982 (p.131); Gardner & Szabo, 1982 (p.187); Brodsky & al.,1983 (p.235); Ferrari, 1992 (p.392, tab.3); Razouls, 1993 (p.310); Bradford-Grieve, 1994 (p.125, Déf., Rem.); Chihara & Murano, 1997 (p.778); Mauchline, 1998 (p.85); Bradford-Grieve & al., 1999 (p.915, 917: clé spp.); Boxshall & Halsey, 2004 (p.92); Vives & Shmeleva, 2007 (p.626, spp. Key) | | Rem.: | Type poorly specified: Ctenocalanus vanus Giesbrecht,1888.
Total: 5 species.
Diagnosis from Bradford-Grieve (1994, p.95) :
- As for the family definition.
- Head and pediger segment 1 fused, pediger segments 4 and 5 fused.
- Rostrum of 2 fine filaments in both sexes.
- A1 female with segments 1 and 2, 9 and 10, and 24 and 25 usually separate ; of the male usually with segments 1 and 2, 8 to 10, and 23 to 25 fused.
- A2 exopodal segments 2 and 3 fused.
- Male P1 exopodal segments 1 and 2 without outer-edge spines.
- Basis of P2 and P3 not enlarged posterodistally but have posterodistal spinules which are very small in female but larger in the male.
- External spines on exopodal segment 3 of P3 and P4 finely toothed.
- Female P5 asymmetrical, present on left.
- Male P5 asymmetrical, uniramous on left, very reduced on right. | | Remarks on dimensions and sex ratio: | | The mean female size is 1.184 mm (n = 8; SD = 0.3057), and the mean male size is 1.282 mm (n = 6; SD = 0.3633), The size ratio (Male: Female) is 1,083; the males thus being slightly longer than the females, that is not the generality in copepods. The sex ratio is 1.25. | | | | Drepanopsis Wolfenden, 1911 | |
| | Ref.: | Wolfenden, 1911 (p.245) | | Rem.: | type: Drepanopsis frigidus Wolfenden,1911. nom. préoc. Cf. Farrania | | | | (3) Drepanopus Brady, 1883 (part.) | |
| | Ref.: | Brady, 1883 (p.76); Giesbrecht, 1892 (p.51, 201); Giesbrecht & Schmeil, 1898 (p.28); van Breemen, 1908 a (p.27); Wolfenden, 1911 (p.245); Rose, 1937 (p.162); Brodsky, 1950 (1967) (p.138); Vervoort, 1951 (p.69); 1957 (p.40); Farran & Vervoort, 1951 f (n°38, p.3); Ramirez, 1966 (p.11); Bayly, 1982 (p.161, Rev.); Razouls, 1982 (p.134); Brodsky & al., 1983 (p.237); Hulsemann, 1985 a (p.910); Ferrari, 1992 (p.392, tab.3); Razouls, 1993 (p.310); Bradford-Grieve, 1994 (p.127, Déf.); Mauchline, 1998 (p.81, 89: M; p.84, 85: F); Boxshall & Halsey, 2004 (p.92) | | Rem.: | type: Drepanopus pectinatus Brady,1883.
Total: 4 species.
Diagnosis from Bradford-Grieve (1994, p.95) :
- As for the family definition.
- Head and pediger segment 1, pediger segments 4 and 5 fused or separate.
- Male rostrum with 2 filaments;
- A1 female 23- or 24-segmented.
- Male A1 slightly asymmetrical, 21- to 23-segmented on the left and 20- to 22-segmented on the right.
- Female genital segment long with distinct anteroventral swelling.
- Both male and female P1 exopods with 3 external spines;
- Endopods of P2 1- or 2-segmented, of P3 2- or 3-segmented.
- Basis of P2 and P3 not widened and without dentiform processes.
- Exopodal segment 3 of P2-P4 with 2 (D. bispinosus) or 3 outer-edge spines.
- Posterior surfaces of P2 and P3 may carry spinules in the female.
