|
|
 |
|
Calanoida ( Order ) |
|
|
|
Clausocalanoidea ( Superfamily ) |
|
|
| |
| | | |
| Scolecitrichidae Giesbrecht, 1892 ( Clausocalanoidea ) | | Syn.: | Scolecithrichina : Giesbrecht, 1892 (p.55, 265);
Scolecithricinae : Esterly, 1905 (p.162);
Scolecithridae : Rose, 1933 a (p.141);
Scolecithricidae : Sars, 1902 (1903) (p.49); 1925 (p.157); Gurney, 1931 a (p.84); Rose, 1942 (p.113, Rem.: p.114); Brodsky, 1950 (1967) (p.83, 239, Genera Key); Tanaka, 1961 a (p.139); Vervoort, 1965 (p.65, Rem.); Mazza, 1967 (p.162); Bradford, 1973 (p.133 & suiv., Rev.); Andronov, 1974 a (p.1005); Roe, 1975 (p.336); Razouls, 1982 (p.299); 1993 (p.310); Bradford & al., 1983 (p.73, Def., Rem. Genera); Park, 1983 (p.191); Zheng Zhong & al., 1984 (1989) (p.239, spp. Key); Mauchline, 1988 (p.738, 740 : cuticular pores); Vyshkvartzeva, 1989 (p.5, Rev.); Huys & Boxshall, 1991 (p.364); non Parkiidae; Mulyadi, 2004 (p.24); | | Ref.: | Madhupratap & al., 1996 (p.863, Table 5: %/copepods); Chihara & Murano, 1997 (p.899); Schulz, 1998 (p.42); Bradford-Grieve & al., 1999 (p.881, 903, 904, 930, key of Genus); Vyshkvartzeva, 1999 (2000) (p.217, Rem.: 2 genera are incorporated in the family: Xantharus and Neoscolecithrix at least for some species ("N." antarctica, "N." farrani, "N." watersae); Vyshkvartzeva, 2001 (p.77, Genera Key)1; 2003 (p.46, Rem. M); Ohtsuka & Huys, 2001 (p.445, 461); Schulz & Kwasniewski (2004, p.158) maintain Xantharus in the Scolecitrichidae; Markhaseva & Dahms, 2004 (Rem.: p.336); Boxshall & Halsey, 2004 (p.15, 49, 185, Def., Key of genera: females only); Vyshkvartzeva, 2004 (p.157, tab.2, 3, 4, 5); Ferrari & Markhaseva, 2005 (p.45, Rem.); Kuriyama & Nishida, 2006 (p.293, table 7, vertical distribution); Vives & Shmeleva, 2007 (p.717, part. Genera Key); Blanco-Bercial & al., 2011 (p.103, Table 1, Fig.2, Biology molecular, phylogeny); Markhaseva & al., 2014 (p.63, 67, 68, Def., Rem.) ; Laakmann & al., 2019 (p.330, Table 1, fig.1, 2, 3, 4, Table A, phylogenetic relationships); Hirai & al., 2020 (p.1, Fig.4: metabarcoding, Fig.8: OTUs distribution patterns, Fig.9: phylogenetic analysis)
Bradford-Grieve J.M., (2002 onwards). Key to calanoid copepod families. Version 1 : 2 oct 2002. http://www.crustacea.net/crustace/calanoida/index.htm  | | Rem.: | Bowman & Abele, 1982 (p.9) correct the spelling of Scolecithricidae in Scolecitrichidae.
Total (2004): 26 G (lato sensu): Amallothrix, Archescolecithrix, Cenognatha, Falsilandrumius, Grievella, Heteramalla, Landrumius, Lophothrix, Macandrewella, Mixtocalanus, "Neoscolecithrix" (part.), Parascaphocalanus, Plesioscolecithrix, Pseudoamallothrix, Puchinia, Racovitzanus, Rythabis, Scaphocalanus, Scolecithricella, Scolecithrix, Scolecitrichopsis, Scolecocalanus, Scopalatum, Scottocalanus, Undinothrix, Xantharus .
Boxshall & Halsey (2004, p.186, 188) give a definition of the family and admit 26 genera. (Neoscolecithrix Canu,1896 and Parkius and the genus Plesioscolecithrix not being included in their key). Vyshkvartzeva (2004, p.176 & suiv.) contests the position of these authors concerning the genus Parkius and re-establishes the status of the Parkiidae family.
Ohtsuka & al. (2003, p.61 & foll.) establish only the core of this family, comprising 18 genera: Amallothrix, Archescolecithrix, Heteramalla, Lophothrix, Macandrewella, Mixtocalanus, Parascaphocalanus, Pseudoamallothrix, Puchinia, Racovitzanus, Scaphocalanus, Scolecithricella, Scolecithrix, Scolecitrichopsis, Scolecocalanus, Scopalatum, Scottocalanus, Undinothrix.
The following genera (marked *): Neoscolecithrix, Cenognatha, Rythabis, Falsilandrumius, Grievella, Landrumius, Xantharus, do not show the characters of this family core, but are more related to this family than to the Tharybidae or Phaennidae. The genus Rythabis is contested in this family by Vyshkvartzeva (2004) who considers it as belonging in the Tharybidae.
Because of the multiple reshuffling within this family all the species cited in the genera are reported. Various corrections and precisions have been introduced in respect to the list in Razouls (1982). When a species is not transferred with certainty into an other genus it is maintained by convenience in its initial genus indicated by its descriptor. According to Bradford (1973) and Bradford & al. (1983) the "sure" species in this genus are marked by a (*), the species for which the genus is doubtful by (**), those that certainly do not belong to the genus by (***).
The genetic analysis realised by Markhaseva & Ferrari (2005 a, p.153 & foll.) is shown in the figure 31 (p.163-164). Three new genera (Brodskius, Byrathis, Omorius) are provisionally included below among the Scolecitrichidae, but the two first genera are included in the Tharybidae by Markhaseva & Schulz (2007, p.732, 737).
Provisionally the under-mentioned list includes 32 genera.
1: Vyshkvartzeva (2001, p.83, pers. comm.: some corrections are to be included in the key to the genera.
38 (37): … … filaments or conical points. Female P5 3- or 2- segmented. …
39 (40): A2 with both rami of about equal length; exopod 6-segmented, Re2 (fused segments Re2-Re3) much longer than Re6.. Re1 of P1 usually without external spines. Female P5 3-segmented, distal segment if longer, only slightly longer than wide. Male mouthparts strongly reduced. Male P5 biramous; right Re1 (sometimes subdevised on 2 segments) without mediodistal projection, Re2 subcylindrical, short, distal spine as long as Re2, triangular; right endopod tapering, as long as or longer than left basipod. ----- Lophothrix.
40 (39): Endopod of A2 not longer than 4/5, usually more shorter, than exopod; Re2 subequal or slightely longer than Re6-7. Re1 of P1 usually with external spine. Female P5 usually 2-segmented, distal segment much longer than wide. Male mouthparts only slightly reduced compared with female. Male P5 biramous; right Re1 usually with mediodistal projection, Re2 frequently curved, distal spine usually shorter than Re2, rudimentary, right endopod shorter than left basipod.
41 (42) ….. --- Pseudoamallothrix
42 (41) ….. --- Amallothrix.
The types of setae on the ramus of Mx2 include this family in the 'Bradfordian' group of families.
Latitudinal distribution in the Indian Ocean see Gopalakrishnan & Balachandran (1992).
Markhaseva & al., 2013 included 18 genera: Amallothrix, Archeoscolecithrix, Diaiscolecithrix, Lophothrix, Macandrewella, Mixtocalanus, Omorius, Parascaphocalanus, Pseudoamallothrix, Racovitzanus, Scaphocalanus, Scolecithricella, Scolecithrix, Scolecitrichopsis, Scolecocalanus, Scopalatum, Scottocalanus, Undinothrix. For these authors the Pakius genus is maintened in the Parkiidae family.
Type genus: Scolecithrix Brady, 1883. The diagnosis of the family is based on the following combination of characters: the apomorphy for the family within the ''Bradfordians'' is the setal formula of the Mxp praecoxal endites as 1, 2 and 1 setae sequence, and the seta on the distal praecoxal endite is brush-like; the male P5 right basis is significantly shorter than the left, oval-rectangular and swollen (3 genera deviating in the latter two characters).
Diagnosis from Boxshall & Halsey (2004, p.185) :
- Usually cephalosome and pedigerous somite 1 separate, pedigerous somites 4 and 5 usually fused, occasionally partly or completely separate.
- Rostrum typically a bifurcate plate with or without filaments, or represented by paired rostral filaments.
- Nauplius eye present.
- Posterolateral angles of prosome usually rounded, produced into strong double pointed processes in Neoscolecithrix.
- Frontal margin of head with median crest in some genera and with conspicuous median lens-like structure in Macandrewella.
- Urosome 4-segmented in female.
- Genital apparatus female comprising common genital aperture located medially on ventral surface of genital double-somite ; copulatory pore co,tained within median genital aperture.
- Urosome 5-segmented in male.
- Single genital aperture male located ventrolaterally at posterior rim of genital somite on left side.
- Caudal rami with up to 7 setae, seta I minute, usually absent.
- A1 19 to 24-segmented in female ; segmental homologies (based on Neoscolecithrix farrani Smirnov) : segment 1 (I) free, segment 2 (II-IV) triple, segment 3 (V) to 7 (IX) separate, segments 8 (X) and 9 (XI) fused, segments 10 (XII) to 23 (XXVI) separate, apical (24th) segment double (XXVII-XXVIII) ; apical segment usually triple, incorporating XXVI to XXVIII. Aesthetascs present on segments II (in male Neoscolecithrix antarctica Hulsemann), III, IV, VII, IX, XI, XIV, XVI, XXI, XXVI-XXVIII. A1 non geniculate on both sides in male ; often with partial segmental fusions in proximal part of antennule, sometimes with segments 20 and 21 (XXIII-XXIV) fused on rifgt side only.
-A2 biramous, coxa and basis separate ; coxa with 1 seta ; basis with 23 setae ; endopod 2-segmented, compound distal segment bilobed, setation formula 1, 7/8, 6/7 ; exopod sometimes markedly longer rgan endopod, indistinctly 6 or 7-segmented, segmental fusions indeterminate except for IX-X, setation formula typically 0, 1, 1, 1, 1, 1, 4, or further reduced ; apparently 1, 3, 1, 1, 1, 3 in Puchinia and 1, 3, 1, 1, 1, 4 in Neoscolecithrix.
- Md biramous ; coxa with well developed gnathobase and distal palp consisting of basis (with 1 to 4 setae ; 2-segmented endopod and 5-segmented exopod ; endopodal segments 1 and 2 with 0-3 and 9/10 setae ; exopodal setation formula 1, 1, 1, 1, 2.
- Md often much reduced in male.
- Mx1 with slender well developed praecoxal arthrite (inner lobe 1) bearing 9 to 14 elements, often including row of up 4 posterior setae, as in Landrumius ; coxa wiyj endite typically bearing 1, 2 to 3 setae, but 5 in Puchinia and in some Neoscolecithrix, with 7 to 9 setae on epipodite (outer lobe) ; basis without outer seta, with proximal endite beari,g 2, 3 or 4 setae and distak group of 3 to 5 setae representing distal endite (rarely 6 setae as in Archescolecithrix ; endopod indistincyly 2 or 3-segmented, maximum setation formula 3, 3, 5, but usually reduced ; exopod 1-segmented with 5 to 10 setae. Setation often reduced.
- Males usually with reduced setation on arthrite and endites.
- Mx2 indistinctly 4-segmented ; praecoxa and coxa partly fused, setation formula of endites 3-5, 3, 3, 3, with 1 element of distal endite sometimes curved, claw-like ; basis with 4 setae or strongly curved claw plus 3 setae(1 or 2 of which typically modified as sensoriform filaments) ; free endopod usually with 3 vermiform and 5 brush-like sensoriform filaments, rarely apparently with 4 vermiform and 4 brush-like sendoriform filaments as in Racovitzanus, additional ninth) setal element present in Neoscolecithrix, Landrumius, Xantharus,, Grievella.
- Mx2 often reduced in males.
- Mxp 7-segmented ; syncoxa with typical endite setation formula 1, 2, 1, 3 in female, 1, 2, 3, 3 in Neoscolecithrix, Landrumius, Xantharus, Grievella ; 1, 2, 2, 3 in Parkius, Rythabis ; setation often reduced in male ; 1 element representing 3rd endite modified as brush-like sensoriform filament ; proximal praecoxal seta and 1 seta of 2 nd endite often transformed as vermiform element ; basis with 3 setae, plus 2 setae on incorporated 1st endopodal segment ; extremely elongate in Parkius : free endopod 5-segmented, segmental setation formula 4, 4, 3, 3+1, 4. Setation, especially on syncoxa, often reducede in males.
- Swimming legs P1 to P4 biramous, typically with 3-segmented rami, except 1-segmented endopod of P1 and 2-segmented endopod of P2 ; rarely with 2-segmented endopod in P3 as in Heteramalla.
- Exopod of P1 sometimes 2-segmented.
- Endopods of P2 to P4 somewhat flattened, typically ornamented with strong spinules on posterior surfaces ; exopods of P2 to P4 sometimes ornamented with spinules.
- Endopod of P1 usually with lobe on outer margin ; sometimes lacking as in Racovitzanus, Landrumius.
- Inner seta on basis of P1 situated on anterior surface and passing across face of endopod ; sometimes absent, as in Mimocalanus, Landrumius.
- P2 to P4 sometimes with fine spinulation on surfaces of both rami ; some species with well developed surface spinulation on posterior surface of P4.
- Female P5 usually small, uniramous, typically 3-segmented ; coxa unarmed ; basis and 1-segmented exopod ; sometimes 1 or 2-segmented by secondary fusion ; exopod sometimes with partial suture (indicating its derivation from 2 exopodal segments. Exopod typically bearing 2 or 3 (rarely 4) elements, often highly ornamented with surface spinules. P5 sometimes reduced or absent, as in Scolecithrix danae.
- Male P5 asymmetrical ; left leg longer than right, usually biramous with rudimentary endopods ; or endopod lost. Left leg primitively 5-segmented, with slender coxa, nasis and 3-segmented exopod ; Right leg 3, 4 or 5-segmented consisting of coxa, basis and 1 to 3-segmented exopod.
- Eggs released into water.
Key to genera after Boxshall & Halsey (2004, p.188) adapted from Vyshkvartzeva (2001) :
Female only :
1 - 3rd endite on syncoxa of Mxp armed with 2 or 3 setal elements ………… 2.
3rd endite on syncoxa of Mxp armed with 1 brush-like drnsory seta only ………. 11.
2 – Mx2 endopod with 1 or 2 of the brush-like sensory setae greatly enlarged …… 3.
- Mx2 endopod without greatly enlarged brush-like sensoty setae, these setae either subequal or with 3 long plus 2 short pattern …… 4.
3 – Posterolateral angles of prosome produced into acute spinous processes extending beyond middle of genital double-somite ; P1 with inner seta on basis ……… Puchina.
- Posterolateral angles of prosome rounded ; P1 without inner seta on basis ……..Heteramalla.4 – Posterolateral angles of prosome produced into 2 widely-separated spinous processes on each side ; 1st exopodal segment of A2 with 1 or 2 setae ……. 25.
- Posterolateral angles of prosome without paired spinous processes ; 1st exopodal segment of A2 unarmed ……. 5.
5 – Mxp with elongate 2nd (basis plus incorporated 1st endopodal segment) bearing 2 setae of basal origin and 2 distal setae of endopodal origin ……. Parkius.
- Mxp without elongate allobasis ; bearing 3 setae of basal origin and 2 distal setae of endopodal origin ……… 6.
6 – P1 with inner seta on basis …….. 8.
- P1 without inner seta on basis ; P5 with 4 setae on exopod …….7.
7 – Rostrum strongly developed, with bifurcate tip, without filaments ; praecoxal arthrite of Mx1 with 4 setae on posterior surface ……. Landrumius.
- Rostrum with 2 long tapering filaments ; praecoxal arthrite of Mx1 with 2 setae on posterior surface. ……… Falsilandrumius.
8 – P5 2- or indistinctly 3-segmented, with 2 or 2 setal elements on exopod …….. 9.
- P5 3-segmented with 4 strong spines on distal exopodal segment …… Rythabis.
9 – Proximal praecoxal endite of Mx2 with 3 long setae plus short element ; basis of Md palp with 2 setae ………Archeoscolecithrix.
- Proximal praecoxal endite of Mx2 with 4 or 5 long setae ; basis of Md palp with 3 setae …….. 10.
10 – P5 with 3 setae around distal margin of exopodal segment ……..Grievella
- P5 with 2 apical spine-like ptocesses and 0-1 subapical inner spine …….Xantharus
11 – Mx2 endopod with 1 or 2 of the brush-like sensory setae greatly enlarged ………. 12.
- Mx2 endopod without gratly enlarged brush-like sensory setae, these setae either subequal or with 3 long plus 2 short pattern ……. 13.
13 – Female with 1 greatly enlarged brush-like sensory setae ; endopod of P1 with small lobe on outer margin …… Scopalatum.
- Female with 2 enlarged brush-like sensory setae ; endopod of P1 with straight outer margin ……..Mixtocalanus.
13 – Head with frontal lens-like organ or with conspicuous eye spots ……. 14.
- Head usually without frontal lens-like or conspicuous eye spots ……. 16.
14 – Head with lens-like organ ; rostrum long, strong and deeply bifurcate ; posterolateral angles of prosome and genital double-somite usually asymmetrical ……. 15.
- Head usually with conspicuous eye spots ; rostrum large, plate-like, terminally bifid; posterolateral angles of prosome and double-somite usually symmetrical ……Scottocalanus.
15 – Frontal margin of head with crest ; P5 present on left side only and comprising short segment bearing long curved spine ; 2 nd and 3rd exopodal segments of P4 with longitudinal row of spinules …….Scolecocalanus.
- Frontal margin of head without crest ; P5 lacking or with small 1-segmented P5 lacking curved spine ; 2 nd and 3rd exopodal segments of P4 without longitudinal row of spinules …….. Macandrewella.
16 – Posterolateral angles of prosome strongly asummetrical ; rostrum strong with 2 triangular processes carrying thick filaments inserted subapically ; P5 3-segmented, segments subequal, distal segment bearing 1 outer and 2 distal long serrate spines ………Undinothrix
- Posterolateral angles of prosome usually symmetrical ; rostrum and P5 of different form …….. 17.
17 – Rostrum a large digitiform process with or not small paired filaments ; anal somite about 1.4 times longer than preceding somite ………Racovitzanus.
- Rostrum not this form ; anal somite not longer than preceding somite ……… 18.
18 – P5 absent or small and 1-segmented …….. 19.
- P5 2- or 3-segmented ……. 20.
19 – Body robust, ovaoid ; genital double-somite with conspicuous swelling and large genital operculum extending posteriorly beyond margin of double-somite ; coxa of P4 without inner seta …….Scolecithrix.
- Body more slender, elliptical ; genital double-somite without swelling and bearing small genital operculum ; coxa of P4 with inner seta ………Scolecithricella.
20 – Rostrum very small, rudimentary, blunt and without filaments ………Parascaphocalanus.
- Rostrum with paired filaments …….. 21.
21 – Rostrum usually of 2 long, smoothly-tapering filaments ………Scaphocalanus.
- Rostrum a short plate with 2 thin filaments, or bifid with 2 strong branches, continuing into conical points or filaments ……… 22.
22 – Rostrum a short plate with 2 thin filaments ; segments of P4 densely ornamented wiith spinules on posterior surface ……….Scolecitrichopsis.
