|
|
 |
|
Calanoida ( Ordre ) |
|
|
|
Clausocalanoidea ( Superfamille ) |
|
|
| |
| | | |
| Clausocalanidae Giesbrecht, 1892 ( Clausocalanoidea ) | | Syn.: | Clausocalanina Giesbrecht, 1892 (p.49); Clausocalaninae : Esterly, 1905 (p.141); Pseudocalanidae Sars, 1900 (p.69); 1901 a (1903) (p.19); Esterly, 1924 (p.88); Gurney, 1931 a (p.84); Rose, 1933 a (p.79); Brodsky, 1950 (1967) (p.83, 110, clé des G.); Farran & Vervoort, 1951 e (n°37, p.3); Tanaka, 1956 c (p.381); Gonzalez & Bowman, 1965 (p.246); Björnberg, 1972 (p.24); Razouls, 1972 (p. Annexe: p.28); Andronov, 1974 a (p.1005); Björnberg & al., 1981 (p.626); Brodsky & al., 1983 (p.144, 147, 218, Genera Key); Sazhina, 1985 (p.113, Nauplius); | | Ref.: | Bowman & Abele, 1982 (p.9); Razouls, 1982 (p.123); 1993 (p.310); Bayly, 1982 (p.162); Andronov & Vyshkvartzeva, 1986 (B.Z.N., 43, 3, p.297); Bowman, 1987 (B.Z.N, 44, 2, p.129); Mauchline, 1988 (p.726: cuticular pores); Huys & Boxshall, 1991 (p.461); Razouls, 1993 (p.310); Vyshkvartzeva, 1994 (p.119); Bradford-Grieve, 1994 (p.106, Def.); Madhupratap & al., 1996 (p.863, Table 5: %/copepods); Chihara & Murano, 1997 (p.775); Bradford-Grieve & al., 1999 (p.878, 902, 904, 914, 915: Genera Key); Ohtsuka & Huys, 2001 (p.461); Boxshall & Halsey, 2004 (p.15, 16; 49; 91: Def.; p.92: Genera Key); Vives & Shmeleva, 2007 (p.611, part. Genera Key); Blanco-Bercial & al., 2011 (p.103, Table 1, Fig. 2, 3, 4, Biol. mol, phylogeny); Laakmann & al., 2019 (p.330, fig.1, 2, 3, phylogenetic relationships); Hirai & al., 2020 (p.1, Fig.4: metabarcoding, Fig.8: OTUs distribution patterns, Fig.9: phylogenetic analysis)
Bradford-Grieve J.M., (2002 onwards). Key to calanoid copepod families. Version 1 : 2 oct 2002. http://www.crustacea.net/crustace/calanoida/index.htm  | | Rem.: | After Madhupratap & al. (1996), la famille des Clausocalanidae représente de 4 à 15,7 % des copepodes selon la saison dans la couche de mélange des eaux océaniques de la région ouest de l'Inde (Mer Arabe), en usant un filet type Multiple Plankton Closing Net à 200 µm de vide de maille (mesh aperture). |  issued from : E.L. Markhaseva & F.D. Ferrari in Invert. Zool., 2005, 2 (2). [p.162, Table 4] Setation of oral parts in females Clausocalanidae (Clausocalanoidea) and ancestral condition of setation. |
 Issued from : G.A. Boxshall & S.H. Halsey in An Introduction to Copepod Diversity. The Ray Society, 2004, No 166, Part. I. [p.91]. Armature formula of swimming legs P1 to P4. Setation of both rami sometimes reduced. Nota: Males typically lacking outer margin spines on 1st and 2nd exopodal segments of P1. 3rd exopodal segment of P2 to P4 with only 2 spines in Spicipes and Drepanopus bispinosus Bayly. Surface of basis of P2 and P3, and of rami of P1 to P4 with or without ornamentation of spinules. - Female P5 uniramous and 3-segmented: comprising fused coxae and intercoxal sclerite, and 2 free segments: 1st free segment (basis) unarmed, 2nd free segment (exopod) with 2 spinous processes apicallyt. P5 sometimes rudimentary, absent in female Pseudocalanus and Microcalanus. - Male P5 rarely biramous with rudimentary endopod, typically uniramous and asymmetrical; short leg, 1 to 3-segmented with minute spinules on apex: long leg elongate, with short coxa fused to body, basis and 3 exopodal segments each elongate; armed with 2 apical setae. Short leg typically on right side and long leg on left, occasionally reversed with long leg on right side and short on left. - Eggs released into water or retained in single ventral mass, as in Pseudocalanus and some species of Clausocalanus. |