- Female P5 symmetrical, 2-segmented with a large curved terminal spine, pectinated along its distal outer half.
- Male P5 prehensile, asymmetrical, exopods 2- or 3-segmented on right ending in a long curved claw, 3-segmented and much shorter on the left; endopods rudimentary or absent. | | Remarks on dimensions and sex ratio: | | The mean female size is 2.038 mm (n = 8; SD = 0.7558), and the mean male size is 1.508 mm (n = 8; SD = 0.4630). The size ratio (Male: Female) is 0.740. The sex-ratio is 1. | | | | | Syn.: | Drepanopsis Wolfenden, 1911(p.245), nom déjà usité; Sewell, 1929 (p.96); Rose, 1933 a (p.84); 1937 (p.161); Brodsky, 1950 (1967) (p.140); Vervoort, 1951 (p.69); Farran & Vervoort, 1951 f (n°38, p.3); Tanaka, 1956 c (p.399); Brodsky & al., 1983 (p.239) | | Ref.: | Sars, 1920 c (p.4); 1925 (p.36); C.B. Wilson, 1950 (p.227); Vervoort, 1957 (p.39); Razouls, 1982 (p.133); Mauchline, 1988 (p.726); Razouls, 1993 (p.310); Mauchline, 1998 (p.81: M; p.84: F); Bradford-Grieve, 1994 (p.129, Déf.); Boxshall & Halsey, 2004 (p.92); Vives & Shmeleva, 2007 (p.630, spp. Key) | | Rem.: | Type: Farrania oblonga Sars,1920. 5 spp. (of which 1 doubtful) + 1 unidentified.
Diagnosis from Bradford-Grieve (1994, p.95) :
- As for the family definition.
- Rostrum or rostral filaments absent.
- Head and pediger segment 1 fused or separate, pediger segments 4 and 5 separate, usually extended into points.
- A1 24-segmented, segments 8-9 fused, all segments with very long setae.
- A2 endopod longer than or equal to the exopod which is 7-segmented.
- Md with a very small endopod.
- Posterior surfaces of coxa and basis and endopods of P2 and P3 may be ornamented with spinules.
- Female P5 3-segmented with 2 or 3 terminal spines.
- Male P5 biramous and styliform and asymmetrical; endopods 1-segmented, exopods 3-segmented, left exopod shorter than the right, terminated by an elongate spine. | | Remarks on dimensions and sex ratio: | | The mean female size is 3.149 mm (n = 9; SD = 0.5780). In the case of only one male: 3.01 mm against 3.56 mm for the female; being a size ratio (M:F) of 0.846. The sex ratio is 0.2 or at best 0.25% if one considers F. oblonga as a synonym. | | | | | (5) Microcalanus Sars, 1901 | |
| | Syn.: | Pseudocalanus (part) : Sars, 1900 | | Ref.: | Sars, 1901 (1903) (p.20); 1903 (p.155); van Breemen, 1908 a (p.26); Wolfenden, 1911 (p.286); Rose, 1933 a (p.80); Brodsky, 1950 (1967) (p.115); Tanaka, 1956 c (p.381, 385); Farran & Vervoort, 1951 e (n°37, p.3); Gardner & Szabo, 1982 (Rem., p.175); Razouls, 1982 (p.126); Brodsky & al., 1983 (p.225); Razouls, 1993 (p.310); Bradford-Grieve, 1994 (p.129, Def.); Chihara & Murano, 1997 (p.779); Mauchline, 1998 (p.88: F; p.89: M); Boxshall & Halsey, 2004 (p.92); Vives & Shmeleva, 2007 (p.632) | | Rem.: | type: Pseudocalanus pygmaeus Sars,1900.
Total: 1 species (with 2 varieties) or 2 species (according to the authors) + 2 unidentified.
Only a genetic analysis could clearly define the extent of the variability, and the validity of two species or more.