- Rostrum bifid with 2 strong branches, continuing into conical points or filaments ; segments of P4 sparsely ornamented with spinules on posterior surface …… 23.
23 – Both rami of A2 anout equal in length ; 1st exopodal segment of P1 usually lacking outer spine ; P5 3-segmented ………. Lophothrix.
- Endopod of A2 usually less than 80% length of exopod ; 1st exopodal segment of P1 usually with outer spine ; P5 usually 2-segmented ………. 24.
24 – Rostrum bifid with short branches : coxa of P4 with oval process on inner margin ; P5 with 2 spines on distal subcylindrical or expanded segment …….Pseudoamallothrix.
- Rostrum bifid with long branches ; coxa of P4 without process on inner margin ; P5 usually with 3 spines on elongated distal segment ……..Amallothrix.
25 – Distal segment of P5 more than twice as long as preceding segment ……….Neoscolecithrix.
- Distal and subdistal segment of P5 subequal in length ………Cenognatha.
After genetic analysis, Laakmann & al. (2019, p.330, Table 1) , 7 genera are transfered in the Diaixidae: Cenognatha, Falsilandrumius, Grievella, Landrumius, Neoscolecithrix, Paraxantharus, Xantharus. Total : 18 G: Amallothrix, Archescolecithrix, Diaiscolecithrix, Lophothrix, Macandrewella, Mixtocalanus, Omorius, Parascaphocalanys, Pseudoamallothrix, Racovitsanus, Scaphocalanus, Scolecithricella, Scolecithrix, Scolecitrichopsis, Scolecocalanus*, Scopalatum, Scottecalanus, Undinothrix*. * Genera not yet completely described. Puchinia (Vyshkvartzeva, 1989), Plesioscolecithrix (Markhaseva & Dahms, 2004) 1995), Rythabis (Schulz & Beckmann), Bradfordiella* (Andronov, 2007), Heteramalla (Sars, 1907) are considered as Insertae sedis genera. The genus Brodskius (Markhaseva & Ferrari, 2005) is transfered in the Tharybidae. The genus Parkius (Ferrari & Markhaseva, 1996) transfered initially in Scolecitrichidae must be considered in the prior family Parkiidae.
Summary after Markhaseva & al. (2014, p.1-73) :
Bradford (1973) included 13 genera in Scolecitrichidae, caracterised by 4 worm-like + 4 brush-like setae at the Mx2 endopod, while all others possess a setation of 3 worm-like + 5 brush-like, a character that Bradford considered being diagnostic for the family. Later, many genera with differing types of Mx2 endopod setation (viz. 3W + 6br, 3w + 5br + 1sc (sclerotzed seta), 5w + 3br + 1sc and 6w + 2br) have been placed in Scolecitrichidae and the family sensu lato was supposed to contain 23-25 genera. The Mx2 endopod 3w + 5br armament was found to be symplesiomorphic [common possession of a derived (apomorphic) homologous character] for scolecitrichids, tharybids, diaixids and parkiids and, thus, considered to be no value as a diagnostic character (Ohtsuka & al., 2003). However, so far no other character was given to confirm the extendede family composition.
Ferrari & Markhaseva (2000b) proposed that the number of setae of the Mxp praecoxa consistently allows differencution between ''Bradfordian'' families: the setal sequence 1, 2, 1 is the scolecithrid armament type (see , p.71 Markhaseva & al., 2014, p.71, figure 6 g).
Ohtsuka & al. (2003) defined the ''main group'' within Scolecitrichidae s.l. on the basis of two synapomorphies, the representation of the 3rd praecoxa endite of the Mxp syncoxa by a single setal element: usually brush-like (i.e. corresponding to the genera with 1, 2, 1 praecoxal setal sequence) and a maximum of 4 setae on the proximal endite of the Mx2. The latter cannot be considered to be a synapomorphy for the family, as 4 setae at the proximal endite of the Mx2 are shared by tharybids (type genus Tharybis Sars, 1902), parkiids (Parkius Ferrari & Markhaseva, 1996), some diaixids (type genus Diaixis Sars, 1902) and some species of the phaennid genus Brachycalanus Farran, 1905.
Ohtsuka & al. (2003) referred to the remaining genera as a ''stem group'' of Scolecutrichidae s.l. which corresponds to the ancestral lineage of ''Bradfordians'', i.e Diaixidae and Tgarybidae sensu Markhaseva & Ferrari (2005) and Markhaseva (2012) with a praecoxal setal sequence of 1, 2, 3 (see Markhaseva @& al. (2014, p.71, fig. 6 e).
Markhaseva & Ferrari (2005) regarded Scolecitrichidae with a Mxp praecoxal armature of 1, 2, 1, of which the most distal seta is brush-like, as a monophyletic lineage. Grnera that belong to this lineage correspond to the family composition of Scolecitrichidae sensu Bradford (1973), except for Heteramalla Sars, 1907, and including layer additions of genera described since 1973 with a praecoxal setation 1, 2, 1. Since the Mxp praecoxal armature in Heteramalla differs from the typical scolecithrid setation, the authors consider the taxonomic status of this genus to be unresolved and exclude this genus from Scolecitrichidae.
Other genera Scolecitrichopsis, Omorius, Diaiscolecithrix currently placed in Scomecitrichidae have the Mxp syncoxa armament sequenxe of 1, 2, 1, yet Omorius and Diaiscolecithrix share no synapomorphy (commpn possession of a derived homologous character) with other members of the family, since the seta at the distal endite of the Mxp syncoxa is sclerotised in these genera. Data on the structure of the male P5, which could give further evidence for their family relationship, are lacking so far, since males are unknown for both genera.
Scolecitrichopsis shares the sequence of the Mxp syncoxa atmament and the brush-like seta at the distal endite with other genera of the Scolecitrichidae; however, the male P5 is not characteristic of this family
The placement of Scolecitrichopsis, Omorius, Diaiscolecithrix within the monophyletic scolecitrichid lineage is therefore unresolved.
Bode & al. (2018, fig.5, p.75) underline that this family is successful colonizers of the entire water column, specialized to detect suspended particles as food items by chemoreceptors on their feeding appendages (Nishida & Ohtsuka, 1997).
|  Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44 [p.870 Fig. 5]. Schematic view of right A1 in males of ''Bradfordian'' family Scolecitrichidae. Black arrows: fused ancestral segments; Roman numerals: ancestral segments; dotted line between antennule segments: incomplete fusion. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44 [p.73, Table 1]. A2 armament (number of seta) in different ''Bradfordian'' genera (females). Scolecitrichidae. c: coxa; b: basis; End1: endoppd segment 1; End2: endopod segment 2; Exp: exopod. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44 [p.74, Table 2]. Md armament (number of seta) in different ''Bradfordian'' genera (females). Scolecitrichidae. b: basis; End1: endopod segment 1; End2: endopod segment 2; Exp: exopod; gn: gnathobase. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44 [p.76, Table 3]. Mx1 armament (number of seta) in different ''Bradfordian'' genera (females). Scolecitrichidae. pa: praecoxal arthrite (setal formula of praecoxal arthrite: terminal+posterior+anterior setae); ce: coxal endite; bp: proximal basal endite; bd: distal basal endite; End: endopod; Exp: exopod; Epi: epipodite. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44 [p.78, Table 4]. Mx2 armament (number of seta) in different ''Bradfordian'' genara (females). Scolecitrichidae. at: attenuation; pe: praecoxal endite; ce: coxal endite; bp: proximal basal endite; bd: distal basal endite; el: enditic-like lobe of proximal endopodal segment; End: endopod; w: worm-like seta; br: brush-like seta; sc: sclerotised seta. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44 [p.80, Table 5]. Mxp setation (number of seta) in different ''Bradfordian'' genera (females). Scolecitrichidae. at: attenuation; pes: praecoxal endites of syncoxa (from proximal to distal); ces: coxal endite of syncoxa; bp: basis proximal; bd: basis distal; End: endopod. For more detailed morphology of the seta on the praecoxal endites of the maxilliped syncoxa see Markhaseva & Ferrari (2005) and Markhaseva & al. (2008). |
 Issued from : G.A. Boxshall & S.H. Halsey in An Introduction to Copepod Diversity. The Ray Society, 2004, No 166, Part. I. [p.186]. Armature formula of swimming legs P1 to P4. |
 Issued from : E.L. Markhaseva & F.D. Ferrari in Invert. Zool., 2005, 2 (2). [p.157, Table 3]. Family placement of Cenognatha, Neoscolecithrix, Rythabis and Xantharus in publications. Remarks: Calanoid copepods belonging to 'bradfordian genera' possess two kinds of poorly-sclerotized, chemosensory setae on the distal endite of the basis and ramus of Mx2; many species also have such setae on the praecoxal endites of the Mxp. Most 'bradfordian' species are members of the Scolecitrichidae (about 200 species in 25 genera) or the Phaennidae (about 110 species in 8 genera), and most of these species are pelagic. Species of Tharybidae (about 50 species in 5 genera), Diaixis (about 10 species in 2 genera) and Parkiidae (1 species in one genus) have been collected in waters above the sea bed. Giesbrecht (1892) diagnosed the first of these families, Scolecitrichidae. When Diaixidae, Phaennidae and Tharybidae were established, Sars (1902) placed Scolecitrichidae and Phaennidae in a different taxonomic section than Diaixidae and Tharybidae, implying two separate monophyletic lineages for calanoids with these chemosensory setae. Fleminger (1957) proposed the four families were closely related, in part based on the chemosensory setae. However, Fleminger noted that it was difficult to separate species of Scolecitrichidae from those of Tharybidae, and recent attempts at family placement of Cenognatha, Neoscolecithrix, Ryrhabis and Xantharus exemplify the problem (Table 3). More recently, Andronov (2002) questioned the validity of both Tharybidae and Diaixidae. The existing diagnostic characters of the two former families are not sufficient to separate them from Scolecitrichidae.With an increasing number of surveys of the immediately deep-sea floor and particularly of hyperbenthic species, number have morphologically diverse chemosensory setae, resulting in several taxa. The most recent ‘bradfornian’ family, Parkiidae was considered monotypic at the time of the discovery of Parkius karenwishneri Ferrari & Markhaseva 1996 ; the diagnosis was based on setarion patterns of Mx2 and Mxp, and the epicuticular extensions of Von Vaupel Klein’s organ. The four other ‘bradfordian’ families continue to be diagnosed using incomplete analyses of the number oand morphology of the chemosensory setae on Mx2 (Bradford & al., 1983 ; Boxshall & halsey, 2004). Observations of these transformed setae on Mx2 do not permit assignment of paryicular setae to the distal endite of the basis or to the presumably multi-segmented ramus, so that homologous setae cannot be identified among different species. The problem of setal homologues on Mx2 presents a significant obstacle to the analysis of ‘bradfordian’ species. Ferrari & Markhaseva (2005) show that within the genus Tharybis, the number and kinds of sensory setae on the distal basal endite plus ramus of Mx2 exhibits the following : 3 worm-like setae, 5 brush-like setae and 1 sclerotized ; 3 worm-lke setae and 6 brush-like setae ; 3 worm-like setae and 5 brush-like setae. This variability further suggests that the number and kinds of sensory setae alone is not adequate to diagnose the ‘bradfordian’ families, or to separate ‘bradfordian’ genera with similar numbers and kinds of sensory setae (see in Ferrari & Markhaseva, 2000c). Two other characters are considered rtio have affected the evolution of ‘bradfordian’ families : number and morphology of the setae on the praecoxal endites of Mxp ; setation and arthrodial membranes on the exopod of A2. The loss and/or transformation of setae to the praecoxal endites of syncoxa of Mxp has been suggested as important to the evolutionary history of the ‘bradfordian’ families (see Ferrari & Markhaseva, 200c). Earlier, Ohtsuka & al (1998) did not consider this character diagnostically useful, nor did Vyshkvartzeva (2000). The ancestral ‘bradfordian’ calanoid is assumed to have had 1, 2, and 3 sclerotized, mechanosensory setae on the proximal, middle and distal praecoxal endites, respectively of the syncoxa of Mxp (Fig. 29, C). The first transformation to the praecoxal endites is assumed to have been the loss of 1 sclerotized seta from the distal endite (Fig. 29, D), and the second transformation is assumed to have been the loss of a second sclerotized seta from that endite. Loss of a sclerotized set ais equivalent to a single transformation. Subsequently, 1 sclerotized seta on each of the three endites may be transformed into a worm-like, chemosensory seta. A brush-like seta, often present on the distal endite, is assumed to be derived from a worm-like seta (Ohtsuka & al., 1998), rather than directly from a sclerotized seta, so a brush-like seta represents two transformations. The exopod of A2 of the ancestral ‘bradfordian’ calanoid (Fig. 30, A) is assumed to have had a proximal segment with a medial seta, followed by a long segment with 3 medial setae arranged linearly. Four short segments eazch with 1 long, thick seta were followed by an elongate segment with its medial seta at mid-length. A small, distal segment with 3 terminal setae completes the ramus. This exopod is assumed to have been derived from a 10-segmented exopod of the ancestral calanoid ; 9 segments, each with a single medial seta and of similar size, was followed by a distal segment with 3 terminal setae (see Ferrari & Markhaseva, 200a). The long segment with 3 medial setae of the ‘bradfordian’ ancestor is assumed to be a complex of 3 segments ; each represented by its medial seta but 2 lack a distal arthrodial membrane. The exopod of A2 of calanoids is patterned during the naupliar phase of development from an area immediately distal to the proximal segment so that the proximal seta of the long segment is the last structure formed (unpublished observations of nauplii of Calanus finmarchicus. A proximal-to-distal loss of setae on the long segment, followed by loss of the arthrodial membrane between the long segment and the proximal of the four small segments, is assumed to represent the progresive transformation of the exopod affected simply a progressively earlier truncation of development. From this model, an exopod for which a medial set ais absent from the proximal segment is the first derived state, followed by one in which the proximal seta of the long segment complex fails to form (Fig. 30, B), and then a long segment complex in which the proximal and middle setae fail to form (Fig. 30, C). Further transformations are a long segment complex in which no setae are present, or one in which the distal arthropodial membrane of the long segment complex fails to form (Fig.30, D), and finally a long segment on which both distal seta and distal arthropodial membrane fail to form so that the long segment is composed of 4 segments with 1 distal seta. The ancestral ‘bradfordian’ calanoid is assumed th have had 9 setae on the distal endite of the basis plus ramus of Mx2, because no more than 9 sclerotized setae are present on any species in the superfamily Clausocalanoidea (see in Table 4). Following the transformation series proposed for syncoxal setae of Mxp, 1 sclerotized seta plus 5 worm-like setae and 3 brush-like setae on the distal endite of the basis plus ramus of Mx2, as in present in Neoscolecithrix japonica Ohtsuka, Boxshall & Fosshagen (2003), represents 11 transformations among the 9 originally sclerotized setae, and is the leastnumber of transformations among extant ‘bradfordian’ species.. Loss of either a sclerotized seta or a worm-like seta from the distal endite of the basis, resulting in 8 setae, is assumed to have occurred early in the evolution of the group. Failure of formation of any set ais equivalent to a single transformation step ; however a sclerotized seta or a worm-like chemosensory set ais much more likely to fail to form than is a brush-like seta due to the latter’s greater complexity (Nishida & Ohtsuka, 1997). In the following analysis, the least derived condition is assigned to a genus exhibiting variable states of any character. For example, 3 different states for the exopod of A2 of Byrathis asre descibed : 1, 1-1-1, 1, 1, 1, 1, 1, 3 ; 1, 1-1-1-1, 1, 1, 1, 1, 3 ; 1, 1-1-1, 1-1, 1, 1, 1, 3. The first state in which 7 arthropodial membranes are present is considered the state of the ancestral Byrathis. In addition, transformations of sclerotized setae on the praecoxal endites of Mxp are assumed to precede changes to the exopod of A2 in all cases. Changes to individual setae of the distal basal endite and ramus of Mx2 are assumed to have been the last to occur during the evolutionary history of ‘bradfordian’ species. |
 Issued from : E.L. Markhaseva & F.D. Ferrari in Invert. Zool., 2005, 2 (2). [p.158, Fig.29]. Ancestral condition of setation of praecoxal endites of tje Mwp syncoxa for various 'bradfordian' genera (Discussion above and source of observations in brackets below). |
 Issued from : E.L. Markhaseva & F.D. Ferrari in Invert. Zool., 2005, 2 (2). [p.159, Fig.29]. Souce of observations for schematic representation of setation of praecoxal endites of Mxp syncoxa for various 'bradfordian' genera (figures above). |
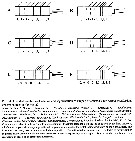 Issued from : E.L. Markhaseva & F.D. Ferrari in Invert. Zool., 2005, 2 (2). [p.160, Fig.30]. Ancestral condition of setation and segmentation of exopod of A2 for various 'bradfordian' genera (sources as for fig. 29 and discussions in text above). |
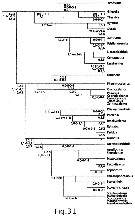 Issued from : E.L. Markhaseva & F.D. Ferrari in Invert. Zool., 2005, 2 (2). [p.164, Fig.31]. Relationships among some 'bradfordian' genera based on : Number and type of setae on praecoxal endites of Mxp; setation of five ancestral segments of the exopod of A2; number and kinds of setae on the distal basal endite olus ramus of Mx2. Sequence of 3 numbers separated by periods are number of setae on the proximal, middle and distal praecoxal endites of Mxp syncoxa (w = worm-like seta; b = brush-like seta; sclerotized seta as a simple number); sequence of 5 numbers separated by commas (artheopodial membrane present) and dashes (arthropodial membrane absent) are segment/seta on the proximal five ancestral segments of the exopod of A2; sequence of 3 numbers separated by commas are the number of sclerotized, worm-like, brush-like setae on the distal basal endite plus ramus of Mx2. Nota: Loss of 1 sclerotized seta to the distal praecoxal endite of Mxp, followed by the loss of a second sclerotized seta to the same endite results in an ancestral group and 2 major derived lineages (Fig. 31). The ancestral group retains 1, 2, 3 setae, respectively, on the proximal, middle and distal praecoxal endites. Byrathis, Diaixis, Xantharus, Falsilandrumius, Landrumius, Neoscolecithrix, Cenognatha share a sensory seta on the distal praecoxal endite of Mxp. Grievella, Tharybis are without a seta on the proximal segment and a proximal seta on the long segment complex of the exopod of A2. Brodskius is derived by loss of the seta on the proximal praecoxal endite of Mxp. Most genera of the first monophyletic lineage, with 1, 2, 2 setae on the praecoxal endites of the syncoxa of Mxp, previously have been placed in the family Phaennidae. Phaennocalanus retains a sclerotized setae on all praecoxal endites; the remaining genera shate a brush-like seta on the distal praecoxal endite. Plesioscolecithrix, Puchinia, Brachycalanus, Rythabis, Parkius share a worm-like seta on the middle praecoxal endite of Mxp. Cornucalanus, Onchocalanus, Phaenna, Cephalophanes, Talacalanus, Xanthocalanus share a derived antennal 2 exopod but are incompletely resolved. It should be pointed out that Xanthocalanus consists of almost 50 species, of which many are poorly described. When the genus is revised, some species will be placed in known genera, including Brachycalanus, while other species will have new genera established for them. Undinella is derived by loss of the seta on the proximal praecoxal endite of Mxp. Genera of the second monophyletic lineage, with 1, 2, 1 setae on the praecoxal endites of the syncoxa of Mxp, have been placed in the Scolecitrichidae, with the exception of the genus Omorius. This genus and Archeoscolecithrix retain sclerotized setae on the praecioxal endites of Mxp ; all other genera share a brush-like seta on the distal praecoxal endite. A worm-like seta on the middle praecoxal endite, pre-sumably homologous to that of first lineage, separates Parascaphocalanus, Scolecithrix, Scolecitrichopsis, Scaphocalanus, Scolecithricella, Scottocalanus, Macandrewella, Pseudoamallothrix from Amallothrix, Scopalatum, Mixtocalanus, Racovitzanus, Lophothrix. The ancestral group and both derived lineages have genera without transformed setae on the praecoxal endites of Mxp : Grievella with 1, 2, 3 sclerotized setae ; Phaennocalanus with 1, 2, 2 sclerotized setae ; Archeoscolecithrix, and Omorius with 1, 2, 1 sclerotized seta. In the ancestral group Grievella, Xantharus, Tharybis, Landrumius, Falsilandrumius, Neoscolecithrix retain the primitive state of 9 setae on the distal basal endite plus ramus of Mx2. In the first derived lineage some species of Brachicalanus retain 9 setae on the distal basal endite plus ramus of Mx2. No genus in the second derived lineage retains 9 setae on the distal basal endite plus ramus of Mx2. 5 worm-like setae on the distal basal endite plus ramus of Mx2 are retained by Brodskius, Byrathis, Neoscolecithrix in the ancestral group, 6 worm-like setae by Rythabis in the first derived lineage and 5 by Omorius in the second derived lineage. Most genera of the first derived lineage are without setae on the three segments of the long segment complex of the exopod of A2, while most genera of the second derived lineage retain the seta on the distal segment of the three segments of the long segment complex. Loss of the seta on the proximal praecoxal endite of Mxp of Brodskius and Undinella is unique to the lineages with 3 or 2 setae, respectively, on the distal praecoxal endite. This loss is assumed to have been independently derived. Due to the paucity of characters, the above hypothesized relationships (Fig. 31) results in undefined lineages and unresolved groups of genera. However ithe aothors’ hypothesis about relationships is correct, then different pelagic or benthopelagic ancestors to the genera comprising both families Phaennidae and Scolecitrichidae suggest these pelagic families are not their onwn closest relatives.