 Issued from : E.L. Markhaseva & J. Renz in Crustaceana, 2015, 88 (9). [p.1042, Table I]. Differences in A1 and Mx2 setation between taxa of Clausocalanidae and Aetididae. The data obtained provided the basis for a primary revision of the clausocalanid characters through the re-examination of the antennule and oral parts of Microcalanus Sars, 1901, Ctenocalanus Giesbrecht, 1888, Farrania Sars, 1920 and a few species of Aetideidae. As a result of the study a combination of characters is proposed to distinguish between the families Clausocalanidae and Aetideidae. The genera Clausocalanus, Drepanopus, Ctenocalanus, Microcalanus and Pseudocalanus of the family Clausocalanidae have been found to share the following combination of characters that distinguish them from the aetideid genera : 1 - Male A1 ancestral segments I-II fused (vs male A1 ancestral segments I-II separate in Aetideiae (Bradford-Grieve & al., 2010). 2 - Female A1 ancestral segments XIII, XVII, XIX, XXI armed as ''1s + 1ae'' or only ''1s'' (vs XIII, XVII, XIX armed as ''2s"", or ''2s + 1ae'' in Aetideidae). 3 - Mx2 praecoxal endite armed with 4 or 5 long setae (vs 3 long setae, or 3 long setae plus 1 very small seta in Aetideidae) ( see in Aetideopsis armata fig.6, C). A further more detailed study of the clausocalanid morphology can add to the list of their diagnostic characters in order to compile the differential diagnosis of the family Clausocalanidae among the other members of the superfamily. |
 Issued from : G.A. Boxshall & S.H. Halsey inThe Ray Society, 2004, N° 166. [p.93, Fig.15]. Clausocalanidae. A, Pseudocalanus elongatus, habitus female; B, habitus male; C, female P2; D, male P5; E, Microcalanus pusillus male P5; F, Drepanopus pectinatus female P5; G, Clausocalanus arcuicornis basis of female P2; H, female P5. Sars, 1901: A-D; Sars 1903a: E; Giesbrecht, 1893a: F-H. | | | | | | (1) Clausocalanus Giesbrecht, 1888 | |
| | Ref.: | Giesbrecht, 1892 (p.50, 185); Giesbrecht & Schmeil, 1898 (p.27); Wheeler, 1901(p.171); Esterly, 1905 (p.142); van Breemen, 1908 a (p.22); A. Scott, 1909 (p.31); Esterly, 1924 (p.88); Wilson, 1932 a (p.42); Rose, 1933 a (p.80); Mori, 1937 (1964) (p.34); Vervoort, 1946 (p.140); Davis, 1949 (p.20); Brodsky, 1950 (1967) (p.117); Farran & Vervoort, 1951 f (n°38, p.3); Tanaka, 1956 c (p.382); Gonzalez & Bowman, 1965 (p.246); Frost & Fleminger, 1968 (p.15, Rev., Key spp.); Carli & Crisafi, 1969 (p.277); Razouls, 1972 (Annexe: p.28, Key F); 1982 (p.127); Gardner & Szabo, 1982 (p.179); Brodsky & al., 1983 (p.228); Zheng Zhong & al., 1984 (1989) (p.234, spp. Key); Mauchline, 1988 (p.726); Ferrari, 1992 (p.392, tab.3); Razouls, 1993 (p.310); Bradford-Grieve, 1994 (p.107, Def.); Chihara & Murano, 1997 (p.775, spp. Key); Mauchline, 1998 (p.85); Bradford-Grieve & al., 1999 (p.915, spp. Key); Boxshall & Halsey, 2004 (p.92); Mulyadi, p.181, Def.); Vives & Shmeleva, 2007 (p.612, spp. Key); Bucklin & Frost, 2009 (p.111, morphology vs molecular analysis, phylogeny). | | Rem.: | type: Calanus mastigophorus Claus,1863. La détermination des espèces avant 1968 est souvent discutable et leurs répartitions géographiques sont établies d'après Frost & Fleminger (1968), complétées par les auteurs ultérieurs.
13 spp.
Frost & Fleminger (1968, p.17, 29, 44, 66) définissent 3 groupes:
Groupe 1: C. mastigophorus, C. lividus, C. ingens and C. laticeps.
Groupe 2: C. arcuicornis, C. farrani, C. jobei, C. minor, C. paululus.