Diagnosis from Bradford-Grieve (1994, p.95) :
- As for the family definition.
- Head and pediger segment 1 fused, pediger segments 4 and 5 fused.
- Female A1 24-segmented with segments 8 and 9 fused.
- Male A1 20-segmented, segments 1-2, 8-11, and 24-25 fused.
- A2 exopod longer than the endopod, exopod segments 2-3 fused.
- P1 exopodal segment 1 without 1 external edge spine, endopod with 4 setae.
- Female P5 absent.
- Male P5 small, asymmetrical, left leg slender, 6-segmented, right leg very small 3-segmented, last segment not styliform. | | Remarks on dimensions and sex ratio: | | The mean female size is 0.755 mm (n = 4; SD = 0.2479), and in male 0.813 mm. The size ratio (male: Female) is 1.044. The sex ratio (F: M) is 1. | | | | (6) Peniculoides Markhaseva & Renz, 2015 | |
| | Ref.: | Markhaseva & Renz, 2015 (p.1033). | | Rem.: | Diagnosis of genus after Markhaseva & Renz (2015, p.1033) :
Female:
- Rostrum as small round-triangular projection bearing 2 filaments.
- Cephalosome and pediger 1, pedigers 4 and 5 separate.- A1 of 24 free segments.
- A2 endopod segment 1 with 1 seta; exopod with 1,1-1-1,1,1,1,1,0 and 3 setae.
- Md gnathobase strong, heavily sclerotisede; basis with 3 setae; endopod segment 1 with 2 setae, segment 2 with 9 setae.
Mx1 praecoxal arthrite larger than the remaining part of the limb, heavily sclerotized, with 9 terminal and 2 posterior setae; coxal epipodite without setae; coxal, proximal basal and distal endites with 2 setae each; endopod with 4 setae: exopod with 4 or 5 setae.
- Mx2 praecoxal to basal endites with distal part flat and round, in coxal and basal endites its surface is densely covered by short spinules, brush-like, setal formula 4, 3, 2, 1 +1, 2; endopod with 3 setae;
- Mxp praecoxal endites setal formula 1, 2 a,d 3, coxal endite with 3 setae; 5 free endopod segments with 4, 4, 3, 3, and 4 setae.
- P1 to P4 segmentation and setation typical of the superfamily Clausocalanodea.
- P5 uniramous, 3-segmented; left leg with 3, right leg with 2 terminal spines.
- Male unknown.
Apomorphies for the genus are: 1- Md gnathobase strong and heavily sclerotized; 2 - Mx1 praecoxal arthrite large and heavily sclerotized; 3 - Mx1 exopod with 4-5 setae, coxal epipodite lacking setae; 4 - Mx2 coxal and basal endites surface densely covered by short spinules, brush-like.
For Markhaseva & Renz (2015, p.1044) The morphology of its mandible gnathobase, maxillule praecoxal arthrite and its specialized maxilla all of which strongly deviate from typical oral limbs of other Clausocalanidae and from those described for the superfamily Clausocalanoidea as a whole. The only other genus in the superfamily with highly specialized oral parts is Chiridiella Sars, 1907 (Aetideidae). The brush-like surfaces of the maxilla endites, which are densely covered by short spinules and provide a large surface for attaching or holding prey, suggest its feeding mode would probably point more towards a carnivorous than detrivorous life style. The maxilla structure might also serve for brushing or cleaning the food of unwanted particles. | | Remarks on dimensions and sex ratio: | | For one female only, the body size is 2.10 mm. | | | | | (7) Pseudocalanus Boeck, 1872 | |
| | Ref.: | Brady, 1878 (p.44); Giesbrecht, 1892 (p.51, 196); Giesbrecht & Schmeil, 1898 (p.28); Sars, 1900 (p.69); 1901 a (1903) (p.19); van Breemen, 1908 a (p.24); Sars, 1903 (p.19); Wilson, 1932 a (p.43); Rose, 1933 a (p.79); Mori, 1937 (1964) (p.36); Davis, 1949 (p.18); Brodsky, 1950 (1967) (p.112); Farran & Vervoort, 1951 e (n°3, p.3); Woods, 1969 (p.543, Rem.); Sheldon & al., 1972 (p.327, fig. 13: size particle vs production's rate); Corkett & McLaren, 1978 (p.3); Klein Breteler, 1982 (p.5, figs.); Robins & McLaren, 1982 (p.529); Razouls, 1982 (p.123); Gardner & Szabo, 1982 (p.171); Brodsky & al., 1983 (p.220); Mauchline, 1988 (p.725); Frost, 1989 (p.525, Rev., Key spp.); Ferrari, 1992 (p.392, tab.3); Razouls, 1993 (p.310); Bradford-Grieve, 1994 (p.130, Déf.); Chihara & Murano, 1997 (p.778); Mauchline, 1998 (p.88: F; p.90: M); Boxshall & Halsey, 2004 (p.92); Vives & Shmeleva, 2007 (p.635) ; Questel & al., 2016 (p.610, fig. 3, genetic analysis, phylogeography). | | Rem.: | Type: Clausia elongata Boeck,1872.