A less derived benthopelagic genus is hypothesized for each family : Omorius for genera in the Scolecitrichidae ; an early species of the ancestral group for genera in the Phaennidae (Fig. 31). This inference suggests that the invasion of the pelagic realm by ‘bradfordian’ copepods has occurred more than once after the colonization of benthopelagic habitats by a tharybid-lke ‘bradfordian’ ancestor (Bradford-Grieve, 2004).
The results of this analysis are considered preliminary because assumptions about the transformations of character states and the order of transformation of different characters have yet to be applied to many ‘bradfordian’ genera.
Assignment to families of the three genera remains tentative. Byrathis belongs to lineage, with Diaixis, Xantharus, Falsilandrumius, Landrumius, Neoscolecithrix, Cenognatha in which 1 of 3 setae on the distal praecoxal endite of Mxp has been transformed to a sensory seta ; Diaixidae is avalable for this lineage.
Brodskius belongs to a lineage with Grievella, Tharybis in which setae on the two proximal segments of the exopod of A2 fail to form ; Tharybidae is avalable for this lineage.
Omorius may be placed in the family Scolecitrichidae as diagnosed with 1, 2, 1 setae on the praecoxal endites of Mxp.
Among ‘bradfordian’ species and genera, parallel transformations of apparently homologous Mxp syncoxal sclerotized setae into poorly-sclerotized setae provide examples of Vavilov’Law (1920) that related species may express a similar variation in derived homologous structures (see Vanilov, 1966).
If the number of setae on each of 3 praecoxal endites of Mxp determines early branching, a modest number of convergences results for stattes of the exopod of A2, and a large number of convergences results for the number of woem-like plus brush-like setae on the distal basal endite plus ramus of Mx2. The convergences in states of the exopod of A2 usually results from presence/absence of the arthropodial membrane between the long segment and the proximal of 4 small segments.
Careful observations of segmentation and setation of the exiopod may reduce the number of these convergences. The same cannot be said for the number of worm-like plus brush-like setae on the distal basal endite plus ramus of Mx2 because determining homologies of these individual setae seems beyond the limits of optical microscopoy.
Detailed descriptions of Mandible, maxilla 1 and swimming leg 1 hold promise as informative states of ‘bradfordian’ genera. Knob or bumps on the distal and posterior faces of Md, and differences in numbers of setae or presence/absence of arthrodial membranes on Mx1 have proven useful in diagnosing genera. Von Vaupel Klein’s organ on P1 also may help resolve relationships among ’bradfordian’ genera. This organ, synapomorphy of gymnoplean copepods, is a transformation of the medial seta of the basis and the anterior face of the proximal endopodal segment which bears one medial seta.
Cornucalanus, Onchocalanus, Phaenna, Cephalophanes, Talacalanus, Xanthocalanus share a derived antennal 2 exopod but are incompletely resolved. It should be pointed out that Xanthocalanus consists of almost 50 species, of which many are poorly described. When the genus is revised, some species will be placed in known genera, including Brachycalanus, while other species will have new genera established for them. Undinella is derived by loss of the seta on the proximal praecoxal endite of Mxp. Genera of the second monophyletic lineage, with 1, 2, 1 setae on the praecoxal endites of the syncoxa of Mxp, have been placed in the Scolecitrichidae,with the exception of the genus Omorius. This genus and Archeoscolecithrix retain sclerotized setae on the praecioxal endites of Mxp ; all other genera share a brush-like seta on the distal praecoxal endite. A worm-like seta on the middle praecoxal endite, pre-sumably homologous to that of first lineage, separates Parascaphocalanus, Scolecithrix, Scolecitrichopsis, Scaphocalanus, Scolecithricella, Scottocalanus, Macandrewella, Pseudoamallothrix from Amallothrix, Scopalatum, Mixtocalanus, Racovitzanus, Lophothrix. The ancestral group and both derived lineages have genera without transformed setae on the praecoxal endites of Mxp : Grievella with 1, 2, 3 sclerotized setae ; Phaennocalanus with 1, 2, 2 sclerotized setae ; Archeoscolecithrix, and Omorius with 1, 2, 1 sclerotized seta. In the ancestral group Grievella, Xantharus, Tharybis, Landrumius, Falsilandrumius, Neoscolecithrix retain the primitive state of 9 setae on the distal basal endite plus ramus of Mx2. In the first derived lineage some species of Brachycalanus retain 9 setae on the distal basal endite plus ramus of Mx2. No genus in the second derived lineage retains 9 setae on the distal basal endite plus ramus of Mx2. 5 worm-like setae on the distal basal endite plus ramus of Mx2 are retained by Brodskius, Byrathis, Neoscolecithrix in the ancestral group, 6 worm-like setae by Rythabis in the first derived lineage and 5 by Omorius in the second derived lineage. Most genera of the first derived lineage are without setae on the three segments of the long segment complex of the exopod of A2, while most genera of the second derived lineage retain the seta on the distal segment of the three segments of the long segment complex. Loss of the seta on the proximal praecoxal endite of Mxp of Brodskius and Undinella is unique to the lineages with 3 or 2 setae, respectively, on the distal praecoxal endite. This loss is assumed to have been independently derived. Due to the paucity of characters, the above hypothesized relationships (Fig. 31) results in undefined lineages and unresolved groups of genera. However ithe aothors’ hypothesis about relationships is correct, then different pelagic or benthopelagic ancestors to the genera comprising both families Phaennidae and Scolecitrichidae suggest these pelagic families are not their onwn closest relatives. A less derived benthopelagic genus is hypothesized for each family : Omorius for genera in the Scolecitrichidae ; an early species of the ancestral group for genera in the Phaennidae (Fig. 31). This inference suggests that the invasion of the pelagic realm by ‘bradfordian’ copepods has occurred more than once after the colonization of benthopelagic habitats by a tharybid-lke ‘bradfordian’ ancestor (Bradford-Grieve, 2004). The results of this analysis are considered preliminary because assumptions about the transformations of character states and the order of transformation of different characters have yet to be applied to many ‘bradfordian’ genera. Assignment to families of the three genera remains tentative. Byrathis belongs to lineage, with Diaixis, Xantharus, Falsilandrumius, Landrumius, Neoscolecithrix, Cenognatha in which 1 of 3 setae on the distal praecoxal endite of Mxp has been transformed to a sensory seta ; Diaixidae is avalable for this lineage. Brodskius belongs to a lineage with Grievella, Tharybis in which setae on the two proximal segments of the exopod of A2 fail to form ; Tharybidae is avalable for this lineage. Omorius may be placed in the family Scolecitrichidae as diagnosed with 1, 2, 1 setae on the praecoxal endites of Mxp. Among ‘bradfordian’ species and genera, parallel transformations of apparently homologous Mxp syncoxal sclerotized setae into poorly-sclerotized setae provide examples of Vavilov’Law (1920) that related species may express a similar variation in derived homologous structures (see Vanilov, 1966). If the number of setae on each of 3 praecoxal endites of Mxp determines early branching, a modest number of convergences results for stattes of the exopod of A2, and a large number of convergences results for the number of woem-like plus brush-like setae on the distal basal endite plus ramus of Mx2. The convergences in states of the exopod of A2 usually results from presence/absence of the arthropodial membrane between the long segment and the proximal of 4 small segments. Careful observations of segmentation and setation of the exiopod may reduce the number of these convergences. The same cannot be said for the number of worm-like plus brush-like setae on the distal basal endite plus ramus of Mx2 because determining homologies of these individual setae seems beyond the limits of optical microscopoy. Detailed descriptions of Mandible, maxilla 1 and swimming leg 1 hold promise as informative states of ‘bradfordian’ genera. Knob or bumps on the distal and posterior faces of Md, and differences in numbers of setae or presence/absence of arthrodial membranes on Mx1 have proven useful in diagnosing genera. Von Vaupel Klein’s organ on P1 also may help resolve relationships among ’bradfordian’ genera. This organ, synapomorphy of gymnoplean copepods, is a transformation of the medial seta of the basis and the anterior face of the proximal endopodal segment which bears one medial seta. |
 Issued from : S. Laakmann, E.L. Markhaseva & J. Renz in Mol. Phylog. Evol., 2019, 130. [p.331, Table 1]. Compilation of information on relationships among ''Bradfordian'' genera from Markhaseva & Ferrari 2005) and Markhaseva & al. (2014). Abbreviations: A2, antenna; Md, mandible; Mx1, maxillule; Mx2, maxilla; Mxp, maxilliped ; P5, leg 5. w, worm-like sensory seta; b, brush-like sensory seta; s, sclerotized seta. |
 Issued from : M. Kuriyama & S. Nishida in Crustaceana, 2006, 79 (3). [p.306, Fig.7] Vertical distribution and abundance of scolecitrichid copepods in Sagami bay (Japan) at 35°00' N, 139°20' E (depth 1450 m). MTD-net (Motoda, 1971) towed horizontally at 14 layers (0, 50, 100, 150, 200, 250, 300, 400, 500, 600, 700, 800, 900, and 1000 m during the day and night, on 9-14 May 2000. See fig. 2 vertical profiles of temperature and salinity; fig. 9 relative abundance of species in groups-migratory function in the water column 0-1000 m; fig. 3: vertical profiles of abundance and species diversity and fig.6: abundance and diversity of three dominant genera. Nota: The author's observations, in addition to the available circumstantial information on morpholohy and food habits, suggest a scenario that the highly diverse scolecitrichid assemblage in Sagami Bay may be structured, through partitioning of vertical habitats and food ressources ( a size factor of 1.5 reflects partitioning of food by size after Von Vaupel Klein, 1998). |
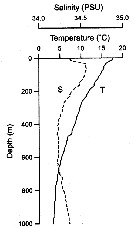 Issued from : M. Kuriyama & S. Nishida in Crustaceana, 2006, 79 (3). [p.301, Fig.2] Vertical profiles of temperature (T) and salinity (S) in Sagami Bay (Japan), in a cruise on 9-14 May 2000. |
 Issued from : M. Kuriyama & S. Nishida in Crustaceana, 2006, 79 (3). [p.308, Fig.9] Relative abundance of scolecitrichid species in the water column 0-1000 m. |
 Issued from : M. Kuriyama & S. Nishida in Crustaceana, 2006, 79 (3). [p.302, Fig.3] Vertical profiles of : A, abundance; abd B, species number of scolecitrichid copepods in Sagami Bay on 9-14 May 2000. Nota: The abundance of scolecithrids, i;e the family as a whole, showed a marked diel pattern, with the major population occurring at 200-400 m and 0-200 m, respectively, at daytime and at night.. In contrast, the number of species showed a similar pattern between day and night, with an abrupt increase from th surface to 100-200 m, followed by a gradual increase with depth to the maximal values at 500-900 m, which was similar to the expected species number normalized for a sample size of 75 individuals [ES], except that the latter lacks near-surface values owing to the extremely low number of sampled specimens (see Fig.4A). The Shannon-Wiener diversity indices (see Fig.4B) also increased with depth, with maximal values at 800 m (day) and 900 m (night) with a trend of increase in shallower depths at night. Pielou's index of eveness was lower at 300-700 m during the day than at night (see Fig.4C). |
 Issued from : M. Kuriyama & S. Nishida in Crustaceana, 2006, 79 (3). [p.304, Fig.6] Abundance and diversity of three dominant genera in scolecitrichid copepods ( Scolecithricella, Scaphocalanus, Amallothrix) in Sagami Bay on 9-14 May 2000. |
 Issued from : M. Kuriyama & S. Nishida in Crustaceana, 2006, 79 (3). [p.312, Fig. 10 D] Vertical distribution, mean body length of females and abundance of genera Scolecitrichidae, others than Scolecithricella (see fig.10 A), Amallothrix (see fig.10 B) and Scaphocalanus (see fig.10 C) collected in Sagami Bay, Japan (9-14 May 2000). The width of the box denotes the density of 50% of population between 25% and 75% distributional depth. |
 Issued from : M. Kuriyama & S. Nishida in Crustaceana, 2006, 79 (3). [p.298, 301] Measurement of species richness, the Shannon-Wiener diversity index, and Morisita-Horn index of similarity. |
 Issued from : M. Kuriyama & S. Nishida in Crustaceana, 2006, 79 (3). [p.303, Figs. 4, 5] Vertical profiles (Fig.4) of: A, expected species number; B, Shannon-wiener diversity index (H'); C, Pielou's index of eveness (J'), of scolecitrichrid copepods in Sagami Bay on 9-14 May 2000. Nota: The cluster analysis based on the Morisita-Horn similarity index (Fig.5) showed 2 distinct groups of assemblages, each comprising the samples from 700-1000 m and from 50-600 m, except for the 0 m (day and night) and 50 m (day) samples, which were distinct from all other samples at the similarity level of <0.2. Deep-mesopelagic samples (700-1000 m) exhibited high similarity indices both day and night. In the upper 600 m, the 400-600 m layers were similar during the day, while the 400 m layer came close to the upper layer at night. | | | | | Amallophora T. Scott, 1894 | |
| | Ref.: | T. Scott, 1894 b (p.54 : comme sous-genre de Scolecithrix); Sars, 1902 (1903) (p.50, Rem.); Wolfenden, 1904 (p.120); Farran, 1908 b (p.48, Rem.); A. Scott, 1909 (p.84); T. Scott, 1909 (p.125, Rem.); Wolfenden, 1911 (p.261); Sars, 1925 (p.140, Rem.); Sewell, 1929 (p.175); Davis, 1949 (p.38); Rose, 1933 a (p.134, Rem.); 1942 (p.115, Rem.); Vervoort, 1957 (p.94, 95, Rem.); Tanaka, 1960 a (p.102 & suiv., Rem.); Bradford, 1973 (p.133, 136, 144); Razouls, 1982 (p.362); Park, 1982 (p.75, 76, Rem.); Roe, 1975 (p.335, 336, Rem.); Mauchline, 1988 (p.735); Razouls, 1993 (p.310) | | Rem.: | Bradford,1973 (p.138,139) originally considers this subgenus, then “genus”, as a synonym of Xanthocalanus. A certain number of forms, formerly placed in the genus Amallophora are divided since, in addition, among Xanthocalanus (Phaennidae), in the other genera of the Scolecitrichidae (see in Bradford et al., 1983, p. 71 & foll.). This way, the genus is emptied of its substance although certain species are difficult to transfer. | | | | (1) Amallothrix Sars, 1925 | |
| | Syn.: | Scaphocalanus (part.); Scolecithricella (part.); Scolecithrix (part.) | | Ref.: | Sars, 1925 (p.176); Sewell, 1929 (p.215); Rose, 1933 a (p.151, spp. Key); 1942 (p.126); Sewell, 1947 (p.154); Davis, 1949 (p.43); Brodsky, 1950 (1967) (p.258, spp. Key); Vervoort,1951 (p.111-112); Bradford, 1973 (p.142, Redef.); Razouls, 1982 (p.300); Bradford & al., 1983 (p.77, Déf.); Mauchline, 1988 (p.737); Schulz, 1991 (p.208: Rem.); Razouls, 1993 (p.310); Mauchline, 1998 (p.82: M; p.84: F); Bradford-Grieve & al., 1999 (part., p.931, spp. Key); Vyshkvartzeva, 1999 (2000) (p. 234, 238, Redef.); 2001 (p.83); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.192: F); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.); Kuriyama & Nishida, 2009 (p.293, table 7, 10, vertical distribution); Vives & Shmeleva, 2007 (p.720, spp. Key) ; Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | Bradford (1973, p.142) redefines this genus (type: Scolecithricella gracilis Sars,1905) what leads to various synonymies. Park, 1980 (p.29) contests the validity of the position of Bradford, preferring following that of Vervoort (1951). The count of the species is very difficult. In Bradford & al. (1983, p.78) one enumerates 10 "sure" (*), 20 probable, 3 that do not totally harmonize with the definition (**) and 2 that have been transferred in new genera. Provisional total of 16 spp + 1 unidentified.
Type species: Scolecithricella gracilis Sars, 1905.
Definition from Bradford & al. (1983, p.77) :
- Pedigerous segments 4 and 5 fused in female, with fusion line sometimes dorsally ; may be separate in male.
- Rostrum of 2 filaments.
- A1 22-23 segments in female ; 19-31 in male.
- Mx1 inner lobe 1 (arthrite) with 2 posterior surface setae ; inner lobe 3 with 4 setae ; endopod segment 1 separated from segments 2 and 3.
- P1 exopodal segment 1 usually with an external spine.
- Male mouthparts slightly reduced.
- Female P5 uniramous, 3-segmented, although 2 orall segments maybe fused ; terminal segment with 2 to 4 spines (inner one of which is longest) ; surface of leg often ornamented with small spinules.
- Male left P5 endopod usually shorter than exopod, not extending past distal part of exopodal segment 2 ; right leg endopod short, at most reaching slighrly further than distal part of basis.
Diagnosis after Vyshkvartzeva (2000, p.234) :
- Pediger somites 4 and 5 in female separte or partially or completely fused.
- Posterolateral corners of last prosomal somite slighly produced, broadly rounded, usually with an incurvation near dorsal side.
- In male, pegiger somites 4 and 5 separte, not produced, broadly rounded.
- Rostrum bifurcated, both long proximal processes strong, sausage-shaped, tapering distally into sensory filament as long as proximal part or about 1/3 its length.
- A1 female 23-24-segmented, 8th and 9th, sometimes also 24th and 25th segments fused. A1 male slightly asymmetrical : in left 8th-12th segments, in right also 20th and 21th segments fused.
- A2 endopod about 2/3-4/5 ,length of exopod ; exopodal segments 2-6 with 1 seta each in female and male ; exopodal segment 7 usually with 1 medial and 3 distal setae.
- Md basipod usually with 3 inner setae ; 2 setae frequently rudimentary ; endopod reachibg about 2/3 length of exopod.