Groupe 3: C. pergens, C. brevipes, C. parapergens, C. furcatus. | | Remarques sur les dimensions et le sex-ratio: | | Le rapport des dimensions Male/Femelle est de 0,772 (n = 13; SD = 0,0761). Le sex-ratio est de 1. |  Issued from : B. Frost & A. Fleminger inBull. Scripps Inst. Oceanogr. Univ. California, 1968, 12. [p.16]. Spines and setae formula of swimming legs P1 to P4. B1 = Coxa; B2 = basis. |
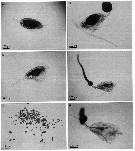 issued from : A. Skovgaard & N. Daugbjerg in Protist, 2008, 159. [p.405, Fig.3 A-F]. Paradinium spp.in Clausocalanus sp. from the NW Mediterranean Sea. A: appearance of infected host at the time of discovery (t = 0h). The host's body cavity is almost completely filled with parasite cell mass. B: t = 22h, the parasite cell mass adheres as a large shere to the host's urosome. C-F: t = 0h, infested host with parasite cell mass; D, t = 19h, parasite cell mass adheres as a string of speres connected to the host's urosome; E, t = 21h, parasite cell mass uncoupled from the host; F, t = 23h, additional parasite cell mass attached to the urosome of the host that now contains only a small amount of parasite cell mass. | | | | | (2) Ctenocalanus Giesbrecht, 1888 | |
| | Ref.: | Giesbrecht, 1892 (p.50,194); Giesbrecht & Schmeil, 1898 (p.28); van Breemen, 1908 a (p.24); Esterly, 1924 (p.90); Rose, 1933 a (p.83); Mori, 1937 (1964) (p.37); Davis, 1949 (p.20); Brodsky, 1950 (1967) (p.121); Farran & Vervoort, 1951 f (n°38, p.3); Tanaka, 1956 c (p.384); Ramirez, 1966 (p.12); Razouls, 1982 (p.131); Gardner & Szabo, 1982 (p.187); Brodsky & al.,1983 (p.235); Ferrari, 1992 (p.392, tab.3); Razouls, 1993 (p.310); Bradford-Grieve, 1994 (p.125, Déf., Rem.); Chihara & Murano, 1997 (p.778); Mauchline, 1998 (p.85); Bradford-Grieve & al., 1999 (p.915, 917: clé spp.); Boxshall & Halsey, 2004 (p.92); Vives & Shmeleva, 2007 (p.626, spp. Key) | | Rem.: | type mal précisé: Ctenocalanus vanus Giesbrecht,1888.
Total: 5 espèces. | | Remarques sur les dimensions et le sex-ratio: | | Le rapport des dimensions M/F est de 1,073 (n = 4) si l'on ne considère que les deux sexes pour chacune des espèces. Les mâles étant ainsi légèrement plus long que les femelles ce qui ne constitue pas le cas général chez les copépodes. | | | | Drepanopsis Wolfenden, 1911 | |
| | Ref.: | Wolfenden, 1911 (p.245) | | Rem.: | type: Drepanopsis frigidus Wolfenden,1911. nom. préoc. Cf. Farrania | | | | (3) Drepanopus Brady, 1883 (part.) | |
| | Ref.: | Brady, 1883 (p.76); Giesbrecht, 1892 (p.51, 201); Giesbrecht & Schmeil, 1898 (p.28); van Breemen, 1908 a (p.27); Wolfenden, 1911 (p.245); Rose, 1937 (p.162); Brodsky, 1950 (1967) (p.138); Vervoort, 1951 (p.69); 1957 (p.40); Farran & Vervoort, 1951 f (n°38, p.3); Ramirez, 1966 (p.11); Bayly, 1982 (p.161, Rev.); Razouls, 1982 (p.134); Brodsky & al., 1983 (p.237); Hulsemann, 1985 a (p.910); Ferrari, 1992 (p.392, tab.3); Razouls, 1993 (p.310); Bradford-Grieve, 1994 (p.127, Déf.); Mauchline, 1998 (p.81, 89: M; p.84, 85: F); Boxshall & Halsey, 2004 (p.92) | | Rem.: | type: Drepanopus pectinatus Brady,1883.