This genus comprises 7 species that have often been confused because of their weak morphological differentiation; there are confined to high latitudes in the northern hemisphere (Cf. Frost, 1989, p.529) + 1 unidentified.
I have restricted the distribution to the most reliable.
Diagnosis from Bradford-Grieve (1994, p.95) :
- As for the family definition.
- Head and pediger segment 1 fused, pediger segments 4 and 5 fused.
- Last metasomal segment with rounded corners.
- Rostrum in male consisting of 2 filaments.
- A1 does not extend beyond the caudal rami, 24-segmented in female (segments 8-9 fused), in male usually 19-segmented.
- Female P5 absent.
- Male P5 uniramous, left slightly longer than the right; terminal segment of left leg much shorter than the preceding segment and bearing a row of spinules and 1 long, slender terminal spine, segments of the right leg taper distally with the terminal segment about as long as the preceding segments and styliform.
Holmborn & al. (2011, p.514) point to when studying the geographic distribution of all Pseudocalanus species, Frost (1989) found that two or more species of this genus commonly co-occur. Therefore, due to the difficulty of visually discriminating between Pseudocalanus species, the reported biology and ecology of single species is fairly unreliable in the litterature. In ecosystems containing multiple species of Pseudocalanus, the use of genetic methods, such as the RFLP protocol and DNA sequencing described by the authors will greatly improve our understanding of the ecological significance of individual species. Protocol of this kind are easy to develop and provide an excellent tool for discriminating between morphologically indistinguishable species.
Questel & al. (2016, p.611) underline that the genus is comprised of 7 species that co-occur as differing assemblages within their geographic ranges; They are herbivorous epipelagic filter-feeders that target a wide size range of food particles, such as diatoms, flagellates and coccolithophores, and opportunistically feed on sea-ice algae in Arctic regions. The species display only very subtle morphological differences in the adult stage with diagnostic features dependent upon the shape of the urosomal segment containing the genital pore as well as the shape of the seminal receptacle ir-tself (Frost, 1989). However, species show typical levels of interspecific genetic divergence for COI sequences of copepods (10-23%) (Bucklin & al., 2003). These extremely subtle morphological differences have created difficulties in accurate species identification and have resulted in a general lack of detailed species-specific distribution data, with co-occuring species typically treated as a species complex and reported simply as Pseudocalanus spp.
P. newmani and P. mimus are considered as temperate species. P. acuspes and P. minutus are Arctic species. Within the northern Gulf of Alaska, the predominant species are P. mimus and P. newmani with P. minutus present in low numbers in both the shelf region and within Prince William Sound (See Napp & al., 2005), and is also found to numerically dominate the outer domains of the Bering Sea (See Bailey & al., 2015).