- Mx1 : inner lobe 1 with 2 (in A. aspinosa, with 4) posterior setae ; inner lobe 3 with 4 (in A. obscura, with 3) setae ; exopod with 8-9 setae.
- Mx2 : inner lobes 1-4 with 3 sclerotized setae ; inner lobe 5 with 3 sclerotized setae (one seta being stronger, hook-like, denticulated) and 1 worm-like sensory filament. 3 endopodal segments with 3 long worm-like and 5 brush-like setae being shorter than 3 others.
- P1 : exopodal segments 1-3 usually with 1 external spine about ½-4/5 length of exopodal segment 2 (in A. lobophora and A. obscura, exopodal segment 1 without external seta ; in A. aspinosa, exopodal segments 1 and 2 without external seta each).
- P2 : distal outer corner of endopodal segment 1 produced into obtuse or acute spine-like process ; external spine of exopodal segment 1 usually long, more than half as long as exopodal segment 2.- Posterior sueface of P2-P4 exopod and endopod with spines and spinules frequently arranged in arcs ; on P4 ornamentation scarcer than on P2-P3.
- P5 female usually 2-segmented ; common basal segment long ; distal segment elongate, curved inward, usually with 3 spines (internal spine the strongest, as long as segment or slightly longer or shorter, with external edge serrated ; apical spine about 1/4 -1/2 length of internal ; external spine small, usually situated opposite to internal spine ; in some species, P5 segments with spines posteriorly. | | Remarks on dimensions and sex ratio: | | The mean female size is 2.659 mm (n = 24; SD = 0.8410), and the mean male size is 3.139 mm (n = 14; SD = 0.9188), except the species of which the genus is not ''sure'' (numbered 5, 7, 14 in the genus list) . The size ratio (Male: Female) is 1.180. The sex ratio is 1.71. | | | | | (2) Archescolecithrix Vyshkvartzeva, 1989 | |
| | Ref.: | Vyshkvartzeva, 1989 (p.6, 8); 2001 (p.79); Mauchline, 1998 (p.82: M; p.84: F); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.); Vives & Shmeleva, 2007 (p.741); Markhaseva & al., 2013 (p.6, Table 1, 2, 3, 4, Rem.) | | Rem.: | Type: Scolecithrix auropecten Giesbrecht,1892; Total: 1 sp. | | | | | (3) Bradfordiella Andronov, 2007 | |
| | Ref.: | Andronov, 2007 (p.628); Markhaseva & al., 2014 (p.83, Table 1, 2, 3, 4, Rem.: p.83); Laakmann & al., 2019 (p.330, Table 1: considered as incertae sedis genera) | | Rem.: | Type: Bradfordiella kurchatovi. 2 spp.
The assignment of this genus to Scolecitrichidae is questionable; Andronov (2007) showed that the A1, with ancestral segments X-XV fused, the reduced distal part of the Mx1 and the huge Mx2 distinguish this genus from other ''Bradfordians'' genus. Markhaseva, Laakmann & Renz (2003, p.21) consider following characters that do not allow to specify its family placement: 1- Md basis lacking setae (versus 1 to 4 setae in other ''Bradfordians''; 2 - Mx1 setal formula 9, 0, 0, 3 (setae on fused distal basal endite and endopod), 2 and 5 setae; Bradfordiella shares a coxal endite without setae with Heteramalla and Pseudophaenna (versus coxal endite with 1-5 setae in other ''Bradfordians''), a proximal basal endite without setae with Kyphocalanus (Kyphocalanidae) (versus 2-4 setae in other ''Bradfordians''), a distal basal endite fused to the endopod with Rostrocalanidae, Brodskius and some species of Rythabis; 3 - Mx2 enditic lobe of endopod with 1 seta is shared with Kyphocalanus (versus usually 4, rarely 3 setae in other ''Bradfordians''; 4 - Mxp syncoxa in Bradfordiella without setae on praecoxal endites (versus setae present in other ''Bradfordians''); 5 - Mxp, endopodal segment 3 with 1 seta (versus 2-3 setae in other ''Bradfordians'' (Table 3-5 in Markhaseva & al., 2014, p.84) . Until a more detailed description of the genus is prepared, its familly placement remains uncertain. | | Remarks on dimensions and sex ratio: | | The mean female size is 1.743 mm (n = 3; SD = 0.3000), and in only one male 1.750 mm. The size ratio (Male: Female) is 1.0057. Only one male is known. | | | | | (4) Brodskius Markhaseva & Ferrari, 2005 | |
| | Ref.: | Markhaseva & Ferrari, 2005 a (p.114: Def., fig.31, Rem.); Markhaseva & Renz, 2011 (p.67, Rem.); Markhaseva & al., 2014 (p.79, Table 1, 2, 3, 4, Rem.); Renz & Markhaseva, 2015 (p.96, Table 4, Fig.3, biogeography); Laakmann & al., 2019 (p.330, Table 1) transfer this genus in the Tharybidae. | | Rem.: | type: Brodskius benthopelagicus Markhaseva & Ferrari, 2005. Total: 6 spp. + 1 unidentified
Markhaseva & Schulz (2007) include this genus in the Tharybidae family.
Markhaseva, Laakmann & Renz (2014, p.79) maintain this genus in Tharybidae family.
Diagnosis from Markhaseva & Ferrari (2005, p.114) :
Female:
- Cephalosome and pediger 1 separate or fused, pedigers 4 and 5 separate.
- Rostrum 2 delicate filaments.
- Posterior corners of prosome laterally as an indented lobe.
- A1 24-segmented.
- A2 coxa and basis without setae.
- Md gnathobase elongate, narrow medially with a knob on distal face, cutting edge narrow, with 2 distinct incisions separating groups of teeth.
- Mx1 distal basal endite fused to unsegmented endopod, setae of distal basal endite inseparable from setae of endopod; 1 seta on proximal basal endite and 2 setae on distal basal endite + endopod long and thick with long setules.
- Mx2 proximal praecoxal endite with 4 sclerotized setaze; both coxal endiotes and proximal basal endite with 1 thick seta, seta on basal endite thickest and claw-like; 5 worm-like setae with well-developeds setules and 2 or 3 short brush-like setae on distal basal endite + ramus.
- Mxp syncoxa without seta on proximal praecoxal endite, 2 setae on middle praecoxal endite, 3 setae on distal praecoxal endite; all praecoxal setae sclerotized; coxal endite with 3 setae.
- P1-P4 clausocalanoidean segmentation and setation.
- P5 3-segmented; distal segment, the exopod, with 1 medial, 1 lateral and 1 subterminal seta, and terminal unarticulated extension.
Male:
- Adult male similar to female except: posterior corners of prosome not indented.
- left A1 24-segmented, right 23-segmented; more and larger aesthetascs.
Md gnathobase poorly-developed.
- Mx1 reduced in size and setation.
- Mxp praecoxal endites of syncoxa with 0, 2, 3 sclerotized setae smaller.
- Von Vaupel klein 's organ of P1 without basal seta and anterior knob.
- P5 biramous , right exopod 2-segmented, left exopod 3-segmented; both endopod 1-segmented, right small and left one longer than exopod.
For Markhaseva & Ferrari (2005, p.115) the synapomorphies of Brodskius are: 1- Md gnathobase narrow, with 2 distinct incisions separating groups of teeth; 2- Mx1 with 1 long, thick and heavily setulated seta on proximal basal endite and 2 such setae on distal basal endite + endopod; setae of distal basal endite inseparable from those of endopod. | | Remarks on dimensions and sex ratio: | | The mean female size is 1.180 mm (n = 9; SD = 0.198), and in male 1.310 mm (n = 3; SD = 0.1529). The size ratio (Male: Female) computed from only one species (numbered 5) is 1.095. The sex ratio (F: M) is 3. | | | | | Byrathis Markhaseva & Ferrari, 2005 | | Rem.: | Cf. Diaixidae | | | | (5*) Cenognatha Bradford-Grieve, 2001 | |
| | Ref.: | Bradford-Grieve, 2001 a (p.792, Def., Rem.); Ohtsuka & al., 2003 (p.61, 62: Rem.); Bradford-Grieve, 2004 (p.287); Boxshall & Halsey, 2004 (p.192: F); Vyshkvartzeva, 2005 (p.166, 168, Table 2); Markhaseva & Ferrari, 2005 a (p.111, Fig.31, Rem.); Markhaseva & Schulz, 2010 (p.5, Rem.); Markhaseva & al., 2014 (p.81, Table 1, 2, 3, 4, Rem.) ; Laakmann & al., 2019 (p.330, Table 1: genus transfered in Diaixidae ) . | | Rem.: | In the broad sens in this family. type: Neoscolecithrix antarctica Hulsemann, 1985. Total: 1 sp. (provisionally).
Diagnosis from Bradford-Grieve (2001a, p.792) :
- Posterior prosome in lateral view extends into 2 posteriorly-directed spines.
- Rostrum short and rounded with 2 filaments.
- Mx2 endite 1 with 5 well-developed setae, endite 5 with 1 strong spine-like seta and 3 setae (2 of these possibly worm-like sensory setae in C. antarctica), endopod usually with 3 long worm-like setae and 5 brush-like sensory setae.
- Mxp coxal endite 3 with 1 or 3 setae with bulbous base.
P5 female with distal segment and its basis subequal in length.
- Male P5 of similar lengths on both sides, styliform o,n right, endopod present on at least one side, 1-segmented and spine-like on right.
- A2 exopod with full complement of ancestral setae as in Clausocalanus: 2 on segment 1, 3 on segment 2, 1 seta each on segments 3-5, segment 6 with 1 proximal seta and 3 terminal setae.
- Edge of gnathobase of Md with dorsal spinulose seta unmodified.
Endite 3 of Mx2 without modified seta.
- Posterodistal border of basis of P1-P3 without spines.
- Mxp praecoxa and coxa fused (based on C. farrani N. Vyshkvartzeva, pers. comm.), coxal endite 2 with 2 setae and 1 brush-like sensory seta.
- Male mouthparts fully developed.
In this genus Bradford-Grieve, 2004 (p.285) includes the two species Neoscolecithrix caetanoi and farrani.
According to Markhaseva & Schulz, 2010 (p.5) Procecenognatha (Diaixidae) and Cenognatha are closely related and share a combination of characters: short rostrum with 2 filaments; biramous right P5 in the male; a similar setation and segmentation pattern of the oral parts.
For Markhaseva, Laakmann (2013, p.19) this genus must be transfered in Diaixidae family. | | | | | (6) Diaiscolecithrix Markhaseva, Schulz & Renz, 2010 | |
| | Ref.: | Markhaseva & al., 2010 (p.114, Def.); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.); Renz & Markhaseva, 2015 (p.96, Table 4, fig.3, biogeography) | | Rem.: | Type: Diaiscolecithrix andeep Markhaseva, Schulz & Renz, 2010. Total: 1 sp. + 1 undet.
Diagnosis from Markhaseva, Schulz & Renz (2010, p.114):
Female:
- Cephalosome and pediger 1 partly fused, pedigers 4 and 5 separate.
- Posterior corners of prosome as short triangular lobes pointed distally.
- Rostrum as a plate with filaments.
- Upper and lower lips well developed and form circular oral cone-like structure).
- Urosome 4-segmented; genital double-somite symmetrical.
- A1 24-segmented, 1st segment with 3 setae.
- A2 exopodal segment 1 witout setae, endopodal segment 1 with 1 seta; exopod nearly 1.5 times as long as endopod.
- Md gnathobase elongate and slender with 2 acute spine-lke teeth distally; basis with 2 setae; endopoal segment 1 without setae; segment 2 with 9 setae; exopod 5-segmented with 1, 1, 1, 1, 2 setae.
- Mx1 praecoxal arthrite with 7 setae; coxal endite, proximal basal endite and distal basal endite with 2 setae each; endopod segments fused with 4-5 setae including at least 1 sensory; exopod with 7 setae; coxal epipodite with 8 setae.
- Mx2 praecoxa with outer hump,; proximal praecoxal endite with 3 setae; distal praecoxal endite with 1 seta; coxal endites with 2 setae each; proximal basal endite with 4 setae; distal basal endite okus endopod with 8 setae (3 long worm-like and 5 brush-like sensory setae).
- Mxp syncoxa with 1, 2, and 1 setae on proximal praecoxal, middle and distal praecoxal endite respectively; coxal endite with 3 setae; basis with 3 medial setae; endopodal segments 2 to 5 with 4, 3, 3+1, 4 setae.
- Segmentation and setation of P1-P4 typical for Clausocalanoidea.
- P5 present, 2-segmented and lacking spines on both segments.
Male unknown. | | Remarks on dimensions and sex ratio: | | The body size for only two females is 1.50 mm and 1.51 mm. | | | | | (7*) Falsilandrumius Vyshkvartzeva, 2001 | |
| | Ref.: | Vyshkvartzeva, 2001 (p.79, 93); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Vyshkvartzeva, 2005 (p.16167, 168, Table 2); Markhaseva & Ferrari, 2005 a (p.111, Fig.31, Rem.); Markhaseva & al., 2014 (p.81, Table 1, 2, 3, 4, Rem.); Renz & Markhaseva, 2015 (p.96, Table 4, Fig.3, biogeography) ; Laakmann & al., 2019 (p.330, Table 1) transfer tis genus in Diaixidae. | | Rem.: | In the broad sense in this family. Type: Scaphocalanus bogorovi. Total: 3 spp.
For Markhaseva, Laakmann & Renz (2014, p.81) this genus must be transfered in Diaixidae family.
Diagnosis after Vyshkvartzeva (2001, p.79) :
- Forehead with a low median crest or without crest.
- Rostrum as a short plate with 2 long filaments.
- Pedigers 4 and 5 separate.
- Posterolateral corners of pediger somite 5 produced distally into triangular lobes with a rounded tip or terminating with a small tooth-like process.
- Urosme of 4 somites, as long as ¼-1/5 of prosome.
- Genital somite (laterally) with a conspicuous genital prominence.
- Caudal rami as long as wide or slightly longer than wide.
- A2 endopod as long as exopod or slightly longer.
- Md basipod with endopod as long as exopod.
- Mx1 with 2 posterior setae on inner lobe 1 ; 3-4 setae on inner lobe 2 and 4 setae on inner lobe 3 ; exopod with 10-11 setae.
- Mx2 inner lobe 1 with 4 ( ?)-5 setae ; inner lobes 2-4 with 3 setae each ; inner lobe 5 with 4 sclerotized setae ; endopod with 3 worm-like and 5-6 brush-like sensory setae.
- Mxp syncoxa in the middle of inner margin with 3 setae, 1 sometimes transformed into a brush-like sensory seta.
- P1 basipod without inner distal seta ; endopod without setose outer lobe ; endopodal segments 1-3 with long, stout outer spine each, orendopodal segments 1 and 2 without outer spine.
- In P2-P4, outer distal corner of all endopodal segments produced as a sharp spine-like process.
- Outer sine of exopodal segment 1 of P2 shorter than outer spines of exopodal segments 2- 3.
- Terminal spines of exopods of P2-P4 longer than exopodal segment 3.
- Posterior surfaces of P2-P4 protopod and exopod segments usually covered with small denticles ; endopod segments with long spinules.
- P5 3-segmented ; distal segment longer than wide and slightly flattened, usually with 4 spines (inner spine the longest, subequal in length to the segment ; outer spine situated before the middle of the outer margin). | | Remarks on dimensions and sex ratio: | | The mean female size is 4.610 mm. (n = 4, SD = 1.150), and probably from one male: 5.11 mm. The sex ratio (Female: Male) = 3:1 | | | | | (8*) Grievella Ferrari & Markhaseva, 2000 | |
| | Ref.: | Ferrari & Markhaseva, 2000 b (p.1080); Ohtsuka & al., 2003 (p.62: Rem.); Bradford-Grieve, 2004 (p.276, 287: Table 2); Boxshall & Halsey, 2004 (p.190: F); Vyshkvartzeva, 2005 (p.167, 168, Table 2); Markhaseva & Ferrari, 2005a (p.111, Fig.31, Rem.); Markhaseva & al., 2014 (p.81, Table 1, 2, 3, 4, Rem.); Laakmann & al., 2019 (p.330, Table 1) transfer th.is genus in Diaixidae | | Rem.: | Type: Grievella shanki Ferrari & Markhaseva, 2000. Total: 1 sp.
In the broad sense in this family. Total: 1 sp.
For Markhaseva, Laakmann & Renz (2014, p.81) this genus must be transfered in Diaixidae family.
Genus' morphologic characters :
- Cephalosome and pediger 1 fused, pedigers 4 and 5 fused.
- Posterior corners of prosome rounded laterally, not reaching beyond the anterior margin of genital complex.
- Genital complex symmetrical in ventral view, asymmetry in lateral view results from small integumental bumps.
- 3 articulating abdominal somites posterior to genital complex.
- Caudal rami with 4 large, terminal setae, 1 small medial-ventral seta, and 1 small lateral-dorsal seta
- A1 24-segmented, reaching thoracic segment 3. Segment 22 with ear-like extension anteriorly with ear cavity facing proximally.
- Mx2 proximal praecoxal endite with 5 setae and attenuation point, distal endite with 3 setae. Proximal coxal endite with 3 setae, distal coxal endite with 3 setae. Proximal basal endite with 1 long, thick seta, 2 long, thin seta and 1 poorly sclerotized setae. Distal basal lobe + exopod with 9 sensory setae, 3 worm-like setae distally and 6 brush-like setae, all about the same length and with short setules; 3 brush-like setae of the same thickness, 2 thinner, 1 very thin brush-like setae.
- Mxp syncoxa with 1 long seta on proximal lobe; 2 long setae on middle lobe, both well-sclerotized; 3 short setae on distal lobe; coxal lobe with 3 setae and denticles on distal face. Basis with 3 setae on unattentuated proximal lobe and 2 setae on distal lobe; proximal denticles short and thick. Endopod 5-segmented from proximal to distal with 4, 4, 3, 4 (1 lateral),4 (1 lateral) setae respectively. .
- P1 without seta but with medial denticlesBasis with medial seta sharply curved.Endopod 3-segmented; endopod 1-segmented complex with 3 medial and 2 terminal setae; quadrate protuberence of Von Vaupel-Klein's organ with anterior row of denticles proximally and posterior pore on lateral edge.
- P2 exopod 3-segmented; endopod 2-segmented.
- P3 exopod 3-segmented; endopod 3-segmented.
- P4 exopod 3-segmented; endopod 3-segmented.
- P5 coxa proximal; basis without seta fused to 1-segmented exopod with distally 1 medial, 1 terminal and 1 lateral setae.
Ferrari & Markhaseva (2000, p.1084) note that 4 derived characters (synapomorphies) states separate this species-type of the genus from other scolecitrichids: small integumental bumps on the genital complex; an ear-like extension on segment 22 of A1; 2 lateral setae on the distal endopodal segment of P2; and a denticle-like attenuation of the proximal praecoxal lobe of Mx2. The authors believe the first of these may be an autapomorphy.
The type-species shares the absence of an outer soine on both the proximal and middle exopodal segments of P1 with a number of other scolecitrichids. This apomorphy that is shared with these other scolecitrichid species results from convergence; it is not evidence for monophyly.
The type-species shares 5 setae on the proximal praecoxal lobe of Mx2 with the scolecitrichids Xantharus renatehaassae, 1 of only 2 species in this genus, with Neoscolecithrix antarctica, 1 of 6 species in this genus, and with all 5 species in this genus, and with all 5 species of the genus Landrumius. The remaining species of Scolecitrichidae have 3 or 4 setae on the proximal praecoxal lobe of Mx2.
Species of the Phaennidae have 5 setae on this proximal praecoxal lobe, except for a few species of Xanthocalanus with 4 setae.