Total: 4 espèces. | | Remarques sur les dimensions et le sex-ratio: | | Le rapport des dimensions M/F est de 0,744 (n = 4; SD = 0,0272) si l'on prend en compte les mesures extrèmes des deux sexes pour chacune des espèces. | | | | | Syn.: | Drepanopsis Wolfenden, 1911(p.245), nom déjà usité; Sewell, 1929 (p.96); Rose, 1933 a (p.84); 1937 (p.161); Brodsky, 1950 (1967) (p.140); Vervoort, 1951 (p.69); Farran & Vervoort, 1951 f (n°38, p.3); Tanaka, 1956 c (p.399); Brodsky & al., 1983 (p.239) | | Ref.: | Sars, 1920 c (p.4); 1925 (p.36); C.B. Wilson, 1950 (p.227); Vervoort, 1957 (p.39); Razouls, 1982 (p.133); Mauchline, 1988 (p.726); Razouls, 1993 (p.310); Mauchline, 1998 (p.81: M; p.84: F); Bradford-Grieve, 1994 (p.129, Déf.); Boxshall & Halsey, 2004 (p.92); Vives & Shmeleva, 2007 (p.630, spp. Key) | | Rem.: | Type: Farrania oblonga Sars,1920. 5 spp. (dont 1 douteuse) + 1 indet.: | | Remarques sur les dimensions et le sex-ratio: | | The mean female size is 3.149 mm (n = 9; SD = 0.5780). In the case of only one male: 3.01 mm against 3.56 mm for the female; being a size ratio (M:F) of 0.846. The sex ratio is 0.2 or at best 0.25% if one considers F. oblonga as a synonym. |  Issued from : E.L. Markhaseva & J. Renz in Crustaceana, 2015, 88 (9). [p.1043, Fig.6, B, D]./em> sp. As Farrania sp. Female: B, Mx2; D, Mx2 praecoxal endite. Scale bars: 0.1 mm. | | | | | (5) Microcalanus Sars, 1901 | |
| | Syn.: | Pseudocalanus (part) : Sars, 1900 | | Ref.: | Sars, 1901 (1903) (p.20); 1903 (p.155); van Breemen, 1908 a (p.26); Wolfenden, 1911 (p.286); Rose, 1933 a (p.80); Brodsky, 1950 (1967) (p.115); Tanaka, 1956 c (p.381, 385); Farran & Vervoort, 1951 e (n°37, p.3); Gardner & Szabo, 1982 (Rem., p.175); Razouls, 1982 (p.126); Brodsky & al., 1983 (p.225); Razouls, 1993 (p.310); Bradford-Grieve, 1994 (p.129, Def.); Chihara & Murano, 1997 (p.779); Mauchline, 1998 (p.88: F; p.89: M); Boxshall & Halsey, 2004 (p.92); Vives & Shmeleva, 2007 (p.632) | | Rem.: | type: Pseudocalanus pygmaeus Sars,1900.
Total: 1 espèce (avec 2 variétés) ou 2 espèces (selon les auteurs)+ 2 indet. | | Remarques sur les dimensions et le sex-ratio: | | The mean female size is 0.755 mm (n = 4; SD = 0.2479), and in male 0.813 mm. The size ratio (male: Female) is 1.044. The sex ratio (F: M) is 1. | | | | (6) Peniculoides Markhaseva & Renz, 2015 | |
| | Ref.: | Markhaseva & Renz, 2015 (p.1033). | | Rem.: | Diagnosis of genus after Markhaseva & Renz (2015, p.1033) :
Female:
- Rostrum as small round-triangular projection bearing 2 filaments.
- Cephalosome and pediger 1, pedigers 4 and 5 separate.- A1 of 24 free segments.
- A2 endopod segment 1 with 1 seta; exopod with 1,1-1-1,1,1,1,1,0 and 3 setae.
- Md gnathobase strong, heavily sclerotisede; basis with 3 setae; endopod segment 1 with 2 setae, segment 2 with 9 setae.
Mx1 praecoxal arthrite larger than the remaining part of the limb, heavily sclerotized, with 9 terminal and 2 posterior setae; coxal epipodite without setae; coxal, proximal basal and distal endites with 2 setae each; endopod with 4 setae: exopod with 4 or 5 setae.
- Mx2 praecoxal to basal endites with distal part flat and round, in coxal and basal endites its surface is densely covered by short spinules, brush-like, setal formula 4, 3, 2, 1 +1, 2; endopod with 3 setae;
- Mxp praecoxal endites setal formula 1, 2 a,d 3, coxal endite with 3 setae; 5 free endopod segments with 4, 4, 3, 3, and 4 setae.
- P1 to P4 segmentation and setation typical of the superfamily Clausocalanodea.
- P5 uniramous, 3-segmented; left leg with 3, right leg with 2 terminal spines.
- Male unknown.
Apomorphies for the genus are: 1- Md gnathobase strong and heavily sclerotized; 2 - Mx1 praecoxal arthrite large and heavily sclerotized; 3 - Mx1 exopod with 4-5 setae, coxal epipodite lacking setae; 4 - Mx2 coxal and basal endites surface densely covered by short spinules, brush-like.
For Markhaseva & Renz (2015, p.1044) The morphology of its mandible gnathobase, maxillule praecoxal arthrite and its specialized maxilla all of which strongly deviate from typical oral limbs of other Clausocalanidae and from those described for the superfamily Clausocalanoidea as a whole. The only other genus in the superfamily with highly specialized oral parts is Chiridiella Sars, 1907 (Aetideidae). The brush-like surfaces of the maxilla endites, which are densely covered by short spinules and provide a large surface for attaching or holding prey, suggest its feeding mode would probably point more towards a carnivorous than detrivorous life style. The maxilla structure might also serve for brushing or cleaning the food of unwanted particles. | | Remarques sur les dimensions et le sex-ratio: | | For one female only, the body size is 2.10 mm. |  Issued from : E.L. Markhaseva & J. Renz in Crustaceana, 2015, 88 (9). [p.1038, Fig.3, C-E]. Peniculoides secundus type species: C, D and E, maxilla in different views, endite setation is not figured in D. Enp, endopod; p, praecoxal endite; c, coxal endite; bp, basal proximal endite; bd, basal distal endite; abd, attenuation of basal distal endite; el, enditic-like lobe of proximal endopodal segment. Scale bar = 0.1 mm. Remarks: The maxilla of the new genus strongly deviates from that of a typical clausocalanoidean an dis strongly specialized distally. The maxilla setal formula can be described as : 4 setae on the praecoxal endite ; 3 setae on the coxal endite ; 2 setae on the proximal basal endite ; 1 seta plus 1 spine-like attenuation on the distal basal endite ; 2 setae on the enditic-like lobe of proximal endopodal segment and 3 endopod setae (terminology follows Ferrari & Ivanenko, 2008). However, homologies of the maxilla distal part are not completely resolved : apparently, the distal basal endite could also be interpreted as lacking armament, then, the spine-like attenuation supplied with 1 stiff set is an enditic-like lobe of an endopod proximal segment, and the endopod bears 2 + 3 setae. Following this interpretation the maximma setal formula is as : 4, 3, 2, 0, 1 + 1 and 2 + 3. | | | | | (7) Pseudocalanus Boeck, 1872 | |
| | Ref.: | Brady, 1878 (p.44); Giesbrecht, 1892 (p.51, 196); Giesbrecht & Schmeil, 1898 (p.28); Sars, 1900 (p.69); 1901 a (1903) (p.19); van Breemen, 1908 a (p.24); Sars, 1903 (p.19); Wilson, 1932 a (p.43); Rose, 1933 a (p.79); Mori, 1937 (1964) (p.36); Davis, 1949 (p.18); Brodsky, 1950 (1967) (p.112); Farran & Vervoort, 1951 e (n°3, p.3); Woods, 1969 (p.543, Rem.); Sheldon & al., 1972 (p.327, fig. 13: size particle vs production's rate); Corkett & McLaren, 1978 (p.3); Klein Breteler, 1982 (p.5, figs.); Robins & McLaren, 1982 (p.529); Razouls, 1982 (p.123); Gardner & Szabo, 1982 (p.171); Brodsky & al., 1983 (p.220); Mauchline, 1988 (p.725); Frost, 1989 (p.525, Rev., Key spp.); Ferrari, 1992 (p.392, tab.3); Razouls, 1993 (p.310); Bradford-Grieve, 1994 (p.130, Déf.); Chihara & Murano, 1997 (p.778); Mauchline, 1998 (p.88: F; p.90: M); Boxshall & Halsey, 2004 (p.92); Vives & Shmeleva, 2007 (p.635) ; Questel & al., 2016 (p.610, fig. 3, genetic analysis, phylogeography). | | Rem.: | type: Clausia elongata Boeck,1872.
Ce genre comprend 7 espèces qui ont été souvent confondues du fait de leur faible différenciation morphologique (Cf. Frost, 1989, p.529) + 1 indét.
Je me suis limité à la distribution la plus sûre. Toutes ces espèces sont, jusqu'à maintenant (2015), confinées à l'hémisphère nord. | | Remarques sur les dimensions et le sex-ratio: | | Le rapport des dimensions M/F est de 0,790 ( n = 7S; SD = 0,0473) si l'on considère la moyenne des extrêmes pour les deux sexes pour chacune des espèces. | 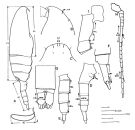 issued from : B.W. Frost in Can. J. Zool., 1989, 67 . [Fig.2, p.528]. mesures utilisées dans la clé de détermination des femelles et des mâles. exemples: A, female (habitus, lateral view) [ P. minutus]; B, C, male (anterior portion of cephalosome, lateral and dorsal views, respectively) [ P. acuspes]; D, female (urosome, lateral view) [ P. minutus]; E, male (urosome, lateral view) [P. minutus]; F, female (posterior segments of urosome and caudal rami, lateral view) [P. minutus]; G, male (posterior segments of urosome and caudal rami, dorsal view) [P. moultoni]; H, male (A1) [ P. minutus]; I, female (basal and coxal segments of P4) [ P. minutus]; J, spermatophore [ P. moultoni]; AS, anterodorsal sensillum; A1 (antennule); B1L (length of basal segment of leg; B2L (length of basipod 2 segment of leg); CL (cephalothorax length); CRL (caudal ramus length); CRW (caudal ramus width); PL (prosome length); SL (spermatophore length); SRL (seminal receptacle length); UL (urosome length); UW (urosome width) Scale bars = 0.1 mm. |
 Issued from : P. Tiselius, E. Saiz & T. Kiørboe in Limnol. Oceanogr., 2013, 58 (5). [p.1661, Fig.4]. Time sequence of the capture of a Heterocapsa triquetra (Dinoflagellate) cell (in circle) by a Pseudocalanus sp. copepodite from Gullmar Fjord (W Sweden). Frame number is shown and the position of A2 (blue), Md (yellow), Mx1 (red) and Mxp (green) shown for clarity. See movie in online Web Appendix, Video 1, Frames 162-242). Nota: Figure shows the sequence of Pseudocalanus sp. motions leading to the capture of the cell of Heterocapsa triquetra was detected, probably by the distal setae of the A2 endopod. Tipically the Mxp and swimming legs were the first to show a capturing movement. Swimming legs started to move out and were fully extended at Frame 182. The A2, Md and Mx1 all made a recovery stroke (Frames 162-187). The Mxp started a typical capture sweep when it moved in a wider circle (Frames 167-187); but in contrast to a regular stroke, the setae were trailing until Frame 187 when the stroke turned into an inward sweeping motion and setae moved water forward (Frames 187-202), meeting the tips of the Md and Mx1. The opposite Mxp (right) moved completely independently and 180° out of phase and helped pushing water toward the capturing area. This can be seen in Frames 177-182, where the right Mxp was in the capturing area. A2 remained in a forward position (Frames 182-242) for the whole capturing event. A suction flow into the capture area was created by the simultaneous opening of the swimming legs (Frames 162-177), the rapid backward stroke of the Mxp (Frames 176-172), and the recovery stroke of A2 and Md (Frames 167-182). This capture area was bounded by the setae on the Mx1 epipodite and rami and by the endites and endopod setae of Mx2. The Mx2 endopod setae were very flexible and spread across a large area (Frames 177-182, 187, 212) and made short beats when trying to capture the cell. The Mx2 endopod and endite setae and the Mx1 handled the particle and brought it toward the mouth (Frames 217-242). During capture attempts, the mx2 moved very quick with a cycle of 10.3 ± 2.2 ms. The capturing event (Frames 162-242) thus consisted of 2 full cycles by the left Mxp, 1-2 cycles in opposite direction by the right Mxp, 3 sweeps by the Mx2, and somewhat unclear motions of the Mx1 and Md. The cell disappeared at Frame 242 in the vicinity of the mouth. The capture of this cell lasted 36.4 ms and the average of all capturing events was 35 ± 19 ms. |
 Issued from : J.M. Questel, L. Blanco-Bercial, R.R. Hopcroft & A. Bucklin in J. Plankton Res., 2016, 38 (3). [p.616, Fig. 3]. Cytochrome oxidase I haplotype networks for wrm>P. acuspes, P. minutus, P. mimus and P. newmani. Each circle represents a unique haplotype; sizes are scaled on the number of individuals expresing that particular haplotype. Each mode represents a single bp mutation. | | | | | (8) Spicipes Grice & Hulsemann, 1965 | |
| | Ref.: | Grice & Hulsemann, 1965 (p.225); Razouls, 1982 (p.135); 1993 (p.310); Bradford-Grieve, 1994 (p.131, Def.); Mauchline, 1998 (p.66: F); Boxshall & Halsey, 2004 (p.92); Vives & Shmeleva, 2007 (p.637) | | Rem.: | type: Spicipes nanseni.
Total: 1 espèce. | | | | | | Remarque sur les tailles :
Les valeurs minimales et maximales en mm des longueurs des adultes femelles de la famille des Clausocalanidae sont indiquées sur la figure 1 avec en abscisse le n° des espèces ( Clausocalanus de 1 à 13, Ctenocalanus de 14 à 18, Drepanopus de 19 à 22, Farrania de 23 à 27, Microcalanus : 28 et 29, Pseudocalanus de 30 à 36, Spicipe s: 37.
figure 1

Tableau I: classement des espèces par groupe de taille
espèces |
Lg (mm) |
Localisations |
Prof. |
Farrania lyra |
3,30 à 4 |
tempéré |
|
Farrania oblonga |
- |
tempéré |
|
Farrania orba |
- |
tempéré |
|
Farrania pacifica |
- |
? Sub-arct., tempéré froid |
B-A |
Farrania frigida |
±2 à 3 |
cosmop., Antarct., sub-tropic |
M-B |
Drepanopus bispinosus |
- |
Antarct. |
|
Drepanopus pectinatus |
- |
Antarct., sub-Antarct. |
N |
Drepanopus forcipatus |
± 1,50 à ± 2,5 |
sub-Antarct. |
|
Pseudocalanus major |
- |
Arct. |
|
Pseudocalanus acuspes |
- |
Arct., sub-arct. |
|
Clausocalanus ingens |
- |
Sub-antarct., tempéré froid |
|
Clausocalanus lividus |
1 à ± 2 |
cosmop., tempéré chaud |
E |
Pseudocalanus minutus |
- |
cosmop., Arct., sub-arct. |
|
Clausocalanus mastigophorus |
- |
tempéré chaud, tropic. |
|
Clausocalanus brevipes |
- |
sub-antarct. |
|
Pseudocalanus mimus |
- |
sub-arct. |
|
Pseudocalanus moultoni |
- |
sub-arct., tempéré froid |
L |
Clausocalanus laticeps |
- |
Antarct., sub-antarct. |
|
Clausocalanus arcuicornis |
- |
cosmop., tempéré |
E |
Pseudocalanus elongatus |
- |
tempéré |
|
Clausocalanus parapergens |
1 à ± 1,5 |
cosmop., sub-tropic. |
E |
Clausocalanus jobei |
- |
cosmop., tropic., sub-tropic. |
E, N |
Clausocalanus furcatus |
- |
cosmop., tropic., sub-tropic. |
|
Ctenocalanus heronae |
- |
tropic. |
|
Ctenocalanus vanus |
- |
cosmop., sub-tropic., tempéré |
E-B |
Pseudocalanus newmani |
- |
Arct., sub-arct. |
|
Drepanopus bungei |
- |
Arct. |
|
Spicipes nanseni |
- |
sub-tropic. |
B |
Ctenocalanus citer |
- |
Antarct., sub-antarct. |
|
Ctenocalanus campaneri |
- |
tropic. |
E-M |
Clausocalanus minor |
0,6 à ± 1 |
tropic., sub-tropic. |
|
Ctenocalanus tageae |
- |
tropic. |
|
Clausocalanus farrani |
- |
tropic., sub-tropic. |
|
Clausocalanus pergens |
- |
tropic., sub-tropic. |
E-B |
Microcalanus pygmaeus |
- |
sub-antarct., sub-tropic., sub-arct. |
E-B |
Clausocalanus paululus |
< à 1 |
sub-antarct., sub-tropic. |
E |
?Microcalanus pusillus |
- |
cosmop., Antarct., sub-trop., sub-arct. |
|
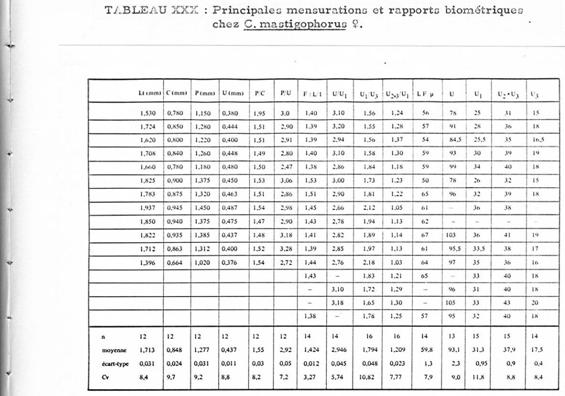
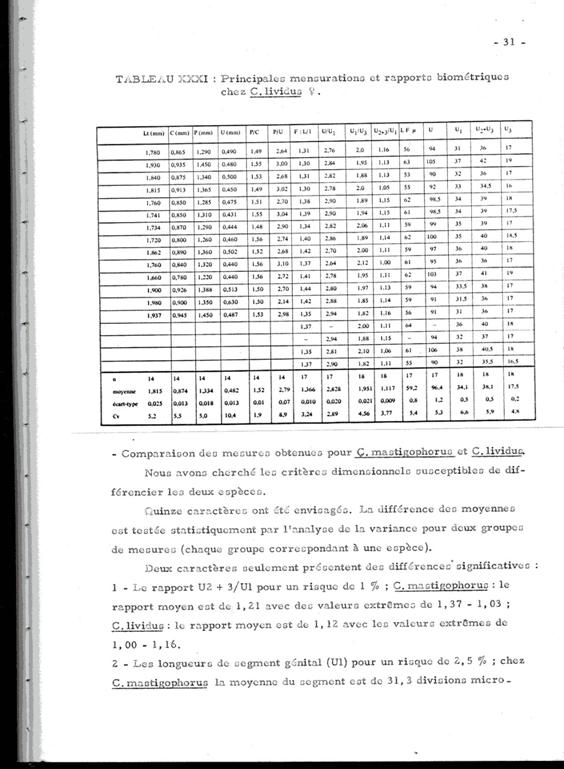
Annexe: Étude comparative entre les femelles de Clausocalanus lividus et Clausocalanus mastigophorus .
La différenciation des deux espèces à Banyuls (Méditerranée: Golfe du Lion) (Razouls, 1972, Annexe: p.30) est malaisée. Il existe une assez forte variablilité dans la forme du rostre pour chacune des deux espèces, par ailleurs la forme des réceptacles séminaux est difficile à observer sans préparation spéciale dans l'une et l'autre espèce. La présence des mâles est aussi très rare.
Les tableaux suivants donnent les mesures et les rapports de certains segments du corps de ces espèces. la nomenclature et le mode de mensuration sont homologues à ceux utilisés par Fleminger (1967) et Frost & Fleminger (1968). Lorsque les mesures ne sont pas exprimées en mm ou micron, une unité = 4,88 microns
Quinze caractères ont été envisagées. la différence des moyennes est testée statistiquement par l'analyse de la variance pour deux groupes de mesures, chaque groupe correspondant à une espèce.
Deux caractères seulement présentent des différences significatives:
1 – Le rapport U2+3/U1 (pour un risque de 1 %). Pour C. mastigophorus le rapport moyen est de 1,21 (valeurs extrêmes: 1,03 -1,37); pour C. lividus le rapport moyen est de 1,12 (valeurs extrêmes: 1,00 -1,16)
2 – Les longueurs du segment génital (U1) pour un risque de 2,5 %. Chez C. mastigophorus la moyenne est de 152 microns (valeurs extrêmes: 122 – 175,68; chez C. lividus la moyenne est de 166 microns (valeurs extrêmes: 151,28 – 185,44).
3 – Le segment génital (U1) chez C. lividus est plus long que chez C. mastigophorus, et par rapport aux deux segments suivants où il apparaît plus long chez C. lividus.
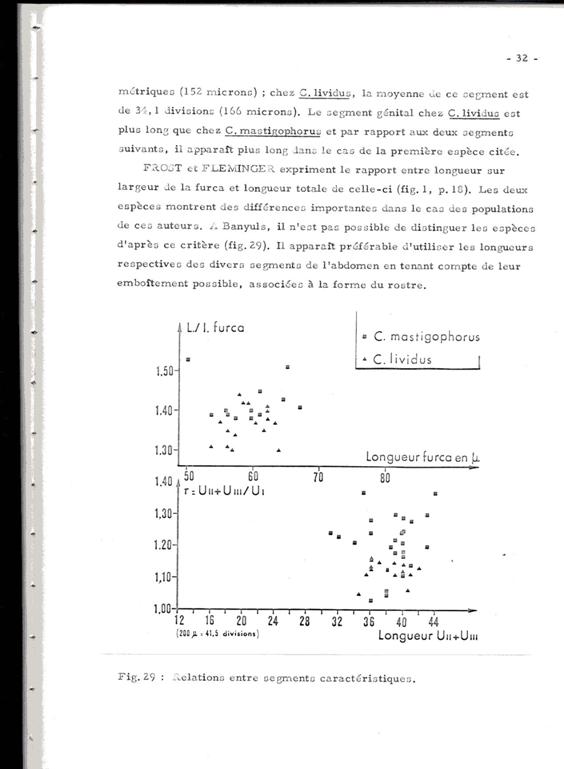
Frost & Fleminger (fig.1, p.18) expriment le rapport entre longueur sur largeur (L/l) de la furca et la longueur totale de celle-ci. Les deux espèces montrent des différences importantes dans le cas des populations analysées par ces auteurs. À Banyuls, il n'est pas possible de distinguer les espèces d'après ce critère (voir figure).
En conclusion, la distinction des deux espèces, le plus souvent coexistante, est particulièrement ardue en l'absence des mâles très rares, sinon absents dans nos prélèvements. Ceci jette un doute sur de nombreuses identifications et à fortiori sur les comptages notés dans divers travaux.
| | | | | | | |
|
|
 Toute utilisation de ce site pour une publication sera mentionnée avec la référence suivante : Toute utilisation de ce site pour une publication sera mentionnée avec la référence suivante :
Razouls C., Desreumaux N., Kouwenberg J. et de Bovée F., 2005-2026. - Biodiversité des Copépodes planctoniques marins (morphologie, répartition géographique et données biologiques). Sorbonne Université, CNRS. Disponible sur http://copepodes.obs-banyuls.fr [Accédé le 08 janvier 2026] © copyright 2005-2026 Sorbonne Université, CNRS
|
|
 |
 |