P. acuspes and P. minutus, P. newmani numerically dominate the species complex in the shallow Chukchi and Beaufort Sea.
In the Pacific, the geographical distribution of P. acuspes is primarily restricted to the North Pacific Ocean and Pacific Arctic Region, and extends south into the Bering Sea (Bailey & al., 2015), an ecosystem heavily influenced by seasonal ice cover. | | Remarks on dimensions and sex ratio: | | The mean female size is 1.531 mm (n = 14; SD = 1.5314), and the mean male size is 1.215 mm (n = 14; SD = 0.4056). The size ratio (Male: Female) is 0.793. The sex-ratio is 1. | | | | | (8) Spicipes Grice & Hulsemann, 1965 | |
| | Ref.: | Grice & Hulsemann, 1965 (p.225); Razouls, 1982 (p.135); 1993 (p.310); Bradford-Grieve, 1994 (p.131, Def.); Mauchline, 1998 (p.66: F); Boxshall & Halsey, 2004 (p.92); Vives & Shmeleva, 2007 (p.637) | | Rem.: | type: Spicipes nanseni Grice & Hulsemann,1965.
Total: 1 species.
Diagnosis from Bradford-Grieve (1994, p.95) :
- As for the family definition.
- Head and pediger segment 1 separate, pediger segments 4 and 5 separate.
- Rostrum small and rounded.
- A1 23-segmented, extending to the end of the caudal rami, segments 8 and 9, 24 and 25 fused.
- Exopod of A2 longer than endopod, segments 1 to 3 apparently fused.
- Endopod and exopod of Md subequal, blade with small uniform teeth.
- Mx1 and Mx2 appear to have a reduced compliment of setae.
- Exopod of P1 1-segmented, of P2-P4 3-segmented.
- Endopod of P1-P4 1-segmented.
- Exopodal segment 3 of P2-P4 with 2 outer edge spines and 4 inner setae.
- The terminal spines of P2 and P3 with outer margins serrate (P4 damaged ?).
- P5 female 3-segmented, the terminal segment bearing a single spiniform seta.
- Male unknown in 2015.
After Bradford-Grieve (1994, p.131) this genus should be close to Farrania in spite of the 1-segmented endopods and exopodal segment 3 of P2 and P3 at least having 2 outer-edge spines. The discovery of Drepanopus bispinosus which also has 2 outer-edge spines on the exopodal segment 3 of P2-P4 indicates that Spicipes is not alone in the Clausocalanidae in blurring the distinction between it and the Paracalanidae (see Bayly, 1982). The apparent reduction in the female mouthparts is not typical for the Clausocalanidae so the position of this genus must remain provisional until more specimens and males are discovered. | | | | | | Remark on the sizes :
The minimum and maximum values in mm for the adult female sizes in the Clausocalanidae family are shown in Figure 1 with the n° of species on the x-axis ( Clausocalanus 1 to 13, Ctenocalanus 14 to 18, Drepanopus 19 to 22, Farrania 23 to 27, Microcalanus : 28 and 29, Pseudocalanus 30 to 36, Spicipe s: 37.