Species of Diaixidae, Parkiidae, and Tharybidae have 3 or 4 setae.
Type -species shares 9 sensory setae on the distal basal lobe plus exopod of Mx2 with all 5 species of Landrumius. The remaining species of Scolecitrichidae have 8 sensory setae on the distal basal lobe plus exopod of Mx2, with the exception of Xantharus renatehaassae which has 8 sensory setae and 1 sclerotized seta. 8 sensory setae is the number most often reported for Phaennidae, Diaixidae, and Tharybidae, although 9 sensory setae have been reported for some phaennids and several tharybid-like copopods (unpubl. obs.).
Species-type shares 1, 2 and 3 setae, from proximal to distal, on the 3 praecoxal lobes of Mxp with Xantharus renatehaassae, with all 5 species of Landrumius, with Neoscolecithrix antarctica and Neoscolecithrix magna; however, these latter 2 species differ quite significantly in other morphological features. Praecoxal lobes with 1, 2 and 3 setae also are known for some diaixids and tharybids. Setation for the remaining Scolecitrichidae usually is 1, 2, and 1 setae. For Phaennidae setal numbers 1, 2 and 2 seem to have been conserved.
If it is assumed that the larger number of elements is the plesiomorphic state (see Monchenko & Von Vaupel-Klein, 1999) for these above 3 characters of the Mx2 and Mxp, then the states for the type-species (Grievella shanki) provide no direct information about its phylogenetic relationships with other copepods sharing the same character states. Among related families Phaennidae, Diaixidae, Tharybidae, and Parkiidae, synapomorphies have been proposed only for the latter family.
The authors place the species Grievella shanki, (consequently the new genus) within the Scolecitrichidae tentativ/ The number and kind of sensory setae on the distal basal lobe plus exopod of Mx2 alone is not adequate to diagnose the Scolecitrichidae, or to separate all of its species from those of the Phaennidae and other families with these kinds of sensory setae.
Careful redescriptions of the setarion of A2, Mx1, Mx2 and Mxp are required before the different synapomorphies of the 5 families with their included genera can be clarified. | | | | | (9) Heteramalla Sars, 1907 | |
| | Syn.: | Heteremalla : Rose, 1929 (p.26); 1933 a (p.135) | | Ref.: | Sars, 1907 a (p.16); A. Scott, 1909 (p.86, 87); Sars, 1925 (p.142); Sewell, 1929 (p.176); Tanaka, 1960 a (p.115); Vervoort, 1965 (p.29, Rem.); Bradford, 1973 (p.138, 140); Roe, 1975 (p.341: Rem.); Razouls, 1982 (p.309); Bradford & al., 1983 (p.87, Déf.); Mauchline, 1988 (p.737, pores cuticulaires); Razouls, 1993 (p.310); Mauchline, 1998 (p.82: F); Vyshkvartzeva, 2001 (p.79); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Vyshkvartzeva, 2004 (2005) §p.166, 168, Table 2); Vives & Shmeleva, 2007 (p.742); Markhaseva & al., 2014 (p.84, Table 1, 2, 3, 4, Rem.: p.84); Laakmann & al., 2019 (p.330, Table 1: considered as incertae sedis genera) | | Rem.: | type: Heteramalla dubia Sars, 1907. 1 sp.
After A. Scott (1909, p.86) the main characteristics of Heteramalla are: 1 - A strong chitinised and slightly bifurcate lamelliform rostrum; 2 - An extraordinary development of two of the sensory appendages on the apex of Mx2; 3 - P5 very small and rudimentary in the female.
For Markhaseva, Laakmann & Renz (2003, p.22) this genus is removed from Scolecitrichidae, but the taxonomic position of this genus will probably clarified when the male is discovered. In this instance the genus has not been attributed to any of the currently known ''Bradfordians families''.
This genus differs from Scolecitrichidae by the following characters: 1- Mx1 coxal endite without setae (versus usually 2 setae in scolecitrichids); 2 - Mx2 setal formula 5, 3, 3, 3, 4 (versus 3-4 setae at praecoxal endite in scolecitrichids); 3 - Mxp praecoxal endites setal formula 1, 1, and 2 (versus 1, 2, and 1 in scolecitrichids), with setae of praecoxal endites as: 1sc + 1w + (1sc + 1w) (versus single brush-like seta present in scolecitrichids) (Markhaseva, pers. com.).
A Heteramalla male has not yet been found, therefore the taxonomic position of this genus will probably be clarified when the male is discovered. | | | | | (10*) Landrumius Park, 1983 | |
| | Syn.: | Lophothrix Giesbrecht,1895 (part.) | | Ref.: | Park, 1983 (p.165,191, Déf.); Razouls, 1993 (p.310); Mauchline, 1998 (p.85: F); Vyshkvartzeva, 2001 (p.79); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Vyshkvartzeva, 2004 (2005) (p.167, 168, Table 2); Markhaseva & Ferrari, 2005a (p.11, fig.31, Rem.); Markhaseva & al., 2014 (p.81, Table 1, 2, 3, 4, Rem.); Laakmann & al., 2019 (p.330, Table 1) transfer tgis genus in Diaixidae. | | Rem.: | In the broad sense in this family. Total: 4 spp.
For Markhaseva, Laakmann & Renz (2014, p.81) this genus must be transfered in Diaixidae family. | | Remarks on dimensions and sex ratio: | | The mean female size is 7.644 mm (n = 5; SD = 0.8359) in species numbered 1 to 3, the species 4 excluded because the size very different (3.750 mm). Males unknown. | | | | | (11) Lophothrix Giesbrecht, 1895 | |
| | Syn.: | Scolecithrix (part.) : Giesbrecht & Schmeil, 1898 (p.41) | | Ref.: | Giesbrecht, 1895 c (p.254); Wolfenden, 1904 (p.120); A. Scott, 1909 (p.98); Wolfenden, 1911 (p.267); Sewell, 1929 (p.193); Sars, 1925 (p.162); Rose, 1933 a (p.144, spp. Key); Davis, 1944 (p.40); Brodsky, 1950 (1967) (p.240); Tanaka, 1961 (p.150); Vervoort, 1965 (p.57, Rem.); Owre & Foyo, 1967 (p.66, clé spp.); Bradford, 1973 (p.140,144, Redef.); Razouls, 1982 (p.310); Gardner & Szabo, 1982 (p.277); Park, 1983 (p.177, 178, Redef.); Bradford al., 1983 (p.88, Def.); Mauchline, 1988 (p.737, cuticular pores); Razouls, 1993 (p.310); Chihara & Murano, 1997 (p.899); Mauchline, 1998 (p.82: M; p.85: F); Bradford-Grieve & al., 1999 (p.931, spp. Key); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.192: F); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.); Vives & Shmeleva, 2007 (p.744, spp. Key); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | type : Lophothrix frontalis Giesbrecht,1895. Bradford & al. (1983) enumerates 2 "sure" species (*), 4 probable (4 having been transferred to the genus Landrumius) and 1 doubtful. Provisional total: 7 soo. (of which 1 doubtful).
Diagnosis from Bradford & al. (1983, p.88) :
- Pedigers 4 and 5 fused or separate.
- Rostrum may have short conical points or elongate filiform appendages.
- Head with or without crest.
- A1 24-segmented in female; about 22 segments in male.
- Mx1 inner lobe 1 with 4 setae on posterior surface; inner lobe 3 with 4 setae.
- P1 exopod segment 1 with or witout external spine;
- Male mouthparts reduced.
- Female P5 3-segmented, the last two of which may be fused; last segment with 3 or 4 spines.
- Male P5 of Scaphocalanus type. | | Remarks on dimensions and sex ratio: | | If one accepts the case of L. latipes, The mean female size is 5.573 mm (n = 12; SD = 1.5082), and 4.169 mm in male (n = 4; SD = 1.4024). The size ratio (Male: Female) established in one case is 0.945. If the species numbered 3 is excluded , we have for female the mean 6.092 mm (n = 10; SD = 0.9785), and 5.260 mm in male. The sex ratio (Female: male) is 3. | | | | | (12) Macandrewella A. Scott, 1909 | |
| | Ref.: | A. Scott, 1909 (p.100); Sewell, 1929 (p.201); Farran, 1936 a (p.104); Tanaka, 1961 a (p.157); Bradford, 1973 (p.140); Gopalakrishnan, 1973 (table spp., p.189: Rem.); Razouls, 1982 (p.313); Bradford & al., 1983 (p.91, Def.); Campaner, 1989 (p.234: Rem.); Razouls, 1993 (p.310); Mauchline, 1998 (p.78, 84: F; 82: M); Vyshkvartzeva, 2001 (p.80); Ohtsuka & al., 2003 (p.62: Rem.); Bradford-Grieve, 2004 (p.287); Ohtsuka & al., 2002 (p.532, Rem.: emend., spp. Key: F,M); Boxshall & Halsey, 2004 (p.190: F); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.); Kuriyama & Nishida, 2006 (p.293, Rem.: p.313); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | Type: Macandrewella joanae A. Scott,1909. Total: 11 sp. (+ 1 ?).
Diagnosis from Bradford & al. (1983, p.91) :
- Head with circular lens-like organs on frontal margin.
- Head and pediger 1 fused, pedigers 4 and 5 separate.
- Rostrum with slender filaments attached to common bifurcate base.
- Posterior part of metasome and genital segment often asymmetrical.
- P5 female absent or small, 1-segmented, attached to basal portion.
- Male generally as female.
- P5 male well developed; right leg endopod well developed, exopod with basal part produced internally and last segment usually forked apically; left leg endopod well developed and exopod with apical spine.
Ohtsuka & al. (2002, p.532, emended) :
- Cephalosome fused to 1st pediger.
- Rostrum bifurcate, bearing pair of long filaments at tip.
- Single distinct cuticular lens present at base of rostrum.
- Anteromedial crest-like plate absent on cephalosome.
- 4th and 5th pedigers fused.
- Last prosomal somite carrying 1 or 2 pairs of acute or lobate processes in female, and pair of short, poineted prominences in male.
- Urosome 4-segmented, at most 1/3 as long as prosome in female; and 5-segmented in male, with anal somite almost telescoped into preceding somite.
- Caudal setae symmetrical or asymmetrical in female.
- A1 23-segmented in female
- A1 male asymmetrical, 18-segmented on right side, 19-segmented on left side.
- Mx2 endopod bearing 3 worm-like and 5 brush-like sensory setae.
- Mouthparts of male similar to those of female, but with some elements on mandibular palp slightly more reduced.
- Terminal elements of Mxp endopod more developed in male than in female.
- Strong processes predent on posterior surface of endopods of P2 and P3 in both sexes.
- Female right/left P5 present or absent.
- Male P5 highly developed, both biramous ; right exopod 3-segmented, left 2-segmented; endopods uni-segmented. Second exopodal segment of right leg forming distinct chela with 3rd segment bearing inner thumb-like process. 3rd exopodal segment left leg armed with 2 membranous elements terminally.
Ohtsuka & al. (2002, p.534) point to the genus is clearly distinguishable from other scolecitrichid genera by the combination of the characters: presence of a single cephalic lens; the highly developed male P5 with the 2nd and 3rd exopodal segments of the right leg forming a chela.
Later, Farran (1936) created a closely related genus Scolecocalanus with a single cephalic lens at the rostral base. Main differences between Macandrewella and Scolecocalanus are (see Campaner, 1989): an anterior crest-like cephalic expansion present in Scolecocalanus and absent in Macandrewella; the female P5 are 2-segmented with a common base or absent in mMcandrewella, whereas in Scolecocalanus, the left leg is uni-segmented with a stout terminal spine and the right leg is reduced. In addition, the rostral filaments are long and slender in Macandrewella but short and thick in Scolecocalanus. | | Remarks on dimensions and sex ratio: | | The mean female size is 3.374 mm (n = 19; SD = 0.294), and the mean male size is 3.370 mm (n = 15; SD = 0,339). The size ratio (M/F) is 0,999. The sex-ratio F/M = 1.2. | | | | (13) Mixtocalanus Brodsky, 1950 | |
| | Ref.: | Brodsky, 1950 (1967) (p.237, Def.); Bradford, 1973 (p.140); Razouls, 1982 (p.314); Bradford & al., 1983 (p.91, Def.); Vyshkvartzeva, 1989 (p.18, 23, Rem.); Razouls, 1993 (p.310); Mauchline, 1998 (p.81: M; p.84: F); Vyshkvartzeva, 2001 (p.79-80); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.); Vives & Shmeleva, 2007 (p.814); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | type: Amallophora altera Farran,1929. Total: 3 spp.
Diagnosis from Brodsky (1967, p.237) :
- Head and 1st thoracic segment separate or fused; 4th and 5th thoracic segments separate.
- Rostrum bifid.
- Exopod of P1 with 3 outer spines.
- Legs with posterior armature both on exopodites and on endopodites.
- P5 female 3-segmented, without spines, except the distal segment, which bears 1 apical spine.
- P5 male uniramous, right leg strongly reduced, left leg 5-segmented, several times longer than the right.
Nota: the male is considered as doubtful as Phaennidae. Mx2 male bears only 2 setae with very large sperical tips
For Brodsky (1967, p.237) the new genus, after male and female found, is closely related to Phaennidae and Scolecitrichidae and occupies ann intermediate position between them.
Mixtocalanus closely resembles Xanthocalanus, but differs from it and from the remaining genera in the structure of the distal setae of Mx2; in the female there are 3 types of setae: 3 are band-shaped, 3 are apically plumose and 2 have spherical tips. Tips of all the types of setae are small, much smaller than in Heteramalla, which also differs in the structure of the rostrum.
Diagnosis from Bradford & al. (1983, p.91) :
- head and pediger 1 fused or separate, pedigers 4 and 5 separate.
- Rostrum bifurcate with 2 filaments in female.
- Female Mx2 endopod with 3 worm-like and 5 brush-like sensoty filaments, 2 of which have spherical tips; all filaments smaller than in Heteramalla.
- Male Mx2 with only 2 brush-like sensory filaments.
- P1 exopod segment 1 with outer edge spine.
- Female P5 3-segmented, with 1 terminal spine.
- Male P5 uniramous, right leg strongly reduced, left leg several times longer than right. | | Remarks on dimensions and sex ratio: | | The mean female size is 2.604 mm. (n = 5, SD = 0.388), and the mean male size is 2.39 mm. (n = 2, SD = 0.127). The size ratio male: female is 0.918. | | | | | (14*) Neoscolecithrix Canu, 1896 (part.) | |
| | Syn.: | Oothrix Farran, 1905 (p.42); van Breemen, 1908 a (p.67); Rose, 1933 a (p.140) | | Ref.: | Brodsky, 1950 (1967) (p.225); Fosshagen, 1972 a (Rem.: p.5-6); Bradford, 1973 (p.147, 149, Rem.); Razouls, 1982 (p.369); Bradford & al., 1983 (Def., p.123); Hulsemann, 1985 a (p.55, Rem.: 2 groups); Alvarez, 1985 a (p.197, Rev., 2 groups: 'koehleri', 'farrani' ); Razouls, 1993 (p.311); Mauchline, 1998 (p.78: F,M; p.87: F); Vyshkvartzeva, 1999 (2000) (p.217-218); Bradford-Grieve, 2001 a (p.781, 791, Def.); Bradford-Grieve, 2004 (p.287); Boxshall & Halsey, 2004 (p.192); Markhaseva & Ferrari, 2005 a (p.111, Fig.31, Rem.); Markhaseva & al., 2014 (p.81, Table 1, 2, 3, 4, Rem.); Renz & Markhaseva, 2015 (p.96, Table 4, fig.3, biogeography); Laakmann & al., 2019 (p.330, Table 1) transfer yjis genus in Diaixodae. | | Rem.: | type: Neoscolecithrix koehleri Canu,1896. 8 spp. + 1 unnamed
A comparison of the characters in the (F) leads Hulsemann (1985, p.60, 61) to define 2 groups: N. koehleri , N. magna , N. sp. Bradford 1973 et N. farrani , N. watersae , N. antarctica . For Vyshkvartzeva these three latter species belong to the Scolecitrichidae.
Diagnosis from Bradford-Grieve (2001a, p.791) :
- Posterior prosome, in lateral view, extends into 2 posteriorly-directed spines.
- Rostrum wide and long with 2 points each with filament.
- Edge of Md gnathobase with very wide dorsal spinulose seta.
- Mx2 endite 1 usually with 4 well-developed setae (sometimes 1 additional small spine present), endite 3 with 1 seta modified as a large brush-like sensory seta directed distally, endite 5 with 1 strong spine-like seta, 2 smaller setae and 1 worm-like sensory seta, and endopod with 5 long worm-like and 3 brush-like sensory setae.
- Mxp coxal endite 3 with 1 of 3 setae with bulbous base.
- Posterodistal border of basis of P1-P3 and often coxa of P2 and P3 with posterior surface spine (s).
- Female P5 with distal segment at least twice as long as basis.
- Male P5 asymmetrical, very short on one side, if endopod present it is rudimentary and fused to basis.
- Male mouthparts fully developed, similar to those of female.
- A2 exopod with full complement of ancestral setae as in Clausocalanus: 2 on segment 1, 3 on segment 2, 1 seta each on segments 3-5, segment 6 with 1 proximal seta and 3 terminal setae.
- Mxp praecoxa and coxa separate, coxal endite 2 with 2 setae and 1 brush-like sensory seta.For Bradford-Grieve (2001a, p.791) the family relationship of the genus Neoscolecithrix s.l. is problematical. These species have been variously placed in the Phaennidae, (e.g. Bradford-Grieve, 1999) or Tharybidae (Bradford & al., 1983 and Vyshkvartzeva (2000) considers that species that are in Group II fit well within the Scolecitrichidae on the basis of the number of 3 worm-like and 5 brush-like sensory setae on the Mx2 endopod. nevertheless Group II species differ from the majority of Scolecitrichidae in important respects.
Ohtsuka, Boxshall & Fosshagen (2003, p.53: History of the genus).
For Markhaseva, Laakmann & Renz (2013, p.19) this genus must be tranfered in Diaixidae family. | | Remarks on dimensions and sex ratio: | | The mean female size is 3.196 mm (n = 13; SD = 0.9578), and the mean male size is 2.804 mm (n = 9; SD = 0.8847). The size ratio (male: female) is ± 1 (n = 5; SD = 0.0480). Species n°2 included in the computation, because it is possible Cenognatha. | | | | | (15) Omorius Markhaseva & Ferrari, 2005 | |
| | Ref.: | Markhaseva & Ferrari, 2005 a (p.111, Def., fig.31, Rem.); Markhaseva & Renz, 2011 (p.68, Rem.); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.); Renz & Markhaseva, 2015 (p.96, Table 4, fig.3, biogeography) | | Rem.: | type: Omorius atypicus. Markhaseva & Ferrari,2005. Total: Total: 2 spp.
Diagnosis adult female from Markhaseva & Ferrari (2005, p.143) :
- Cephalosome and pediger 1 fused dorsally, separate laterally; pedigers 4 and 5 fused dorsally and separate laterally.
- Posterior corners of prosome as rounded lobes in lateral view.
- Rostrum without filaments.
- A1 24-segmented.
- A2 coxa with 1 seta; basis with 2 setae; exopod with 1, 3, 1, 1, 1, 1, 1, 3 setae.
- Md gnathobase with a tooth-like knob on posterior face, cutting edge wide proximal to distal.
- Mx1 praecoxal endite with 4 posterior setae and 9 terminal setae, 3rd and 4th (2nd and 3rd from proximal seta) thin and slightly curved; coxal epipodite with 9 setae; proximal basal endite with 2 setae; distal basal endite with 3 setae; endopod articulating with basis with groups of 2 and 5 setae; exopod with 4 setae.
- Mx2 praecoxal endite with 4 setae; proximal and distal coxal endites with 3 setae, 1 thicker; and proximal basal endite with 4 setae, 1 thicker, 1 worm-like; distal basal endite + ramus with 5 long, worm-like setae and 3 short, brush-like setae.
- Mxp praecoxal endites of syncoxa with 1, 2, 1 sclerotized setae; coxal endite with 3 setae; endopod of 5-segmented with 4, 4, 3, 3+1 and 4 setae.
- P1-P4 clausocalanoidean segmentation and setation.
- P5 3-segmented, distal segment with 3 setae and terminal attenuation.
For Markhaseva & Ferrari (2005, p.143), at present3rd and 4th thin and slightly curved setae on the praecoxal arthrite of Mx1 is the only proposed synapomorphy for the monotypic Omorius. A sclerotized seta on distal praecoxal endite of Mxp syncoxa is shared with Archeoscolecithrix although the latter genus has 5 brush-like and 3 worm-like setae on the distal basal endite + ramus of Mx2, a situation reversed for Omorius. On the remaining 17 scolecitrichid-like genera, a brush-like seta replaces the sclerotized seta on the distal praecoxal endite of Mxp syncoxa. On the remaining 'bradfordian' genera, 2 or 3 setae are found on the endite. | | Remarks on dimensions and sex ratio: | | The mean female size is 1.715 mm (n = 2). Males unknown. | | | | | (16) Parascaphocalanus Brodsky, 1955 (? Scolecitrichidae ) | |
| | Ref.: | Brodsky, 1955 a (p.195); Bradford, 1973 (p.140); Roe, 1975 (p.321, Rem.); Razouls, 1982 (p.314); Brodsky & al., 1983 (p.135); Bradford & al., 1983 (p.91, Rem.); Razouls, 1993 (p.310); Mauchline, 1998 (p.82: M; p.85: F); Vyshkvartzeva, 2001 (p.80, 87, 93); Ohtsuka & al., 2003 (p.61, 62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Markhaseva & Ferrari, 2005 a (p.111, fig.31, Rem.); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | Type: Parascaphocalanus zenkevitchi Brodsky, 1955. Total: 1 sp.
Diagnosis from Vyshkvartzeva (2001, p.93) :
- Forehead with a low median crest or without crest.
- Rostrum as a short plate with 2 long filaments.
- Pedigers 4 and 5 separate.
- Posterolateral corners of pediger somite 5 produced distally into triangular lobes with a rounded tip or terminating with a small tooth-like process.
- Urosme of 4 somites, as long as ¼-1/5 of prosome.
- Genital somite (laterally) with a conspicuous genital prominence.
- Caudal rami as long as wide or slightly longer than wide.
- A2 endopod as long as exopod or slightly longer.
- Md basipod with endopod as long as exopod.
- Mx1 with 2 posterior setae on inner lobe 1 ; 3-4 setae on inner lobe 2 and 4 setae on inner lobe 3 ; exopod with 10-11 setae.
- Mx2 inner lobe 1 with 4 ( ?)-5 setae ; inner lobes 2-4 with 3 setae each ; inner lobe 5 with 4 sclerotized setae ; endopod with 3 worm-like and 5-6 brush-like sensory setae.
- Mxp syncoxa in the middle of inner margin with 3 setae, 1 sometimes transformed into a brush-like sensory seta.
- P1 basipod without inner distal seta ; endopod without setose outer lobe ; endopodal segments 1-3 with long, stout outer spine each, orendopodal segments 1 and 2 without outer spine.
- In P2-P4, outer distal corner of all endopodal segments produced as a sharp spine-like process.
- Outer sine of exopodal segment 1 of P2 shorter than outer spines of exopodal segments 2- 3.
- Terminal spines of exopods of P2-P4 longer than exopodal segment 3.
- Posterior surfaces of P2-P4 protopod and exopod segments usually covered with small denticles ; endopod segments with long spinules.
- P5 3-segmented ; distal segment longer than wide and slightly flattened, usually with 4 spines (inner spine the longest, subequal in length to the segment ; outer spine situated before the middle of the outer margin).
After Bradford & al. (1983, p.91) it is not clear that Parascaphocalanus is a scolecithrid. Brodsky (1955) figures the terminal part of Mx2 with 4 worm-like and 2 brush-like sendsory filaments, endopod segment 1 of P2 is small and narrow, and the male P5 very like that of some tharybids. On the other hand Parascaphocalanus appears to differ from most tharybids in that the A2 rami are of similar length and exopod segment 1 of P1 is without an outer edge spine. | | Remarks on dimensions and sex ratio: | | Body size from one species: female 2 mm, and male 2mm. | | | | (17) Paraxantharus Schulz, 2006 | |
| | Ref.: | Schulz, 2006 (p.48); Markhaseva, 2010 (p.269, 275, Table 1, Rem.); Markhaseva & al., 2014 (p.81, Table 1, 2, 3, 4, Rem.); Renz & Markhaseva, 2015 (p.96, Table 4, fig.3, biogeography); Laakmann & al., 2019 (p.330, Table 1) transfer this genus in Diaixidae. | | Rem.: | type-species: Paraxantharus brittae Schulz,2006. Total: 2 spp.
According to Markhaseva (2010, p.209) this genus is tentatively placed in the Diaixidae lineage (sensu Markhaseva & Ferrari, 2005);
For Markhaseva, Laakmann & Renz (2014, p.81) this genus must be transfered in Diaixidae family.
Diagnosis male from Schulz (2006, p.48) :
- cephalosome and pediger 1 fused, pedigers 4 and 5 incompletely coalescent.
- Rostrum a bifurcate plate with 2 tiny filaments distinctly separate.
- Urosome of 5 somites.
- Anal somite very short (almost ''invisible'').
- A1 extending to urosome, 23-segmented on left, 22-segmented on right side (due to fusion of ancestral segments XXII-XXIII); left segment XXII smaller than each of remaining segments.
- A2 with exopod nearly 2 times length of endopod, ancestral segment I-IX armed with 1 seta each.
- Mouthparts not reduced.
- Md with gnathobase armed with 8 sharply pointed teeth.
Mx2 with proximal praecoxal endite bearing 5 setae; endopod with 5 brush-like and 3 long worm-like plus 1 sclerotized setae.
- Mxp syncoxa with 1, 2, 3, and 3 setae, endite 3 with 2sclerotized and 1 sensory setae bearing small terminal brush.
- Legs of clausocalanoidean segmentation, 2nd and 3rd exopodal segments with strong outer spines, terminal exopodal spine large with comparatively few widely spaced teeth.
- P1 endopod wih large outer lobe.
P5 biramous with 1-segmented endopods, exopods 3-segmented; right exopod slender, with long terminal spine-like segment; left exopod shorter, compact. | | Remarks on dimensions and sex ratio: | | The female size in only one species is 1.560 mm. and the male of another species is 1.445 mm. The size ratio (Male: Female) probably near of 0.93. | | | | | (18) Parkius Ferrari & Markhaseva, 1996 | |
| | Ref.: | Ferrari & Markhaseva, 1996 (p.266); Bradford-Grieve, 2004 (p.287); Boxshall & Halsey, 2004 (p.186, 188, 190); Vyshkvartzeva, 2004 (p.176); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.); Renz & Markhaseva, 2015 (p.96, Table 4, fig.3, biogeography) | | Rem.: | Boxshall & Halsey, 2004 (p.188; 190: F) deem that the Parkiidae family should be included in the Scolecitrichidae in the absence of a phylogenetic analysis. Total: 1 sp. + 1 undetermined
For Markhaseva, Laakmann & Renz (2014, p.75, 77) this family is not included in the Scolecitrichidae, but in the Parkiidae family. | | Remarks on dimensions and sex ratio: | | For one female the mean female size is 1.975 mm, and for one male from another species the size is 1.92 mm. Apparently the size ratio (male : female) should be 0.972. | | | | (19) Plesioscolecithrix Markhaseva & Dahms, 2004 | |
| | Ref.: | Markhaseva & Dahms, 2004 (p.328, Def.); Vyshkvartzeva, 2004 (2005) (p.166, 168, Table 2); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.); Markhaseva & al., 2013 (p.22, Table 1, 2, 3, 4, Rem.: p.84); Laakmann & al., 2019 (p.330, Table 1: considered as incertae sedis genera) | | Rem.: | Type: Plesioscolecithrix juhlae Markhaseva & Dams, 2004. Total: 1 sp.
Not included in the generic key in Boxshall & Halsey (2004, p.188).
Earlier assigned to the Scolecitrichidae, later analysis of ''Bradforddians'' relationships showed that this genus does not belong to the monophyletic scolecitrichid lineage (Markhaseva & Ferrari, 2005). The taxonomic position of this genus therefore remains unresolved.
After Markhaseva, Laakmann & Renz (2013, p.22) this genus should be placed outside the Scolecitrichidae because of the following characters: 1 - the proximal part of right A1 in males has only ancestral segments X-XI fused (more segments fusions in scolecitrichids); 2 - A2 endopodal segment 1 with 1 seta (versus usually 2 setae in scolecitrichids); 3 - Mx1 praecoxal arthrite has no posterior setae (versus usually 1-4 posterior setae in scolecitrichids); 4 - Mx2 praecoxal artrite has 5 setae (versus 3-4) in scolecitrichids); 5 - Mxp praecoxa setal formula: 1, 2 and 2 setae (versus 1, 2 and 1 setae in scolecitrichids) (see Table 1-5), with setae of praecoxa as: 1sc + (1w+1sc) + (1sc+1br); 6 - male P5 right basipod not swollen (versus swollen in scolecitrichids); legs of silmple structure (versus complex in scolecitrichids); right and left coxa and basis nearly the same length (versus right basis significantly shorter than left bin scolecitrichids). | | | | | (20) Pseudoamallothrix Vyshkvartzeva, 2000 | |
| | Syn.: | Amallothrix : Bradford-Grieve & al., 1999 (part., p.881, 931, spp. Key); Scolecithricella : Bradford-Grieve & al., 1999 (part., p.881, 932) | | Ref.: | Vyshkvartzeva, 1999 (2000) (p.227); 2001 (p.83); 2003 (p.46: Rem.: M); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.192: F); Markhaseva & Ferrari, 2005 a (p.111, fig.31, Rem.); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | type: Amallothrix profunda Brodsky,1950. Total: 15 spp. (of which 1 doubtful).
Diagnosis from Vyshkvartzeva (2000, p.227):
- Pedigerous somites 4 and 5 usually fused in female ( articulation suture sometimes visible dorsally), usually separate in male and some females of large species.
- Posterolateral corners of pedigerous somite 5 not produced, broadly rounded, in the middle part with a shallow incurvation (incision), or slightly produced, narrowly rounded with indentation near dorsolateral part.
- Rostrum as a short plare with 2 strong but short rami continuing in 2 thick aesthetasc-like filaments; the latter longer than the strong proximal part and frequently notched at apex.
- A1 female with 23-24 segments; 8th and 9th segments fused, 24th and 25th segme,ts sometimes fused. A1 of male slightly asymmetrical: left A1 20-segmented (8th-12th segments and 24-25th segments fused; right A1 with 19-segmented (also 20th-21th segments fused).
- A2 endopod 1/2-2/, the length of exopod; exopodal segment 1 without seta, usually with a rounded swelling on internal margin (without swelling in P. cenotelis, P. longispina, P. ovata); exopodal segments 2-6 frequently with 1 seta each in female and male; seta of exopodal segment 2 usually shorter in female, than in male.
- Mx1: inner lobe 1 with 2 or 4 posterior setae; inner lobe 3 usually with 4, sometimes with 3 setae (P. ovata, P. cenotelis); exopod usually with 8 setae (5 setae in P. ovata, P. cenotelis).
- Mx2: inner lobes 1-4 with 3 setae each; inner lobe 5 with 2-3 sclerotized setae and 1 or sometimes 2 worm-like sensory setae; on inner lobe 3 , sometimes one of the 3 sclerotized setae transformed into sensory seta; 3-segmented endopod with 3 long worm-like and 5 brush-like sensory setae; distal brush of brush-like setae always small, but 3 of 5 setae have ''stem'' slightly or significantly longer and sometimes worm-like.
- Male mouthparts similar to those of female in meristic details, but some setae are shorter , slightly reduced.
- Each of the three segments of P1 exopod with external spine of about 1/2-4/5 the length of the segment, in P. longispina longer than segment (exopodal segment 1 without seta in P. birshteini; exopodal segments 1-2 without setae in P. canariensis.
- External distal corner of endopodal segment 1 of P2 produced into long, spine-like process; external spine on endopodal segment 1 of P2 straight, usually short.
- P2-P3 coxopod with a distinct indentation about in the middle of external margin ; internal margin usually with well marked projection distally ( without projection in P. longispina) ; the latter often with a notch ; dorsal surface of exopod and endopod segments with spines arranged in arcs and with numerous spinules.
- P4 coxopod with external margin smooth, internal margin bearing a rounded lamelliform lobe ( absent in P. longispina) ; dorsal surface of segments with few spinules.
- P5 female uniramous with 1 or sometimes 2 free subcylindrical segments and 2 (apical and ubapical) spines ; common basal segment long.
- P5 male biramous, asymmetrical. In right leg, 1st and 2nd exopodal segments almost completely fused, 2nd segment with or without short distal projection, 3rd exopodal segment curved, bearing lamella-like spine on distal end ; right 1-segmented endopod usually longer than 3-segmented exopod or sometimes as long as exopod. Right P5 sometimes significantly shorter than left (P. indica). Sometimes (in P. ovata, ?P. cenotelis) P5 uniramous, right leg much shorter than left.
The author in 2000 transfered 13 species in this genus. | | Remarks on dimensions and sex ratio: | | The genus shows two groups of body size:
1st Group: Mean size < 3.5 mm (species numbered 1, 2, 3, 6, 7, 9, 10, 12, 13, 14), the mean female size is 2.491 mm (n = 19; SD = 0.6668), and the mean male size is 2.285 mm (n = 13; SD = 0.6788). The size ratio (Male: Female) is 0.979 (n = 3; SD = 0.1112). The sex ratio (Female: Male) = 1.43.
In the 2nd Group (body size > 3. 5 mm in species numbered 4, 5, 8, 11): The mean female size is 4.551 mm (n = 7; SD = 1.1465), and the mean male size is 3.808 mm (n = 4; SD = 0.2300). The size ratio (Male: Female) is 0.994. The sex ratio (Female: Male) is 2. | | | | | (21) Puchinia Vyshkvartzeva, 1989 | |
| | Ref.: | Vyshkvartzeva, 1989 a (p.29); 2001 (p.79); Mauchline, 1998 (p.82: F); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Vyshkvartzeva, 2004 (2005) (p.166, 168, Table 2); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.); Markhaseva & Schulz, 2010 (p.17, Rem); Markhaseva & al., 2014 (p.44, Table 1, 2, 3, 4, Rem.: p.85); Laakmann & al., 2019 (p.330, Table 1: in incertae sedis genera) | | Rem.: | type: Puchinia obtusa. Total: 1 sp.
The sensitive setae of the Mx2 indicate that this genus belongs to the Scolecithricidae and presents the most evolved characters in this family. Exop. 1 & 2 of the A2 with numerous small setae and the Ri of the Mx1 with 3 segments reveal archaic characters. 5 setae on the 2nd internal lobe of Mx; 2 sensory filaments and 1 usual chaeta in the middle protopodite of the Mxp; 1 sensory filament present on the 2nd-4th endites of Mx2. For Vyshkvartzeva, analysis of the evolutionary importance of the morphological features of Puchinia has revealed that it is characterized by a combination of a number of highly specialized features of its structure with the archaic ones, typical for the ancestor of the whole superfamily, by some parallelisms not only with the genera of its own family but with other Pseudocalanoidea.
The bad condition of the type specimen makes it impossible to clarify the taxonomic position of the genus (Markhaseva pers. obs.).
For Markhaseva, Laakmann & Renz (2014, p.23) the attribution of this genus to scolecitrichids is doubtful as Mx1 coxal endite bears 5 setae (versus 2 or rarely 4 setae in scolecitrichids) and Mxp praecoxa setal formula is 1, 2 and 2 (versus 1, 2 and 1 setae in scolecitrichids). | | Remarks on dimensions and sex ratio: | | The female size, for only one individual, is about 2.95 mm. | | | | | (22) Racovitzanus Giesbrecht, 1902 | |
| | Ref.: | Giesbrecht, 1902 (p.26); Wolfenden, 1911 (p.259); Brodsky, 1950 (1967) (p.266); Tanaka, 1961 a (p.185); Bradford, 1973 (p.140); Razouls, 1982 (p.314); Gardner & Szabo, 1982 (p.295); Park, 1983 (p.171, Redef.); Bradford & al., 1983 (Def., p.91); Mauchline, 1988 (p.737, pores cuticulaires); Razouls, 1993 (p.310); Chihara & Murano, 1997 (p.899); Mauchline, 1998 (p.80, 85: F; p.82: M); Vyshkvartzeva, 2001 (p.80); Ohtsuka & al., 2003 (p.61, 62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.);Vives & Shmeleva, 2007 (p.816, spp. Key); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | type: Racovitzanus antarcticus Giesbrecht,1902.
Total provisionally: 2 spp.
For Tanaka (1961 a , p.185) the genus is closely allied to Scaphocalanus in the structure of the male P5 and intermediate between Scaphocalanus and Scolecithrix in having a rather long outer edge spine on the 2nd exopodal segment of P1.
Diagnosis from Bradford & al. (1983, p.91) ;
- Rostrum cylindrical with 2 small filaments terminally.
- Mx1 inner lobe 1 with 2 setae on posterior surface; inner lone 3 with 3 setae.
- Mx2 endopod with worm-like and brush-like sensory filaments.
- P1 exopod segment 1 without external spine.
- Female P5 absent or 2-segmented, with 1 or 2 spines, neither of which are external.
- Male P5 of the Scaphocalanus type.
After Bradford-Grieve (1983, p.91) the nature of the sensory filaments on Mx2 is difficult to observe and there is considerable variation in the number and type recorded by various authors. The male which Vervoort (1957) attributes erroneously to R. antarcticus appears to differ from other Racovitzanus in having an outer edge spine on exopod segment 1 of P1. | | Remarks on dimensions and sex ratio: | | The mean female size is 2,038 mm (n=2; S= 0,124; Cv= 0,061) and the mean male size is 1,850 mm (n= 2; S= 0,198; Cv= 0,107). The size ratio (M/F) is 0,936 ou 93,6 % (n= 2; S= 0,042; Cv= 0,046). The sex-ratio F/M= 1. | | | | | (23*) Rythabis Schulz, 1995 | |
| | Ref.: | in Schulz & Beckmann, 1995 (p.199); Ohtsuka & al., 2003 (p.61, 62: Rem.); Bradford-Grieve, 2004 (p.287); Boxshall & Halsey, 2004 (p.190: F); Markhaseva & Ferrari, 2005 a (p.11, fig.31, Rem.: p.149, 157, 164); Markhaseva & al., 2014 (p.44, Table 1, 2, 3, 4, Rem.: p.85); Renz & Markhaseva, 2015 (p.96, Table 4, Fig.3, biogeography); Laakmann & al., 2019 (p.330, Table 1: considered as incertae sedis genera) | | Rem.: | Type: Rythabis atlantica Schulz & Beckmann, 1995. Total: 4 spp.
Diagnosis from Schulz, 1995 (1995, p.199) :
- Body compact (tharybids).
- Cephalosome and pediger 1 separate, pedigers 4 and 5 mostly separate.
- Anal somite as long as preceding somite.
- Rostrum a knob-like prominence lacking filaments.
- A1 with fusion patterns of segments typical of superfamily Clausocalanoidea, and with ancestral segments XXVI and XXVII-XXVIII separated.
- Exopod of A2 more than twice the length of endopod.
- Md gnathobase bearing 3 acute teeth ventrally; basis with 2 long curved setae.
- Mx1 well equipped with setal armature.
- Mx2 comprising short praecoxa and relatively large endopod .
- Mxp slender.
- Legs of typical clausocalanoid segmentation.
- Inner endopodal lobe of P1 reduced.
- Outer exopodal spines of P1 and P2 (presumably P3 and P4 as well) exceptionally strong and ornamented with small marginal denticles.
- Posterior face of P2-P5 spinose.
- P5 uniramous, 3-segmented, terminal segment bearing 2 distal, 1 inner and 1 outer denticulated spines.
- Male unknown (up to 2015).
For Schulz & Beckmann (1995, p.203) this genus shows closest affinities to the genus Tharybis, particularly on account of its compact ovate body form, the conical rostral knob lacking filaments, the separated segments XXVI and XXVII-XXVIII of A1, presence of identical numbers of large and massive spines on the praecoxal arthrite of Mx1, occurrence of 2 different types of maxillar aesthetascs and presence of 3 basal setae on Mxp, and non-broadened exopods of P1 to P4. However, the following are distinguishing characters taht separate members of Rythabis from Tharybis: 1- 4th and 5th pedigers mostly separated; 2- anal somite as long as preceding somite; 3- basis of Md equpped with only 2 setae; 4- maxillular epipodite, basis, endopod and exopod comparatively well developed and armed with higher numbers of setae; 5- brush-like aesthetascs of Mx2 and Mxp of different structure; 6- outer exopodal spines of P1 and P2 ornamented with denticles; 7- P5 having the exopod armed with 1 inner, 1 lateral and 2 distal articulated spines. In members of the genus Tharybis the terminal segment of P5 always carries a single inner distal spine in addition to usually 2 spine-like protrusions.
Markhaseva & Schulz (2007, p.742) consider the family placement as incompletely resolved. For Markhaseva, Laakmann & Renz (2014, p.85) the characters following prevent the placement of this genus in Tharybidae (placed in this family by Schulz & Beckmann, 1995, followed by Bradford-Grieve, 2004 and Vyshkvartzeva, 2005), as in the Scolecitrichidae (placed by Ohtsuka & al. 2003, and Boxshall & Halsey, 2004). The following characters prevent a placement of the genus Rythabis into the Scolecitrichidae: 1 - Mx2 endopod setal formula has usually 6 worm-like + 2 brush-like sensory setae (versus usually 3w + 5br sensory setae in scolecitrichids, however 1 species of Rythabis shares the 4w + 4br setation with the scolecitrichid genus Racovitzanus) and the Mxp praecoxal setal formula 1, 2 and 2 (versus 1, 2, and 1 setae in scolecitrichids), setae at praecoxa 1w + 2 sclerotised setae + (1sc +1br). Except for the differences in limbs setation, Rythabis is distinguised from scolecithrichids: 1 - The short A2 endopod (shared with Pseudophaenna, Diaixis and Tharybis: 2 - Mxp syncoxa with a row of long thin spinules proximal of coxal endite (absent in scolecitrichids); 3 - Md gnathobase with a row of spinules along cutting edge (absent in scolecitrichids); 4 - female P5 with 4 spines at the exopod (usually 3 spines in scolecitrichids).
The following characters prevent the placement of Rythabis in Tharybidae: 1 - Mx1 coxal and distal basal endites with 4-5 setae (versus 2-3 setae in Tharybis), in some species the distal basal endite and endopod are fused (versus separate in Tharybis); 2 - Mx2 endopod setal formula 6w + 2br or 4w + 4br (versus 3w + 5br, 3W + 6br, or 3w + 5br + 1sc in Tharybis); 3 - Mxp praecoxal setal formula 1, 2, 2 (versus 1, 2 and 3 in Tharybis).
Laakmann, Markhaseva & Renz (2019, p.330, Table 1) consider this genus as incertae sedis genera in the ''Bradfodians families" .
| | Remarks on dimensions and sex ratio: | | The mean female size is 1.248 mm (n = 5; SD = 0.1117). Males unknown. | | | | | (24) Scaphocalanus Sars, 1900 | |
| | Syn.: | Amallophora (part.) Sars, 1902 (1903) (p.50) | | Ref.: | Sars, 1900 (p.35); A. Scott, 1909 (p.95); Sars, 1920 c (p.8); 1925 (p.169); Farran, 1926 (p.257); Sewell, 1929 (p.205); Wilson, 1932 a (p.76, clé spp.); Rose, 1933 a (p.146, clé spp.); Mori, 1937 (1964) (p.49); Rose, 1942 (p.115); Davis, 1949 (p.41); Rose, 1942 (p.115); Brodsky, 1950 (1967) (p.246, spp. Key ); Tanaka, 1961 a (p.157); Vervoort, 1965 (p.65, Rem.); Bradford, 1973 (p.140,143, Redéf.); Séret, 1979 (p.113); Razouls, 1982 (p.316); Gardner & Szabo, 1982 (p.281); Park, 1982 (p.78, Redef., spp. Key, Rem.: p.125); Bradford & al., 1983 (p.93, Def.,); Schulz, 1987 (p.105, Rem.); Mauchline, 1988 (p.737, cuticular pores); Razouls,1993 (p.310); Chihara & Murano, 1997 (p.900); Mauchline, 1998 (p.80, 84, 85: F, figs.198, 232; p.82: M); Bradford-Grieve & al., 1999 (p.931, 932, spp. Key); Vyshkvartzeva, 2001 (p.80); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.192: F); Markhaseva & Ferrari, 2005 a (p.111, fig.31, Rem.); Kuriyama & Nishida, 2009 (p.293, table 7, 10, vertical distribution); Vives & Shmeleva, 2007 (p.750, spp. Key); Markhaseva & Renz, 2011 (p.67, Rem.); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | type: Amallophora magna T. Scott, 1894.
For Bradford & al (1983) there are 14 'sure' species(*) and 14 'probable'(**). Provisional total 32 spp. (of which 1 doubtful) + 5 undet.
After Tanaka (1961 a, p.157) the species of the genus resemble each other so closely in general appearance and in the structure of P5 that is very difficult to discriminate them. Moreover, P5 female exhibit a considerable degree of variability in the proportional lengths and the number of spines on the distal segment. The P5 os often furnished with abnormal endopod as illustrated by Vervoort (1957) in S. brevicornis Sars.
Diagnosis after Bradford & al. (1983, p.93) :
- Pedigerous segments 4 and 5 fused in female, usually separate in male.
- Head may have crest.
- Rostrum of 2 filaments.
- A1 22-segmented in female, with segments 4-20 flattened and expanded posteriorly; of 19-21 segments in male.
- Mx1 inner lobe 1 usually with 3 setae on posterior surface; inner lobe 3 with 3 or 4 setae; endopod segment 1 separated from segments 2 and 3.
- Mx2 endopod with 3 worm-like and 5 brush-like sensory filaments.
- P1 exopod segment 1 without outer spine.
P2 endopod segment 1 without pointed nouter distal extension.
Male mouthparts reduced, especially Mx2 and inner lobes of Mx1.
- Female P5 absent or, more usually, present and 3-segmented, often with last two segments fused; terminal segment with 2 to 4, usually strong spines.
- Male left P5 endopod longer than exopod; right P5 endopod long, usually reaching segment 2, exopod segment 3 long and blade-like.
Diagnosis after Park (1982, p.78) :
Female:
- Head and 1st pedigeous segment fused; 4th and 5th thoracic segments fused, often with a line of segmentation visible dorsally.
- Urosome 4-segmented, followed by moderately developed caudal rami.
- Rostrum of 2 filaments, usually well-developed.
A1 with segments 8 through 10 fused and with a transparent strip along posterior edges of segments 5 through 22.
- Both endopod and exopod of A2 well developed; 1st exopodal segment without a seta.
- Md with strong masticatory blade and well-developed basis followed by small endopod and large exopod.
- Mx1 with coxa bearing 3 inner lobes and 1 outer lobe, and basis carrying endopod and exopod.
- Mx2 with 5 well-developed lobes and a small endopod; endopod with 3 long vermiform and 5 short brush-like sensory setae.
- Mxp with large coxa and basis followed by 5 small endopodal segments; basis with 3 middle and 2 terminal setae.
- P1 with 1-segmented endopod and 3-segmented exopod; 1st exopodal segment without spines or setae.
- P2 with 2-segmented endopod and 3-segmented exopod; coxa fringed medially with hair followed by a well-developed seta; basis devoid of setae and hair along inner margin.
- P3 and P4, both endopod and exopod 3-segmented; basis of P3 similar to P2; in P4, coxa without inner marginal hair but with a moderately developed inner seta;
- P5 absent or more usually 2-segmented; distal segment often divided partially into 2, with strong inner and terminal spines and ofren with 1 or 2 small outer spines.
Male:
- Head and 1st pedigerous segment fused, 4th and 5th thoracic segments incompletely fused with line of segmentation clearly visible dorsally.
- Urosome 5-segmented, followed by small caudal rami;
- Rostrum of 2 filaments, usually well-developed.
- A1 with segments 8 through 12 fused, without a transparent strip along posterior edge; segments 20 and 21 fused on right A1, but separate on left.- Md blade poorly developed, with small teeth on its distal edge; palp well-developed with a broad basis followed by strong endopod and exopod.
- Mx1 with poorly developed inner lobes and well-developed outer lobe and exopod.
- Mx2 poorly developed, with 5 lobes followed by an endopod bearing 3 vermiform and 5 brush-like sensory filaments.
- Mxp relatively small; setae poorly developed, except those on last 2 endopodal segments; basis with 3 middle anfd 2 terminal setae.
- P1 to P4 similar to those of female.
- P5 asymmetrical, each leg with 2-segmented basipod followed by endopod and exopod; basis elongate in left leg but short in right. In left leg, exopod3-segmented; distal segment with hair and bristles; endopod 1-segmented with a small terminal spine and longer than exopod; In right leg, exopod 2-segmented with well-developed distal spine; 1st segment ofthen divided into two by a line; endopod 1-segmented with a terminal spine.
Bradford & al. (p.93) note that the number of setae on Mx1 inner lobes 1 and 3 appear not to be as constant (see Bradford, 1973). Scaphocalanus difficilis Roe, 1975 has 2 posterior surface setae on inner lobe 1 and S. difficilis, S. curtus, S. similis, S. invalidus all have 3 setae on inner lobe 3 (see Hure & Scotto di Carlo, 1968). | | Remarks on dimensions and sex ratio: | | If we consider only the species attributed to this genus there are two groups of sizes. In the first group (species body length > 3 mm, species numbered 1, 4, 6, 7, 8, 9, 11, 12, 13, 14, 15, 16, 17, 18,20, 21, 23, 24, 26, 28, 29,30, 31, 32, 33, 35, 36, 37), the average lengths of the females is 4.683 mm (n = 14; SD = 0.8334), and 4.799 mm for males (n = 10, SD = 0.8254). The lentgh ratio (Male: Female) is 1.0248. The sex ratio (F: M) is 2.25. In the second group (species body length < 3 mm, numbered 2, 3, 5, 10, 19, 22, 25, 27, 34), the average length of females is 1.997 mm (n = 47; SD = 0.5306), and 2.059 mm for males (n = 30; SD = 0.6522). The length ratio (Male: Female) is 1.031. The sex ratio (F: M) is 1.625. | | | | | (25) Scolecithricella Sars, 1902 | |
| | Syn.: | Scolecithrix (part.) | | Ref.: | Sars, 1902 (1903) (p.54); A. Scott, 1909 (p.88); Sars, 1925 (p.187); Sewell, 1929 (p.211); Wilson, 1932 a (p.83); Rose, 1933 a (p.156); Mori, 1937 (1964) (p.50); Rose, 1942 (p.149); Davis, 1949 (p.45); Brodsky, 1950 (1967) (p.268, spp. Key); Vervoort, 1951 (p.111-112, Rem.); Tanaka, 1962 (p.38); Vervoort, 1965 (p.65, Rem.); Owre & Foyo, 1967 (p.60); Bradford, 1973 (p.142, Redef.); Park, 1980 (p.26, spp. Key); Razouls, 1982 (p.325); Gardner & Szabo, 1982 (p.299); Bradford & al., 1983 (p.102, Def.); Campaner, 1984 a (p.171, Key of F); Zheng Zhong & al., 1984 (1989) (p.240); Mauchline, 1988 (p.737, cuticular pores); Schulz, 1991 (p.208); Razouls, 1993 (p.310); Chihara & Murano, 1997 (p.900); Mauchline, 1998 (p.81: M, figs.176, 194; p.84: F); Bradford-Grieve & al., 1999 (part., p.881, 931, 932, spp. Key); Vyshkvartzeva, 1999 (2000) (p.233, 238, Redef.); Vyshkvartzeva, 2001 (p.83); Ohtsuka & al., 2003 (p.61, 62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.); Kuriyama & Nishida, 2009 (p.293, table 7, 10, vertical distribution); Vives & Shmeleva, 2007 (p.773, spp. Key); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | Bradford & al. (1983) numbers 9 'safe' species (*) in this genus and 16 probable, 3 whose generic status would require to be clarified, and 2 congeneric species of a group : 'Scolecithrix ctenopus' near Scolecithricella.
Type: Scolecithrix minor Brady, 1883. Provisional total 31 spp. + 6 undet.
Sano & al. (2013, p.23) note that the genus is considerd omnivore/detritivore in previous studies, and their chemo-sensory setae are considered to be involved in the detection of detritus (see in Nishida & Ohtsuka, 1997).
Diagnosis after Vyshkvartzeva (2000, p. 233):
- Pediger somites 4 and 5 in female completely fused, more or less produced distally, narrowly or broadly rounded, sometimes with a incision near the dorsal side, in male separate, broadly rounded.
- Rostrum bifurcated, both long proximal processes strong, sausage-shaped, tapering distally into sensory filaments, varying from short to 3/5 length of proximal part.
- A1 female 20-23-segmented, 8-10th segments of the typical 25-segmented calanoid antennule fused ; 24th and 25th, 1st and 2nd, and 12th and 13th segments sometimes also fused, partly or completely. A1 male symmetrical , with 18-19-segmented (segments 8-12th, 20-21th completely fused, 24-25th sometimes partly fused.
- A2 : endopod about 2/3 exopod. Exopodal segment 1 without seta or swelling ; exopdal segments 2-6 with 1 seta each in both sexes ; in female, seta of exopodal segment 2 sometimes shorter than in male.
- Md endopod reaching the end of exopod ; basis with 1 inner seta reduced in male.
- Mx1 : inner lobe 1 with 1 or 2 posterior setae ; inner lobe 3 with 3 setae ; exopod with 5, 6, 7 or 8 setae.
- Mx2 : inner lobe 2 with 3 setae ; inner lobes 3-4 with 2-3 setae ; inner lobe 5 with 3 setae (one of them more stout) and usually with 1 worm-like sensory seta ; distal 3 segments of endopod with 3 worm-like and 5 brush-like sensory setae ; 2 of 5 brush-like setae shortyer, their brushes slightly larger.
- P1 exopod with 2 outer spines (exopdal segment 1 without outer spine) ; spine of exopodal segment 2 not longer than 2/3 length of exopodal segment 3.
- Outer distal corner of endopodal segment 1 of P2 produced distally into obtuse or acute spine-like process ; external spine of exopodal segment 1 of P2 usually long, more than half as long as exopodal segment 2.
- P4 coxopod near outer distal margin with obtuse, knob-like process. Anterior and posterior surfaces of protopod, endopod and exopod with sparse small spines.
P5 female uniramous, 1-segmented ; segment flat, attached tp common, very short coupler, armed with long inner spine (slightly shortyer, equal or slightly longer than segment), shorter apical spine and a minute outer spine, the latter frequently absent. P5 sometimes completely absent.
- P5 male biramous, asymmetrical ; length of right and left legs subequal or right leg slightly shorter ; legs shorter than, about equal to or a little longer than urosome. Endopods of both legs short and rudimentary ; exopods usually 3-segmented, segments subcylindrical. In right leg, exopodal segments 1 and 2 partially or completely fused ; exopodal segment 2 with or more frequently without short medio-distal projection ; exopodal segment 3 usually with a minute seta distally, or tapering as a long spiniform process, or termibating with a characteristic grooved structure. Distal segment of left leg exopod usually the shortest, with spinules. Left endopod ginger-like, not longer than half of exopod, usually with a minute seta distally.
For the author 11 species are included in this genus, and possibly 4 species belong to this genus but their descriptions are insufficient and usually males unknown : S. farrani (Rose, 1942), (= ? S. longipes) ; S. longipes Giesbredht, 1892 ; S. longispina Chen & Zhang, 1965 ; S. pearsoni Sewell, 1914 ; S. sarsi (Rose, 1942) ( = ? Scolecithricella globulosa) ; S. vespertina Tanaka, 1962.
Definition from Bradford, Haakonssen & Jillett (1983, p.102) :
- Pedigerous segments 4 and 5 fused.
- Rostrum of 2 filaments.
- A1 22 or 23-segmented in female ; 19 segments in male.
- Mx1 inner lobe 1 (arthrite) with 2 posterior surface setae ; inner lobe 3 (basal endite) with 3 setae ; endopod segment 1 usually separate from segments 2 and 3.
- Mx2 endopod with 3 worm-like and 5 brush-like elements.
- P1 exopod segment 1 usually without an external spine.
- Male mouthparts slightly reduced compared with female.
- Female P5 uniramous, 1-segmented, flattened, plate-like, attached to common basal segment.
- Male P5 biramous on both sides, both endopods very short. | | Remarks on dimensions and sex ratio: | | If one takes into account only the most likely species in this genus species (numbered 1, 4, 6, 9, 10, 12, 14, 16, 17, 18, 19, 20, 24, 28, 29, 30, 32, 33), the average lengths of the females is 1.369 mm (n = 29; SD = 0.4251), and 1.485 mm for males (n = 24; SD = 0.4809). The length ratio (Male: Female) is 1.385. | | | | | (26) Scolecithrix Brady, 1883 | |
| | Ref.: | Brady, 1883 (p.56); Giesbrecht, 1892 (part., p.56, 265); Giesbrecht & Schmeil, 1898 (part., p.41); Sars, 1900 (p.45); Sars, 1902 (1903) (p.49, Rem.); Wolfenden, 1904 (p.120); Esterly, 1905 (p.163, spp. Key); 1906 a (p.64); van Breemen, 1908 a (p.69); A. Scott, 1909 (p.87); Esterly, 1911 (p.327); Wolfenden, 1911 (p.250); Sars, 1925 (p.175, Rem.); Sewell, 1929 (p.209, Rem.); Wilson, 1932 a (p.81); Rose, 1933 a (p.150); Mori, 1937 (1964) (p.53); Rose, 1942 (p.115); Oliveira, 1946 (1949) (p.459); Brodsky, 1950 (1967) (p.273, Rem.); Tanaka, 1962 (p.35); Vervoort, 1965 (p.65, Rem.); Bradford, 1973 (p.140, 141, Déf.); Razouls, 1982 (p.347); Bradford & al., 1983 (p.112, Def.); Park, 1983 (p.166, Redef.); Zheng Zhong & al., 1984 (1989) (p.240, Rem.); Mauchline, 1988 (p.737: pores cuticulaires); Razouls, 1993 (p.310); Chihara & Murano, 1997 (p.902); Mauchline, 1998 (p.78, 84: F; p.81: M); Bradford-Grieve & al., 1999 (p.931, 933, spp. Key); Vyshkvartzeva, 2001 (p.80, 83); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.); Vives & Shmeleva, 2007 (p.799, spp. Key); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | Type: Undina danae Lubbock,1856.
Bradford & al. (1983, p.113) include 2 "sure" spp. (*), more 1 likely (**) in this genus. Transfers probable in other genera (***).
Provisional total 13 spp.
Defintion from Bradfoird & al. (1983, p.112) :
- Pedigerous segments 4 and 5 usually separate.
- A1 female 19 or 20 segments ; A1 male 17-19 segments..
- Mx1 inner lobe (arthrite) with 1 seta on posterior surface ; endopod with all joinys fused.
- P1 exopod segment 1 with or without external spine.
- Mouthparts only very slightly reduced compared with female.
- Female P5 absent or, if present, small and asymmetrical.
- Male P5 tends to be uniramous on right ; biramous on left side.
Diagnosis from Park (1983, p.166) :
Female:
- Cephalosome and pediger 1 fused, pedigers 4 and 5 distinctly separate.
- Urosome 4-segmented
- Small caudal rami.
- Rostrum biramous, eavh ramus tapering into a long filament.
- A1 with 1st and 2nd, 8th through 12th segments fused, resulting in 20 free segments.
- A2 with both rami well developed, exopod slightly longer than endopod, 1st exopodal segment without a seta.
- Md with strong masticatory blade and roughly triangular basis bearing small 2-segmented endopod and large 5-segmented exopod.
- Mx1 with 3 inner lobes and 1 outer lobe on coxa and endopod and exopod on basis. Endopod unsegmented and fused with basis.
- Mx2 with 5 well-developed basipodal lobes and a small endopod. Endopod with 3 vermiform and 5 brush-form sensory filaments.
- Mxp with strong coxa and basis followed by 5-segmented endopod. Coxa with a short brush-form sensory filament and 2 proximal setae modified into vermiform sensory filaments.
P1 with 1-segmented endopod and 3-segmented exopod. Endopod produced distally into a prominent spiniform process along outer margin. 1st exopodal segment with or without an outer spine; 2nd and 3rd each with an outer spine.
- P2 to P4 similar with 3-segmented exopod terminated with a strong spine. Basis with a prominent spiniform process distally along inner margin. Endopod produced distally into a sharp spiniform process along outer margin. 1st and 2nd exopodal segment each with an inner seta and an outer spine; 3rd with 4 inner setae and 3 outer spine of equal size.
- Coxa with an inner seta in P2 and P3.
- Endopod 2-segmented in P2 but 3-segmented in P3 and P4.
- Posterior surface armed with strong spinules in P2 and P3, and with small spinules in P4.
- P5 absent or if present small, unsegmented, and asymmetrical.
Male:
- Body similar to that of female.
- Cephalosome and pediger 1 fused, pedigers 4 and 5 distinctly separate.
- Urosome 5-segmented, 5th segment (anal somite) almost telescoped in preceding segment.
- Rostrum biramous , each ramus tapering into a long filament as in female.
- A1 with 8th through 12th segments fused as in female, but 1st and 2nd separate; 20th and 21th separate on left but fused on right.
- A2, Md, Mx1, Mx2 and Mxp agree in morphological details with those of female and almost as well developed as in female.
P1 to P4 similar to those of female except that outer exopodal spines relatively small;
- P5 asymmetrical each with 2-segmented basipod followed in left leg by 3-segmented exopod and 1-segmented endopod and in right only by 2-segmented exopod. | | Remarks on dimensions and sex ratio: | | Only two species (numbered 3 and 4 in the genus list) are considered for the measurments. The mean female size is 1.753 mm (n = 4; SD = 0.5955), and 1.663 mm in male (n = 4; SD = 0.5928). The size ratio (Male: Female) is 0.945. The sex ratio (F: M) is 1. | | | | | (27) Scolecitrichopsis Vyshkvartzeva, 2000 | |
| | Syn.: | Scolecithricella : Bradford-Grieve & al., 1999 (p.932) | | Ref.: | Vyshkvartzeva, 1999 (2000) (p.219); 2001 (p.83); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.192: F); Schulz, 2005 (Rem.: p.68-69); Markhaseva & Ferrari, 2005 a (p.111, fig.31, Rem.); Blanco-Bercial & al., 2011 (p.103, Table 1, Fig.2, 3, 4, molecular biology, phylogeny); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.); Renz & Markhaseva, 2015 (p.96, Table 4, Fig.3, sex ratio, biogeography) | | Rem.: | type: Scolecithrix ctenopus Giesbrecht,1888. Total: 7 spp. [+ 3 probable].
Diagnosis from Vyshkvartzeva (2000, p.219) :
- Pedigerous somites 4 end 5 separate or fused (if fused, , a well marked notch on ventro-lateral margin indicates the border of each somite).
- Posterolateral corners of last prosomal somite produced distally, triangular, sometimes with a spine-like process.
Rostrum as a short plate with 2 thin filaments (in S. difficilis, conical, without filaments).
- A1 female 24-segmented (8th and 9th segments of the ancestral fused). A1 male asymmetrical, left one 21-segmented (8-12th segments fused), right one 20-segmented (additional to those of left, 20th and 21th segments fused).
- A2 exopod of female with 4 medial setae (in segments 3-4), of male with 5 medial setae (in segments 2-6). Exopod slightly longer than endopod.
- Mx1: inner lobe 1 with 1-2 posterior setae; inner lobe 3 with 2-3 setae; exopod usually with 7 setae (in S. tenuipes with 6 setae; in S. difficilis with 10 setae).
- Mx2: endopod distally with 3 worm-like and 5 small, subequal, brush-like sensory filaments; inner lobe 5 with 3 sclerotized setae (frequently 2 of them stout, hook-like, with denticles) and 1 worm-like sensory seta or with 2 sclerotized and 2 sensory setae; on inner lobes 2-4 sometimes 1 or 3 sclerotized setae transformed into worm-like one. (Mx2 of S. difficilis wiyh differing armament.
- Male mouthparts almost not reduced compared with those of female..
P1: exopod al segments 1-3 usually with long external spine each (S. ctenopus without external spine on exopodal segment 1).
- Outer distal corner of endopodal segment 1 of P2 not produced, rounded; external spine on exopodal segment 1 of P2 short, not longer than half of exopodal segment 2..
- Posterior surfaces of P2-P4 endopod and exopod segments usually with numerous spines and spinules; posterior surfaces of endopodal segments 2-3 and exopodal segments 2-3 of P4 sometimes with flat, large, lancet-like spines; P4 segments of protopod with numerous spines and spinules; anterior surface of endopodal segment 2 of P3 with long spines distally.
- P5 female uniramous, 2-3-segmented, of variable shape; common basal segment long; surface of all or distal segments with spinules.
- P5 male uniramous, strongly asymmetrical; left leg much longer than the right one, 5-segmented; 4 proximal segments subcylindrical; 4th segment sometimes with long spines along inner margin, 5th segment short; right leg of 3-5 segments, not reaching beyond the middle of 2nd proximal segments of left leg. | | Remarks on dimensions and sex ratio: | | The average lengths of the females is 1.873 mm (n = 11, SD = 0.5186), and 2.035 mm for males (n = 10; SD = 0.5489). The length ratio (Male : Female) is 1.087. The males belonging to this genus seem to be slightly longer than females. The sex ratio is 1.2. | | | | | (28) Scolecocalanus Farran, 1936 | |
| | Ref.: | Farran, 1936 a (p.102); Tanaka, 1961 a (p.156); Bradford, 1973 (p.140); Razouls, 1982 (p.361); Bradford & al., 1983 (p.113, Def.); Campaner, 1989 (p.234: Rem.); Vervoort, 1990 (p.177, 182, Rem.); Razouls, 1993 (p.310); Mauchline, 1998 (p.80: F; p.81: M); Vyshkvartzeva, 2001 (p.80); Ohtsuka & al., 2002 (p.534); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Kuriyama & Nishida, 2006 (p.293, fig.7: vertical distribution, Rem.: p.313); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | Total: 5 spp.
Definition from Bradford & al. (1983, p.113), after Farran, 1936 and C.B. Wilson, 1950 :
- Rostral spines large and tapered.
- Lenticular thickeningt at base of rostrum (as in Macandrewella.
- Genital segment asymmetrical in dorsal view).
- P4 exopod segments 3 and 4 bear longitudinal row of spinules on anterior surface.
- Female P5 present only on left side ; short basal segment bearing a long curved spine.
- Male right P5 with basis swollen ; endopod well developed , extending almost as far as exopod ; left leg endopod well developed. | | Remarks on dimensions and sex ratio: | | The average lengths of the females is 4.227 mm (n = 6, SD = 0.4399), and 4.150 mm for males (n = 3; SD = 0.1732). The length ratio (Male: Female) is 0.99. | | | | (29) Scopalatum Roe, 1975 | |
| | Syn.: | 'Amallophora altera' Group: Bradford, 1973 (part., p.144, 145) | | Ref.: | Roe, 1975 (p.335); Razouls, 1982 (p.299); Bradford & al., 1983 (p.114, Déf.); Razouls, 1993 (p.311); Mauchline, 1998 (p.81: M; p.82: F); Vyshkvartzeva, 2001 (p.79); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.190: F; p.189: fig.F); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.); Vives & Shmeleva, 2007 (p.804, spp. Key); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | type: Scopalatum gibbera Roe,1975. Total: 5 spp. + 1 undet.
Nota after Boxshall & Halsey (2004, p.190):
- Mx2 female with 1, male with 2 greatly enlarged brush-like sensory setae.
Diagnosis after Vives & Shmeleva (2007, p.804) :
- Cephalosome and pediger segment 1 fused, pedigers 4 and 5 fused or separte.
- A2 exopod slightly longer than endopod.
- Mx2 endopod showing 5 setae as powder-puff and 3 flattened vermiform setae ; in female, one of the seta is brush-shape and very large ; and 2 in male.
- P1 endopod 1-segmented ; P2 endopod 2-segmentd ; P3 and P4 endopod 3-segmented
- P2 with large external terminal spines ; exopod segments 2 and 3 and endopod segment 2 bear spines on the surface.
- Coxa of P3 with group of thick and large spines ; endopod segments 2 and 3 bear large spine on the surface ; but are small and rare on the same segments of P4. Exopodal segments lacking of spine. | | Remarks on dimensions and sex ratio: | | The mean female size is 2.628 mm (n = 8; SD = 0.7365). The mean male size is 2.550 mm (for the rare alone males of the same species). The size ratio (Male: Female) is probably near of 0.97. | | | | | (30) Scottocalanus Sars, 1905 | |
| | Syn.: | Scottcalanus : Tanaka, 1961 a (p.140) | | Ref.: | Sars, 1905 c (p.7); A. Scott, 1909 (p.103); Sars, 1925 (p.157); Sewell, 1929 (p.183); Wilson, 1932 a (p.80); Rose, 1933 a (p.143); Mori, 1937 (1964) (p.48); Brodsky, 1950 (1967) (p.241); Vervoort, 1965 (p.35, Rem.); Owre & Foyo, 1967 (p.63, clé spp.); Morris, 1970 (p.2309: Rem.); Bradford, 1973 (p.140); Razouls, 1982 (p.342); Gardner & Szabo, 1982 (p.273); Bradford & al., 1983 (p.115, Def.); Park, 1983 (p.197, Redef., Key of F); Mauchline, 1988 (p.737, cuticular pores); Campaner, 1989 (p.234: Rem.); Ferrari, 1992 (p.392, tab.3); Razouls, 1993 (p.311); Chihara & Murano, 1997 (p.902); Mauchline, 1998 (p.82: M; p.85: F); Vyshkvartzeva, 2001 (p.80: Def.); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Mulyadi, 2004 (p.25); Markhaseva & Ferrari, 2005 a (p.111, fig.31, Rem.); Kuriyama & Nishida, 2006 (p.293, fig.7: vertical distribution, Rem.: p.313); Vives & Shmeleva, 2007 (p.807, spp. Key); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | Type: Scolecithrix securifrons T. Scott,1894. 15 spp. (with 1 doubtful) + 1 undet.
Sano & al. (2013, p.23) note that the genus is considerd omnivore/detritivore in previous studies, and their chemo-sensory setae are considered to be involved in the detection of detritus (see in Nishida & Ohtsuka, 1997).
Diagnosis after Bradford & al. (1983, p.115) and Vives & Shmeleva (2007, p.807) :
- Head usually with crest ; Pedigegous segments 4 and 5 partly or completely fused.
- Rostrum large, bifurcate with notch more or less marked, with or without short processes.
- Corners of last thoracic segment pôinted sometimes slightly rounded.
- Mx1 inner lobe 1 (arthrite) with 3 setae on posterior surface ; inner lobe 3 with 3 setae ; endopod segments 2 and 3 with 4 setae ;
- P1 exopod segment 1 with external spine.
- P5 female imperfectly 3-segmented ; terminal segment wide, with long sub-apical spine directed backwards on the inner edge, and very small apical spine.
- Male P5 asymmetrical, biramous on both sides ; right leg endopod usually well developed, reaching exopod segment 2 ; left leg endopod small, terminal part of exopod complex, prehensile, formed from distal part of segment 2 and small segment 3. | | Remarks on dimensions and sex ratio: | | Apparently the genus shows two groups. The first group with a body size > 4 mm (species numbered 1, 7, 8, 9, 10, 14, 15): The average lengths of the females is 4.860 mm (n = 15; SD = 0.8323), and 4.749 mm for males (n = 15; SD = 0.7003). The length ratio (Male: Female) is 0.98. The sex ratio (Female: Male) is 1,17.
In the second group with a body size< 4 mm (species numbered 2,4, 5, 11, 12, 13): The mean female size is 3.557 mm (n = 11; SD = 0.3558), and for the males 3.908 mm. (n = 6; SD = 0.5113). The size ratio (Male; Female) is 1.099.
| | | | | (31) Undinothrix Tanaka, 1961 | |
| | Ref.: | Tanaka, 1961 a (p.153); Bradford, 1973 (p.140); Razouls, 1982 (p.362); Bradford & al., 1983 (p.122); Razouls, 1993 (p.311); Mauchline, 1998 (p.84: F); Vyshkvartzeva, 2001 (p.80); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | Type species: Undinothrix spinosa. Total: 1 sp.
Definition from Tanaka (1961 a, p.153) after female of the type species:
- Head and 1st pedigerous segment fused, 4th and 5th pediger segments incompletely separate.
- Posterolateral corners of last thoracic segment produced into a triangular expansion.
- Rostrum bifurcate, pointed at apex, to which a filament-like spines are attached.
- Urosome 4-segmented.
- Caudal rami furnished with ordinary 4 setae and a short appendicular seta.
- A1 23-segmented.
- P1 3-segmented exopod and 1-segmented endopod.
- Mx1 with 5 setae on the exopod.
- Mx2 as in Lophothrix Giesbrecht, with sensory appendages on the distal lobes.
- Mxp 1st basal segment with a slender bud-like sensory appendage about the middle of the anterior margin; the proximal outer margin of the 2nd basal segment rather coarsely serrated, the distal 2 segments of endopod with each a well developed outer marginal seta.
- P1 3-segmented exopod and 1-segmented endopod; the segment of the exopod with no outer edge spine.
- P2 3-segmented exopod and 2-segmented endopod.
- P3 and P4 have each 3-segmented exopod and endopod.
- Posterior surface of the exopod and endopod of P2 to P4 furnished with spinules.
- P5 symmetrical, cpmposed of 3 segments; the terminal segment furnished with 3 spines, of which the apical two are very strong and coarsely denticulated.
Male always unknown (C. R., 2017) | | | | | (32*) Xantharus Andronov, 1981 | |
| | Ref.: | Andronov, 1981 (p.1719); Bradford & al., 1983 (p.60, 104, Rem.); Mauchline, 1998 (p.81: M; p.84: F); Schulz, 1998 (p.42: Rem.: Scolecithrichidae); Vyshkvartzeva, 1999 (2000) (p.217: Rem: Scolecitrichidae); 2001 (p.79); Ohtsuka & al., 2003 (p.61: Rem.); Bradford-Grieve, 2004 (p.287); Boxshall & Halsey, 2004 (Rem.: p.160; p. 190: F); Vyshkvartzeva, 2004 (2005) (p.167, 168, Table 2); Markhaseva & Ferrari, 2005 a (p.111, Fig.31, Rem.); Schulz, 2006 (p.55: emend.); Markhaseva & Schulz, 2010 (p.13, Table 1, Rem); Markhaseva & al., 2013 (p.20, Table 1, 2, 3, 4, Rem.); Laakmann & al., 2019 (p.330, Table 1) transfer this genus in Diaixidae. | | Rem.: | Type: Xantharus formosus Andronov,1981. Total; 4 spp.
For Markhaseva, Laakmann & Renz (2013, p.20) this genus must be transfered in Diaixidae family.
Diagnosis from Schulz (2006, p.55, emend.) :
- Rostrum produced ventrally, bifurcate, triangular-shaped, with 2 short, slender filaments.
- Cephalosome and pediger 1 fused, pedigers 4 and 5 with suture line visible at least dorsally.
- Posterolateral corners of prosome produced into angular or rounded lappets.
- Urosome with anal somite very short (nearly ''invisible'').
- Caudal rami usually with 7 setae including minute seta I at midlength, seta II at laterodistal edge and seta VII subdistally on ventral surface.
- A1 female 24-segmented, symmetrical, usually relatively short, not extending to posterior border of prosome; of greater lengths in male, with ancestral segmentsXXII and XXIII comparatively small, segment XXII hardly 0.5 times length of XXIII, and both segments each having 1 exceptionally long seta in female.
- Male A1 dimorph, left 24-segmented, right one 23-segmented, due to fusion of ancestral segments XXII and XXIII.
- A2 with exopod slightly longer than endopod and bearing 9 setae; endopodal segment 1 distinctly longer than terminal exopodal compound segment.
- Md basis with 3-4 setae.
- Mx1 with 2-3 posterior setae on praecoxal arthrite; coxal and 1st basal endite with 2 and 4 setae.
- Mx2 with 4-5 setae on 1st praecoxal endite; endopod with 8 sensory setae including 5 brush-like and 3 vermiform; 1 sclerotized seta may be present.
- Mxp syncoxa with setal formula 1, 2, 3, 3; 1 seta of lobe 3 sensory with very small brush-like tip.
- Male mouthparts not reducede.
- Sementation of legs as typical of superfamily.
- P1 with subterminal outer lobehaving angular shape distally and long endopod; exopodal segments 1-3 each with 1 large outer spine.
- P2-P4 with fine spinulation on posterior surfaces of endopods.
- P4 with spinulation on posterior surfaces of both rami.
- P2-P4 each with long, slender terminal spine bearing coarsely to densely serrate outer margin.
- Female P5 symmetrical, uniramous, typically 3-segmented but basis and exopod may be fused, exopod armed with 2 apical spine-like processes and 0-1 subapical inner spine.
- Male P5 biramous, slightly asymmetrical, 5-segmented, with rudimentary, unarmed endopods or leg uniramous; both legs with protopods about subequal in size, coxa with posterior surface spinulation distally; exopopds 3-segmented, 1st exopodal segmens each with 1 small outer distal spine; 3rd exopodal segment of right leg bifurcated in proximal third, with each branch tapering to unequal lengths. | | Remarks on dimensions and sex ratio: | | The mean female size is 1.183 mm (n = 6; SD = 0.2282), and in males 0.930 mm (n = 4; SD = 0.2894). The size ratio (Male: Female) is 0.79.
The sex ratio (Female: Male) is 2. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2026. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed February 20, 2026] © copyright 2005-2026 Sorbonne University, CNRS
|
|
 |
 |



