figure 1

Table I: classification of species by size groups
espèces |
Lg (mm) |
Localization |
Depth |
Farrania lyra |
3,30 à 4 |
temperate |
|
Farrania oblonga |
- |
temperate |
|
Farrania orba |
- |
temperate |
|
Farrania pacifica |
- |
? Sub-arct., cold temperate |
B-A |
Farrania frigida |
±2 à 3 |
cosmop., Antarct., sub-tropic |
M-B |
Drepanopus bispinosus |
- |
Antarct. |
|
Drepanopus pectinatus |
- |
Antarct., sub-Antarct. |
N |
Drepanopus forcipatus |
± 1,50 à ± 2,5 |
sub-Antarct. |
|
Pseudocalanus major |
- |
Arct. |
|
Pseudocalanus acuspes |
- |
Arct., sub-arct. |
|
Clausocalanus ingens |
- |
Sub-antarct., cold temperate |
|
Clausocalanus lividus |
1 à ± 2 |
cosmop., warm temperate |
E |
Pseudocalanus minutus |
- |
cosmop., Arct., sub-arct. |
|
Clausocalanus mastigophorus |
- |
warm temperate, tropic. |
|
Clausocalanus brevipes |
- |
sub-antarct. |
|
Pseudocalanus mimus |
- |
sub-arct. |
|
Pseudocalanus moultoni |
- |
sub-arct., cold temperate |
L |
Clausocalanus laticeps |
- |
Antarct., sub-antarct. |
|
Clausocalanus arcuicornis |
- |
cosmop., temperate |
E |
Pseudocalanus elongatus |
- |
temperate |
|
Clausocalanus parapergens |
1 à ± 1,5 |
cosmop., sub-tropic. |
E |
Clausocalanus jobei |
- |
cosmop., tropic., sub-tropic. |
E, N |
Clausocalanus furcatus |
- |
cosmop., tropic., sub-tropic. |
|
Ctenocalanus heronae |
- |
tropic. |
|
Ctenocalanus vanus |
- |
cosmop., sub-tropic., temperate |
E-B |
Pseudocalanus newmani |
- |
Arct., sub-arct. |
|
Drepanopus bungei |
- |
Arct. |
|
Spicipes nanseni |
- |
sub-tropic. |
B |
Ctenocalanus citer |
- |
Antarct., sub-antarct. |
|
Ctenocalanus campaneri |
- |
tropic. |
E-M |
Clausocalanus minor |
0,6 à ± 1 |
tropic., sub-tropic. |
|
Ctenocalanus tageae |
- |
tropic. |
|
Clausocalanus farrani |
- |
tropic., sub-tropic. |
|
Clausocalanus pergens |
- |
tropic., sub-tropic. |
E-B |
Microcalanus pygmaeus |
- |
sub-antarct., sub-tropic., sub-arct. |
E-B |
Clausocalanus paululus |
< à 1 |
sub-antarct., sub-tropic. |
E |
?Microcalanus pusillus |
- |
cosmop., Antarct., sub-trop., sub-arct. |
|
Annex:
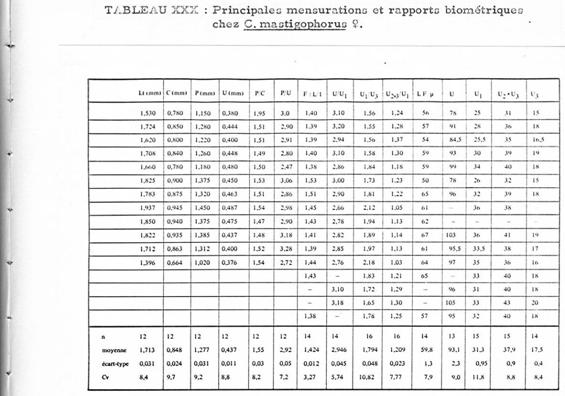
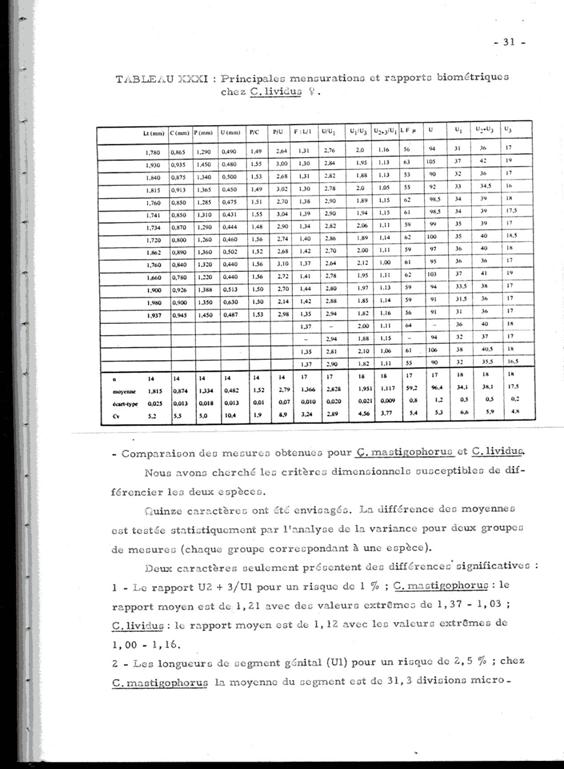
Comparative study between the females of Clausocalanus lividus and Clausocalanus mastigophorus.
The differentiation of the two species near Banyuls (Mediterranean Sea , Golfe du Lion) (Razouls 1972, Annex: p. 30) is uneasy. A rather strong variability exists in the form of the rostrum for each of the two species, in addition the form of the seminal receptacles is difficult to observe without special preparation of both species. The presence of males is also very rare.
The following tables give the measures and the ratios of certain body segments of these species. The nomenclature and the measurements are homologous to those used by Fleminger (1967) and Frost & Fleminger (1968). When the measurements are not expressed in mm or µm, one unit = 4.88 µm.
Fifteen characters were considered, the difference in means was statistically tested by analysis of variance for the two groups of measurements, each group corresponding to a species.
Only two characters show significant differences:
1 – The proportion U2+3/U1 (for a 1% risk). For C. mastigophorus the mean ratio is 1.21 (extreme values: 1.03-1.37); for C. lividus the mean ratio is 1.12 (extreme values: 1.00-1.16)
2 – The length of the genital segment (U1) for a risk of 2.5%. In C. mastigophorus lthe mean length is 152 µm (extreme values: 122 – 175.68); in C. lividus the mean length is 166 µm (extreme values: 151.28 – 185.44).
3 – The genital segment (U1) in C. lividus is longer than that in C. mastigophorus, and in respect to the following segments where it appears longer in C. lividus.
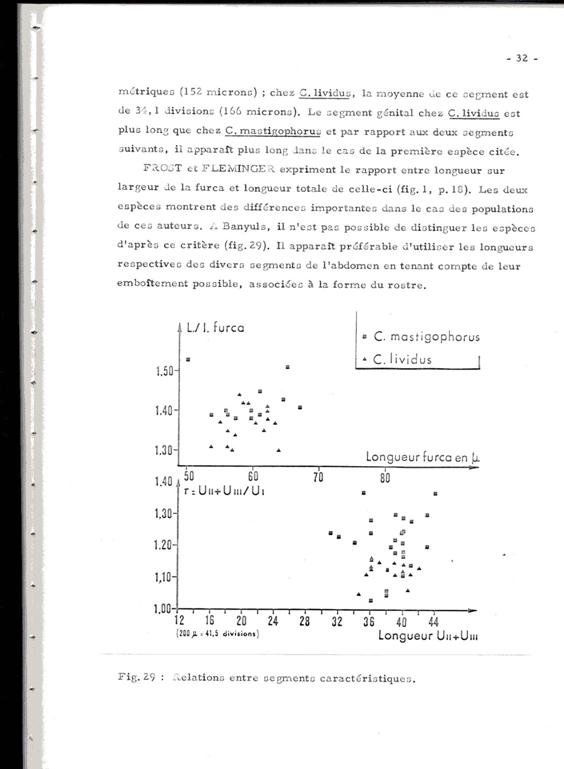
Frost & Fleminger (fig.1, p.18) express the ratio between length to width (L/w) of the furca and its total length. The two species show important differences in the case of the populations analysed by these authors. In Banyuls it is not possible to distinguish the species after this criterium (see Figure).
In conclusion, the distinction between the two species, most often co-existant, is particularly arduous in the absence of the very rare males, if not absent in our samples. This throws a doubt on the numerous identifications and all the more on the counts, noted in various works.
| | | | | | | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed February 03, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |