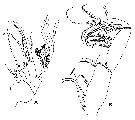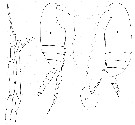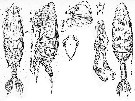|
|
 |
|
Calanoida ( Ordre ) |
|
|
|
Clausocalanoidea ( Superfamille ) |
|
|
| |
| | | |
| Scolecitrichidae Giesbrecht, 1892 ( Clausocalanoidea ) | | Syn.: | Scolecithrichina : Giesbrecht, 1892 (p.55, 265);
Scolecithricinae : Esterly, 1905 (p.162);
Scolecithridae : Rose, 1933 a (p.141);
Scolecithricidae : Sars, 1902 (1903) (p.49); 1925 (p.157); Gurney, 1931 a (p.84); Rose, 1942 (p.113, Rem.: p.114); Brodsky, 1950 (1967) (p.83, 239, Genera Key); Tanaka, 1961 a (p.139); Vervoort, 1965 (p.65, Rem.); Mazza, 1967 (p.162); Bradford, 1973 (p.133 & suiv., Rev.); Andronov, 1974 a (p.1005); Roe, 1975 (p.336); Razouls, 1982 (p.299); 1993 (p.310); Bradford & al., 1983 (p.73, Def., Rem. Genera); Park, 1983 (p.191); Zheng Zhong & al., 1984 (1989) (p.239, spp. Key); Mauchline, 1988 (p.738, 740 : cuticular pores); Vyshkvartzeva, 1989 (p.5, Rev.); Huys & Boxshall, 1991 (p.364); non Parkiidae; Mulyadi, 2004 (p.24); | | Ref.: | Madhupratap & al., 1996 (p.863, Table 5: %/copepods); Chihara & Murano, 1997 (p.899); Schulz, 1998 (p.42); Bradford-Grieve & al., 1999 (p.881, 903, 904, 930, key of Genus); Vyshkvartzeva, 1999 (2000) (p.217, Rem.: 2 genera are incorporated in the family: Xantharus and Neoscolecithrix at least for some species ("N." antarctica, "N." farrani, "N." watersae); Vyshkvartzeva, 2001 (p.77, Genera Key)1; 2003 (p.46, Rem. M); Ohtsuka & Huys, 2001 (p.445, 461); Schulz & Kwasniewski (2004, p.158) maintain Xantharus in the Scolecitrichidae; Markhaseva & Dahms, 2004 (Rem.: p.336); Boxshall & Halsey, 2004 (p.15, 49, 185, Def., Key of genera: females only); Vyshkvartzeva, 2004 (p.157, tab.2, 3, 4, 5); Ferrari & Markhaseva, 2005 (p.45, Rem.); Kuriyama & Nishida, 2006 (p.293, table 7, vertical distribution); Vives & Shmeleva, 2007 (p.717, part. Genera Key); Blanco-Bercial & al., 2011 (p.103, Table 1, Fig.2, Biology molecular, phylogeny); Markhaseva & al., 2014 (p.63, 67, 68, Def., Rem.) ; Laakmann & al., 2019 (p.330, Table 1, fig.1, 2, 3, 4, Table A, phylogenetic relationships); Hirai & al., 2020 (p.1, Fig.4: metabarcoding, Fig.8: OTUs distribution patterns, Fig.9: phylogenetic analysis)
Bradford-Grieve J.M., (2002 onwards). Key to calanoid copepod families. Version 1 : 2 oct 2002. http://www.crustacea.net/crustace/calanoida/index.htm  | | Rem.: | Bowman & Abele, 1982 (p.9) corrigent l'orthographe de Scolecithricidae en Scolecitrichidae.
Total (2004): 26 G (largo sensu): Amallothrix, Archescolecithrix, Cenognatha, Falsilandrumius, Grievella, Heteramalla, Landrumius, Lophothrix, Macandrewella, Mixtocalanus, "Neoscolecithrix"(part.), Parascaphocalanus, Plesioscolecithrix, Pseudoamallothrix, Puchinia, Racovitzanus, Rythabis, Scaphocalanus, Scolecithricella, Scolecithrix, Scolecitrichopsis, Scolecocalanus, Scopalatum, Scottocalanus, Undinothrix, Xantharus .
Boxshall & Halsey (2004, p.186, 188) donnent une définition de la famille et admettent 26 genres (Neoscolecithrix Canu,1896 et Parkius. Le genre Plesioscolecithrix n'étant pas inclus dans leur clé). Vyshkvartzeva (2004, p.176 & suiv.) conteste la position des auteurs précédents concernant le genre Parkius et rétablit le statut de la famille des Parkiidae.
Ohtsuka & al. (2003, p.61 & suiv.) établissent que le noyau de cette famille comprend 18 genres: Amallothrix, Archescolecithrix, Heteramalla, Lophothrix, Macandrewella, Mixtocalanus, Parascaphocalanus, Pseudoamallothrix, Puchinia, Racovitzanus, Scaphocalanus, Scolecithricella, Scolecithrix, Scolecitrichopsis, Scolecocalanus, Scopalatum, Scottocalanus, Undinothrix.
Les genres suivants (notés *): Neoscolecithrix, Cenognatha, Rythabis, Falsilandrumius, Grievella, Landrumius, Xantharus, ne présentent pas les caractères du noyau de la famille, mais sont apparentés à cette famille plus qu'aux Tharybidae ou Phaennidae. Le genre Rythabis est contesté dans cette famille par Vyshkvartzeva (2004) qui le considère comme appartenant aux Tharybidae.
Du fait des remaniements multiples au sein de cette famille toutes les espèces citées dans les genres ont été signalées. Diverses corrections et précisions ont été introduites par rapport à la liste in Razouls (1982). Lorsque une espèce n'a pas été transférée avec certitude dans un autre genre elle est maintenue, "par commodité", dans le genre initialement indiqué par son descripteur. D'après Bradford (1973) et Bradford & al. (1983) les espèces "sûres" dans le genre sont indiquées par (*), les espèces dont le genre est douteux par (**), celles qui n'appartiennent certainement pas au genre par (***).
L'analyse phylogénétique réalisée par Markhaseva & Ferrari (2005 a, p.153 & suiv.) est exprimée dans la figure 31 (p.163-164). Trois nouveaux genres (Brodskius, Byrathis, Omorius) sont provisoirement inclus ci-dessous parmi les Scolecitrichidae, mais les deux premiers genres sont inclus parmi les Tharybidae par Markhaseva & Schulz (2007, p.732, 737). La famille comprendrait alors 25 genres.
Provisoirement la liste mentionnée ci-dessous comprend 32 genres.
1: Vyshkvartzeva (2001, p.83, comm. pers.: quelques corrections sont à introduire dans la clé des genres.
38 (37): … filaments or conical points. Female P5 3- or 2- segmented. …
39 (40): A2 with both rami of about equal length; exopod 6-segmented, Re2 (fused segments Re2-Re3) much longer than Re6.. Re1 of P1 usually without external spines. Female P5 3-segmented, distal segment if longer, only slightly longer than wide. Male mouthparts strongly reduced. Male P5 biramous; right Re1 (sometimes subdevised on 2 segments) without mediodistal projection, Re2 subcylindrical, short, distal spine as long as Re2, triangular; right endopod tapering, as long as or longer than left basipod. ----- Lophothrix.
40 (39): Endopod of A2 not longer than 4/5, usually more shorter, than exopod; Re2 subequal or slightely longer than Re6-7. Re1 of P1 usually with external spine. Female P5 usually 2-segmented, distal segment much longer than wide. Male mouthparts only slightly reduced compared with female. Male P5 biramous; right Re1 usually with mediodistal projection, Re2 frequently curved, distal spine usually shorter than Re2, rudimentary, right endopod shorter than left basipod.
41 (42) ….. --- Pseudoamallothrix
42 (41) ….. --- Amallothrix.
Genre type: Scolecithrix Brady, 1883.
After Madhupratap & al. (1996), la famille des Scolecitrichidae représente de 0,1 à 0,8 % des copepodes selon la saison dans la couche de mélange des eaux océaniques de la région ouest de l'Inde (Mer Arabe), en usant un filet type Multiple Plankton Closing Net à 200 µm de vide de maille (mesh aperture). |  Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44 [p.870 Fig. 5]. Schematic view of right A1 in males of ''Bradfordian'' family Scolecitrichidae. Black arrows: fused ancestral segments; Roman numerals: ancestral segments; dotted line between antennule segments: incomplete fusion. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44 [p.73, Table 1]. A2 armament (number of seta) in different ''Bradfordian'' genera (females). Scolecitrichidae. c: coxa; b: basis; End1: endoppd segment 1; End2: endopod segment 2; Exp: exopod. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44 [p.74, Table 2]. Md armament (number of seta) in different ''Bradfordian'' genera (females). Scolecitrichidae. b: basis; End1: endopod segment 1; End2: endopod segment 2; Exp: exopod; gn: gnathobase. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44 [p.76, Table 3]. Mx1 armament (number of seta) in different ''Bradfordian'' genera (females). Scolecitrichidae. pa: praecoxal arthrite (setal formula of praecoxal arthrite: terminal+posterior+anterior setae); ce: coxal endite; bp: proximal basal endite; bd: distal basal endite; End: endopod; Exp: exopod; Epi: epipodite. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44 [p.78, Table 4]. Mx2 armament (number of seta) in different ''Bradfordian'' genara (females). Scolecitrichidae. at: attenuation; pe: praecoxal endite; ce: coxal endite; bp: proximal basal endite; bd: distal basal endite; el: enditic-like lobe of proximal endopodal segment; End: endopod; w: worm-like seta; br: brush-like seta; sc: sclerotised seta. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014, 44 [p.80, Table 5]. Mxp setation (number of seta) in different ''Bradfordian'' genera (females). Scolecitrichidae. at: attenuation; pes: praecoxal endites of syncoxa (from proximal to distal); ces: coxal endite of syncoxa; bp: basis proximal; bd: basis distal; End: endopod. For more detailed morphology of the seta on the praecoxal endites of the maxilliped syncoxa see Markhaseva & Ferrari (2005) and Markhaseva & al. (2008). |
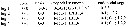 Issued from : G.A. Boxshall & S.H. Halsey in An Introduction to Copepod Diversity. The Ray Society, 2004, No 166, Part. I. [p.186]. Armature formula of swimming legs P1 to P4. |
 Issued from : E.L. Markhaseva & F.D. Ferrari in Invert. Zool., 2005, 2 (2). [p.157, Table 3]. Family placement of Cenognatha, Neoscolecithrix, Rythabis and Xantharus in publications. Remarks: Calanoid copepods belonging to 'bradfordian genera' possess two kinds of poorly-sclerotized, chemosensory setae on the distal endite of the basis and ramus of Mx2; many species also have such setae on the praecoxal endites of the Mxp. Most 'bradfordian' species are members of the Scolecitrichidae (about 200 species in 25 genera) or the Phaennidae (about 110 species in 8 genera), and most of these species are pelagic. Species of Tharybidae (about 50 species in 5 genera), Diaixis (about 10 species in 2 genera) and Parkiidae (1 species in one genus) have been collected in waters above the sea bed. Giesbrecht (1892) diagnosed the first of these families, Scolecitrichidae. When Diaixidae, Phaennidae and Tharybidae were established, Sars (1902) placed Scolecitrichidae and Phaennidae in a different taxonomic section than Diaixidae and Tharybidae, implying two separate monophyletic lineages for calanoids with these chemosensory setae. Fleminger (1957) proposed the four families were closely related, in part based on the chemosensory setae. However, Fleminger noted that it was difficult to separate species of Scolecitrichidae from those of Tharybidae, and recent attempts at family placement of Cenognatha, Neoscolecithrix, Ryrhabis and Xantharus exemplify the problem (Table 3). More recently, Andronov (2002) questioned the validity of both Tharybidae and Diaixidae. The existing diagnostic characters of the two former families are not sufficient to separate them from Scolecitrichidae.With an increasing number of surveys of the immediately deep-sea floor and particularly of hyperbenthic species, number have morphologically diverse chemosensory setae, resulting in several taxa. The most recent ‘bradfornian’ family, Parkiidae was considered monotypic at the time of the discovery of Parkius karenwishneri Ferrari & Markhaseva 1996 ; the diagnosis was based on setarion patterns of Mx2 and Mxp, and the epicuticular extensions of Von Vaupel Klein’s organ. The four other ‘bradfordian’ families continue to be diagnosed using incomplete analyses of the number oand morphology of the chemosensory setae on Mx2 (Bradford & al., 1983 ; Boxshall & halsey, 2004). Observations of these transformed setae on Mx2 do not permit assignment of paryicular setae to the distal endite of the basis or to the presumably multi-segmented ramus, so that homologous setae cannot be identified among different species. The problem of setal homologues on Mx2 presents a significant obstacle to the analysis of ‘bradfordian’ species. Ferrari & Markhaseva (2005) show that within the genus Tharybis, the number and kinds of sensory setae on the distal basal endite plus ramus of Mx2 exhibits the following : 3 worm-like setae, 5 brush-like setae and 1 sclerotized ; 3 worm-lke setae and 6 brush-like setae ; 3 worm-like setae and 5 brush-like setae. This variability further suggests that the number and kinds of sensory setae alone is not adequate to diagnose the ‘bradfordian’ families, or to separate ‘bradfordian’ genera with similar numbers and kinds of sensory setae (see in Ferrari & Markhaseva, 2000c). Two other characters are considered rtio have affected the evolution of ‘bradfordian’ families : number and morphology of the setae on the praecoxal endites of Mxp ; setation and arthrodial membranes on the exopod of A2. The loss and/or transformation of setae to the praecoxal endites of syncoxa of Mxp has been suggested as important to the evolutionary history of the ‘bradfordian’ families (see Ferrari & Markhaseva, 200c). Earlier, Ohtsuka & al (1998) did not consider this character diagnostically useful, nor did Vyshkvartzeva (2000). The ancestral ‘bradfordian’ calanoid is assumed to have had 1, 2, and 3 sclerotized, mechanosensory setae on the proximal, middle and distal praecoxal endites, respectively of the syncoxa of Mxp (Fig. 29, C). The first transformation to the praecoxal endites is assumed to have been the loss of 1 sclerotized seta from the distal endite (Fig. 29, D), and the second transformation is assumed to have been the loss of a second sclerotized seta from that endite. Loss of a sclerotized set ais equivalent to a single transformation. Subsequently, 1 sclerotized seta on each of the three endites may be transformed into a worm-like, chemosensory seta. A brush-like seta, often present on the distal endite, is assumed to be derived from a worm-like seta (Ohtsuka & al., 1998), rather than directly from a sclerotized seta, so a brush-like seta represents two transformations. The exopod of A2 of the ancestral ‘bradfordian’ calanoid (Fig. 30, A) is assumed to have had a proximal segment with a medial seta, followed by a long segment with 3 medial setae arranged linearly. Four short segments eazch with 1 long, thick seta were followed by an elongate segment with its medial seta at mid-length. A small, distal segment with 3 terminal setae completes the ramus. This exopod is assumed to have been derived from a 10-segmented exopod of the ancestral calanoid ; 9 segments, each with a single medial seta and of similar size, was followed by a distal segment with 3 terminal setae (see Ferrari & Markhaseva, 200a). The long segment with 3 medial setae of the ‘bradfordian’ ancestor is assumed to be a complex of 3 segments ; each represented by its medial seta but 2 lack a distal arthrodial membrane. The exopod of A2 of calanoids is patterned during the naupliar phase of development from an area immediately distal to the proximal segment so that the proximal seta of the long segment is the last structure formed (unpublished observations of nauplii of Calanus finmarchicus. A proximal-to-distal loss of setae on the long segment, followed by loss of the arthrodial membrane between the long segment and the proximal of the four small segments, is assumed to represent the progresive transformation of the exopod affected simply a progressively earlier truncation of development. From this model, an exopod for which a medial set ais absent from the proximal segment is the first derived state, followed by one in which the proximal seta of the long segment complex fails to form (Fig. 30, B), and then a long segment complex in which the proximal and middle setae fail to form (Fig. 30, C). Further transformations are a long segment complex in which no setae are present, or one in which the distal arthropodial membrane of the long segment complex fails to form (Fig.30, D), and finally a long segment on which both distal seta and distal arthropodial membrane fail to form so that the long segment is composed of 4 segments with 1 distal seta. The ancestral ‘bradfordian’ calanoid is assumed th have had 9 setae on the distal endite of the basis plus ramus of Mx2, because no more than 9 sclerotized setae are present on any species in the superfamily Clausocalanoidea (see in Table 4). Following the transformation series proposed for syncoxal setae of Mxp, 1 sclerotized seta plus 5 worm-like setae and 3 brush-like setae on the distal endite of the basis plus ramus of Mx2, as in present in Neoscolecithrix japonica Ohtsuka, Boxshall & Fosshagen (2003), represents 11 transformations among the 9 originally sclerotized setae, and is the leastnumber of transformations among extant ‘bradfordian’ species.. Loss of either a sclerotized seta or a worm-like seta from the distal endite of the basis, resulting in 8 setae, is assumed to have occurred early in the evolution of the group. Failure of formation of any set ais equivalent to a single transformation step ; however a sclerotized seta or a worm-like chemosensory set ais much more likely to fail to form than is a brush-like seta due to the latter’s greater complexity (Nishida & Ohtsuka, 1997). In the following analysis, the least derived condition is assigned to a genus exhibiting variable states of any character. For example, 3 different states for the exopod of A2 of Byrathis asre descibed : 1, 1-1-1, 1, 1, 1, 1, 1, 3 ; 1, 1-1-1-1, 1, 1, 1, 1, 3 ; 1, 1-1-1, 1-1, 1, 1, 1, 3. The first state in which 7 arthropodial membranes are present is considered the state of the ancestral Byrathis. In addition, transformations of sclerotized setae on the praecoxal endites of Mxp are assumed to precede changes to the exopod of A2 in all cases. Changes to individual setae of the distal basal endite and ramus of Mx2 are assumed to have been the last to occur during the evolutionary history of ‘bradfordian’ species. |
 Issued from : E.L. Markhaseva & F.D. Ferrari in Invert. Zool., 2005, 2 (2). [p.158, Fig.29]. Ancestral condition of setation of praecoxal endites of tje Mwp syncoxa for various 'bradfordian' genera (Discussion above and source of observations in brackets below). |
 Issued from : E.L. Markhaseva & F.D. Ferrari in Invert. Zool., 2005, 2 (2). [p.159, Fig.29]. Souce of observations for schematic representation of setation of praecoxal endites of Mxp syncoxa for various 'bradfordian' genera (figures above). |
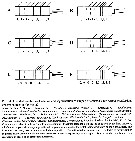 Issued from : E.L. Markhaseva & F.D. Ferrari in Invert. Zool., 2005, 2 (2). [p.160, Fig.30]. Ancestral condition of setation and segmentation of exopod of A2 for various 'bradfordian' genera (sources as for fig. 29 and discussions in text above). |
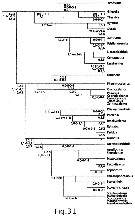 Issued from : E.L. Markhaseva & F.D. Ferrari in Invert. Zool., 2005, 2 (2). [p.164, Fig.31]. Relationships among some 'bradfordian' genera based on : Number and type of setae on praecoxal endites of Mxp; setation of five ancestral segments of the exopod of A2; number and kinds of setae on the distal basal endite olus ramus of Mx2. Sequence of 3 numbers separated by periods are number of setae on the proximal, middle and distal praecoxal endites of Mxp syncoxa (w = worm-like seta; b = brush-like seta; sclerotized seta as a simple number); sequence of 5 numbers separated by commas (artheopodial membrane present) and dashes (arthropodial membrane absent) are segment/seta on the proximal five ancestral segments of the exopod of A2; sequence of 3 numbers separated by commas are the number of sclerotized, worm-like, brush-like setae on the distal basal endite plus ramus of Mx2. Nota: Loss of 1 sclerotized seta to the distal praecoxal endite of Mxp, followed by the loss of a second sclerotized seta to the same endite results in an ancestral group and 2 major derived lineages (Fig. 31). The ancestral group retains 1, 2, 3 setae, respectively, on the proximal, middle and distal praecoxal endites. Byrathis, Diaixis, Xantharus, Falsilandrumius, Landrumius, Neoscolecithrix, Cenognatha share a sensory seta on the distal praecoxal endite of Mxp. Grievella, Tharybis are without a seta on the proximal segment and a proximal seta on the long segment complex of the exopod of A2. Brodskius is derived by loss of the seta on the proximal praecoxal endite of Mxp. Most genera of the first monophyletic lineage, with 1, 2, 2 setae on the praecoxal endites of the syncoxa of Mxp, previously have been placed in the family Phaennidae. Phaennocalanus retains a sclerotized setae on all praecoxal endites; the remaining genera shate a brush-like seta on the distal praecoxal endite. Plesioscolecithrix, Puchinia, Brachycalanus, Rythabis, Parkius share a worm-like seta on the middle praecoxal endite of Mxp. Cornucalanus, Onchocalanus, Phaenna, Cephalophanes, Talacalanus, Xanthocalanus share a derived antennal 2 exopod but are incompletely resolved. It should be pointed out that Xanthocalanus consists of almost 50 species, of which many are poorly described. When the genus is revised, some species will be placed in known genera, including Brachycalanus, while other species will have new genera established for them. Undinella is derived by loss of the seta on the proximal praecoxal endite of Mxp. Genera of the second monophyletic lineage, with 1, 2, 1 setae on the praecoxal endites of the syncoxa of Mxp, have been placed in the Scolecitrichidae, with the exception of the genus Omorius. This genus and Archeoscolecithrix retain sclerotized setae on the praecioxal endites of Mxp ; all other genera share a brush-like seta on the distal praecoxal endite. A worm-like seta on the middle praecoxal endite, pre-sumably homologous to that of first lineage, separates Parascaphocalanus, Scolecithrix, Scolecitrichopsis, Scaphocalanus, Scolecithricella, Scottocalanus, Macandrewella, Pseudoamallothrix from Amallothrix, Scopalatum, Mixtocalanus, Racovitzanus, Lophothrix. The ancestral group and both derived lineages have genera without transformed setae on the praecoxal endites of Mxp : Grievella with 1, 2, 3 sclerotized setae ; Phaennocalanus with 1, 2, 2 sclerotized setae ; Archeoscolecithrix, and Omorius with 1, 2, 1 sclerotized seta. In the ancestral group Grievella, Xantharus, Tharybis, Landrumius, Falsilandrumius, Neoscolecithrix retain the primitive state of 9 setae on the distal basal endite plus ramus of Mx2. In the first derived lineage some species of Brachicalanus retain 9 setae on the distal basal endite plus ramus of Mx2. No genus in the second derived lineage retains 9 setae on the distal basal endite plus ramus of Mx2. 5 worm-like setae on the distal basal endite plus ramus of Mx2 are retained by Brodskius, Byrathis, Neoscolecithrix in the ancestral group, 6 worm-like setae by Rythabis in the first derived lineage and 5 by Omorius in the second derived lineage. Most genera of the first derived lineage are without setae on the three segments of the long segment complex of the exopod of A2, while most genera of the second derived lineage retain the seta on the distal segment of the three segments of the long segment complex. Loss of the seta on the proximal praecoxal endite of Mxp of Brodskius and Undinella is unique to the lineages with 3 or 2 setae, respectively, on the distal praecoxal endite. This loss is assumed to have been independently derived. Due to the paucity of characters, the above hypothesized relationships (Fig. 31) results in undefined lineages and unresolved groups of genera. However ithe aothors’ hypothesis about relationships is correct, then different pelagic or benthopelagic ancestors to the genera comprising both families Phaennidae and Scolecitrichidae suggest these pelagic families are not their onwn closest relatives.
A less derived benthopelagic genus is hypothesized for each family : Omorius for genera in the Scolecitrichidae ; an early species of the ancestral group for genera in the Phaennidae (Fig. 31). This inference suggests that the invasion of the pelagic realm by ‘bradfordian’ copepods has occurred more than once after the colonization of benthopelagic habitats by a tharybid-lke ‘bradfordian’ ancestor (Bradford-Grieve, 2004).
The results of this analysis are considered preliminary because assumptions about the transformations of character states and the order of transformation of different characters have yet to be applied to many ‘bradfordian’ genera.
Assignment to families of the three genera remains tentative. Byrathis belongs to lineage, with Diaixis, Xantharus, Falsilandrumius, Landrumius, Neoscolecithrix, Cenognatha in which 1 of 3 setae on the distal praecoxal endite of Mxp has been transformed to a sensory seta ; Diaixidae is avalable for this lineage.
Brodskius belongs to a lineage with Grievella, Tharybis in which setae on the two proximal segments of the exopod of A2 fail to form ; Tharybidae is avalable for this lineage.
Omorius may be placed in the family Scolecitrichidae as diagnosed with 1, 2, 1 setae on the praecoxal endites of Mxp.
Among ‘bradfordian’ species and genera, parallel transformations of apparently homologous Mxp syncoxal sclerotized setae into poorly-sclerotized setae provide examples of Vavilov’Law (1920) that related species may express a similar variation in derived homologous structures (see Vanilov, 1966).
If the number of setae on each of 3 praecoxal endites of Mxp determines early branching, a modest number of convergences results for stattes of the exopod of A2, and a large number of convergences results for the number of woem-like plus brush-like setae on the distal basal endite plus ramus of Mx2. The convergences in states of the exopod of A2 usually results from presence/absence of the arthropodial membrane between the long segment and the proximal of 4 small segments.
Careful observations of segmentation and setation of the exiopod may reduce the number of these convergences. The same cannot be said for the number of worm-like plus brush-like setae on the distal basal endite plus ramus of Mx2 because determining homologies of these individual setae seems beyond the limits of optical microscopoy.
Detailed descriptions of Mandible, maxilla 1 and swimming leg 1 hold promise as informative states of ‘bradfordian’ genera. Knob or bumps on the distal and posterior faces of Md, and differences in numbers of setae or presence/absence of arthrodial membranes on Mx1 have proven useful in diagnosing genera. Von Vaupel Klein’s organ on P1 also may help resolve relationships among ’bradfordian’ genera. This organ, synapomorphy of gymnoplean copepods, is a transformation of the medial seta of the basis and the anterior face of the proximal endopodal segment which bears one medial seta.
Cornucalanus, Onchocalanus, Phaenna, Cephalophanes, Talacalanus, Xanthocalanus share a derived antennal 2 exopod but are incompletely resolved. It should be pointed out that Xanthocalanus consists of almost 50 species, of which many are poorly described. When the genus is revised, some species will be placed in known genera, including Brachycalanus, while other species will have new genera established for them. Undinella is derived by loss of the seta on the proximal praecoxal endite of Mxp. Genera of the second monophyletic lineage, with 1, 2, 1 setae on the praecoxal endites of the syncoxa of Mxp, have been placed in the Scolecitrichidae,with the exception of the genus Omorius. This genus and Archeoscolecithrix retain sclerotized setae on the praecioxal endites of Mxp ; all other genera share a brush-like seta on the distal praecoxal endite. A worm-like seta on the middle praecoxal endite, pre-sumably homologous to that of first lineage, separates Parascaphocalanus, Scolecithrix, Scolecitrichopsis, Scaphocalanus, Scolecithricella, Scottocalanus, Macandrewella, Pseudoamallothrix from Amallothrix, Scopalatum, Mixtocalanus, Racovitzanus, Lophothrix. The ancestral group and both derived lineages have genera without transformed setae on the praecoxal endites of Mxp : Grievella with 1, 2, 3 sclerotized setae ; Phaennocalanus with 1, 2, 2 sclerotized setae ; Archeoscolecithrix, and Omorius with 1, 2, 1 sclerotized seta. In the ancestral group Grievella, Xantharus, Tharybis, Landrumius, Falsilandrumius, Neoscolecithrix retain the primitive state of 9 setae on the distal basal endite plus ramus of Mx2. In the first derived lineage some species of Brachycalanus retain 9 setae on the distal basal endite plus ramus of Mx2. No genus in the second derived lineage retains 9 setae on the distal basal endite plus ramus of Mx2. 5 worm-like setae on the distal basal endite plus ramus of Mx2 are retained by Brodskius, Byrathis, Neoscolecithrix in the ancestral group, 6 worm-like setae by Rythabis in the first derived lineage and 5 by Omorius in the second derived lineage. Most genera of the first derived lineage are without setae on the three segments of the long segment complex of the exopod of A2, while most genera of the second derived lineage retain the seta on the distal segment of the three segments of the long segment complex. Loss of the seta on the proximal praecoxal endite of Mxp of Brodskius and Undinella is unique to the lineages with 3 or 2 setae, respectively, on the distal praecoxal endite. This loss is assumed to have been independently derived. Due to the paucity of characters, the above hypothesized relationships (Fig. 31) results in undefined lineages and unresolved groups of genera. However ithe aothors’ hypothesis about relationships is correct, then different pelagic or benthopelagic ancestors to the genera comprising both families Phaennidae and Scolecitrichidae suggest these pelagic families are not their onwn closest relatives. A less derived benthopelagic genus is hypothesized for each family : Omorius for genera in the Scolecitrichidae ; an early species of the ancestral group for genera in the Phaennidae (Fig. 31). This inference suggests that the invasion of the pelagic realm by ‘bradfordian’ copepods has occurred more than once after the colonization of benthopelagic habitats by a tharybid-lke ‘bradfordian’ ancestor (Bradford-Grieve, 2004). The results of this analysis are considered preliminary because assumptions about the transformations of character states and the order of transformation of different characters have yet to be applied to many ‘bradfordian’ genera. Assignment to families of the three genera remains tentative. Byrathis belongs to lineage, with Diaixis, Xantharus, Falsilandrumius, Landrumius, Neoscolecithrix, Cenognatha in which 1 of 3 setae on the distal praecoxal endite of Mxp has been transformed to a sensory seta ; Diaixidae is avalable for this lineage. Brodskius belongs to a lineage with Grievella, Tharybis in which setae on the two proximal segments of the exopod of A2 fail to form ; Tharybidae is avalable for this lineage. Omorius may be placed in the family Scolecitrichidae as diagnosed with 1, 2, 1 setae on the praecoxal endites of Mxp. Among ‘bradfordian’ species and genera, parallel transformations of apparently homologous Mxp syncoxal sclerotized setae into poorly-sclerotized setae provide examples of Vavilov’Law (1920) that related species may express a similar variation in derived homologous structures (see Vanilov, 1966). If the number of setae on each of 3 praecoxal endites of Mxp determines early branching, a modest number of convergences results for stattes of the exopod of A2, and a large number of convergences results for the number of woem-like plus brush-like setae on the distal basal endite plus ramus of Mx2. The convergences in states of the exopod of A2 usually results from presence/absence of the arthropodial membrane between the long segment and the proximal of 4 small segments. Careful observations of segmentation and setation of the exiopod may reduce the number of these convergences. The same cannot be said for the number of worm-like plus brush-like setae on the distal basal endite plus ramus of Mx2 because determining homologies of these individual setae seems beyond the limits of optical microscopoy. Detailed descriptions of Mandible, maxilla 1 and swimming leg 1 hold promise as informative states of ‘bradfordian’ genera. Knob or bumps on the distal and posterior faces of Md, and differences in numbers of setae or presence/absence of arthrodial membranes on Mx1 have proven useful in diagnosing genera. Von Vaupel Klein’s organ on P1 also may help resolve relationships among ’bradfordian’ genera. This organ, synapomorphy of gymnoplean copepods, is a transformation of the medial seta of the basis and the anterior face of the proximal endopodal segment which bears one medial seta. |
 Issued from : S. Laakmann, E.L. Markhaseva & J. Renz in Mol. Phylog. Evol., 2019, 130. [p.331, Table 1]. Compilation of information on relationships among ''Bradfordian'' genera from Markhaseva & Ferrari 2005) and Markhaseva & al. (2014). Abbreviations: A2, antenna; Md, mandible; Mx1, maxillule; Mx2, maxilla; Mxp, maxilliped ; P5, leg 5. w, worm-like sensory seta; b, brush-like sensory seta; s, sclerotized seta. |
 Issued from : M. Kuriyama & S. Nishida in Crustaceana, 2006, 79 (3). [p.306, Fig.7] Vertical distribution and abundance of scolecitrichid copepods in Sagami bay (Japan) at 35°00' N, 139°20' E (depth 1450 m). MTD-net (Motoda, 1971) towed horizontally at 14 layers (0, 50, 100, 150, 200, 250, 300, 400, 500, 600, 700, 800, 900, and 1000 m during the day and night, on 9-14 May 2000. See fig. 2 vertical profiles of temperature and salinity; fig. 9 relative abundance of species in groups-migratory function in the water column 0-1000 m; fig. 3: vertical profiles of abundance and species diversity and fig.6: abundance and diversity of three dominant genera. Nota: The author's observations, in addition to the available circumstantial information on morpholohy and food habits, suggest a scenario that the highly diverse scolecitrichid assemblage in Sagami Bay may be structured, through partitioning of vertical habitats and food ressources ( a size factor of 1.5 reflects partitioning of food by size after Von Vaupel Klein, 1998). |
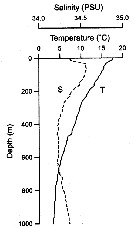 Issued from : M. Kuriyama & S. Nishida in Crustaceana, 2006, 79 (3). [p.301, Fig.2] Vertical profiles of temperature (T) and salinity (S) in Sagami Bay (Japan), in a cruise on 9-14 May 2000. |
 Issued from : M. Kuriyama & S. Nishida in Crustaceana, 2006, 79 (3). [p.308, Fig.9] Relative abundance of scolecitrichid species in the water column 0-1000 m. |
 Issued from : M. Kuriyama & S. Nishida in Crustaceana, 2006, 79 (3). [p.302, Fig.3] Vertical profiles of : A, abundance; abd B, species number of scolecitrichid copepods in Sagami Bay on 9-14 May 2000. Nota: The abundance of scolecithrids, i;e the family as a whole, showed a marked diel pattern, with the major population occurring at 200-400 m and 0-200 m, respectively, at daytime and at night.. In contrast, the number of species showed a similar pattern between day and night, with an abrupt increase from th surface to 100-200 m, followed by a gradual increase with depth to the maximal values at 500-900 m, which was similar to the expected species number normalized for a sample size of 75 individuals [ES], except that the latter lacks near-surface values owing to the extremely low number of sampled specimens (see Fig.4A). The Shannon-Wiener diversity indices (see Fig.4B) also increased with depth, with maximal values at 800 m (day) and 900 m (night) with a trend of increase in shallower depths at night. Pielou's index of eveness was lower at 300-700 m during the day than at night (see Fig.4C). |
 Issued from : M. Kuriyama & S. Nishida in Crustaceana, 2006, 79 (3). [p.304, Fig.6] Abundance and diversity of three dominant genera in scolecitrichid copepods ( Scolecithricella, Scaphocalanus, Amallothrix) in Sagami Bay on 9-14 May 2000. |
 Issued from : M. Kuriyama & S. Nishida in Crustaceana, 2006, 79 (3). [p.312, Fig. 10 D] Vertical distribution, mean body length of females and abundance of genera Scolecitrichidae, others than Scolecithricella (see fig.10 A), Amallothrix (see fig.10 B) and Scaphocalanus (see fig.10 C) collected in Sagami Bay, Japan (9-14 May 2000). The width of the box denotes the density of 50% of population between 25% and 75% distributional depth. |
 Issued from : M. Kuriyama & S. Nishida in Crustaceana, 2006, 79 (3). [p.298, 301] Measurement of species richness, the Shannon-Wiener diversity index, and Morisita-Horn index of similarity. |
 Issued from : M. Kuriyama & S. Nishida in Crustaceana, 2006, 79 (3). [p.303, Figs. 4, 5] Vertical profiles (Fig.4) of: A, expected species number; B, Shannon-wiener diversity index (H'); C, Pielou's index of eveness (J'), of scolecitrichrid copepods in Sagami Bay on 9-14 May 2000. Nota: The cluster analysis based on the Morisita-Horn similarity index (Fig.5) showed 2 distinct groups of assemblages, each comprising the samples from 700-1000 m and from 50-600 m, except for the 0 m (day and night) and 50 m (day) samples, which were distinct from all other samples at the similarity level of <0.2. Deep-mesopelagic samples (700-1000 m) exhibited high similarity indices both day and night. In the upper 600 m, the 400-600 m layers were similar during the day, while the 400 m layer came close to the upper layer at night. | | | | | Amallophora T. Scott, 1894 | |
| | Ref.: | T. Scott, 1894 b (p.54 : comme sous-genre de Scolecithrix); Sars, 1902 (1903) (p.50, Rem.); Wolfenden, 1904 (p.120); Farran, 1908 b (p.48, Rem.); A. Scott, 1909 (p.84); T. Scott, 1909 (p.125, Rem.); Wolfenden, 1911 (p.261); Sars, 1925 (p.140, Rem.); Sewell, 1929 (p.175); Davis, 1949 (p.38); Rose, 1933 a (p.134, Rem.); 1942 (p.115, Rem.); Vervoort, 1957 (p.94, 95, Rem.); Tanaka, 1960 a (p.102 & suiv., Rem.); Bradford, 1973 (p.133, 136, 144); Razouls, 1982 (p.362); Park, 1982 (p.75, 76, Rem.); Roe, 1975 (p.335, 336, Rem.); Mauchline, 1988 (p.735); Razouls, 1993 (p.310) | | Rem.: | Bradford,1973 (p.138,139) considère ce sous-genre à l'origine, puis "genre", comme synonyme de Xanthocalanus. Un certain nombre de formes anciennement placées dans le genre Amallophora sont réparties désormais, outre parmi les Xanthocalanus (Phaennidae), dans les autres genres de Scolecitrichidae (voir in Bradford & al., 1983, p.71 & suiv.). Ce genre se trouve ainsi vidé de sa substance bien que certaines espèces soient difficiles à transférer. | | | | (1) Amallothrix Sars, 1925 | |
| | Syn.: | Scaphocalanus (part.); Scolecithricella (part.); Scolecithrix (part.) | | Ref.: | Sars, 1925 (p.176); Sewell, 1929 (p.215); Rose, 1933 a (p.151, spp. Key); 1942 (p.126); Sewell, 1947 (p.154); Davis, 1949 (p.43); Brodsky, 1950 (1967) (p.258, spp. Key); Vervoort,1951 (p.111-112); Bradford, 1973 (p.142, Redef.); Razouls, 1982 (p.300); Bradford & al., 1983 (p.77, Déf.); Mauchline, 1988 (p.737); Schulz, 1991 (p.208: Rem.); Razouls, 1993 (p.310); Mauchline, 1998 (p.82: M; p.84: F); Bradford-Grieve & al., 1999 (part., p.931, spp. Key); Vyshkvartzeva, 1999 (2000) (p. 234, 238, Redef.); 2001 (p.83); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.192: F); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.); Kuriyama & Nishida, 2009 (p.293, table 7, 10, vertical distribution); Vives & Shmeleva, 2007 (p.720, spp. Key) ; Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | Bradford (1973, p.142) redéfinit ce genre (type: Scolecithricella gracilis Sars,1905) ce qui conduit à diverses synonymies. Park, 1980 (p.29) conteste le bien fondé de la position de Bradford, préférant suivre celle de Vervoort (1951). Le décompte des espèces est difficile. In Bradford & al. (1983, p.78) on en dénombre 10 'sûres'(*), 20 probables, plus 3 qui ne s'accordent pas totalement avec la définition (**) et 2 qui ont été transférées dans de nouveaux genres. Total provisoire de 14 spp. + 1 indet. | | Remarques sur les dimensions et le sex-ratio: | | Le rapport des longueurs (M/F) est de 1,136 (n = 6; SD = 0,1946) si l'on ne prend en compte que les cas où les espèces présentent les deux sexes. |  Issued from : N.V. Vyshkvartzeva in Zoosyst. Rossica, 2000, 8 (2). [p.238, Table 2]. Diagnostic features of Amallothrix. SmP5 : pediger somite of P5; A: 8-12th fused in both A1, also 20-21st in right A1. Nota: 20 (male: 15) species included definitely and 2 probably included in the genus. |
 Issued from : G.O. Sars in Résult. Camp. scient. Prince Albert I, 69, 1924. [Pl. XLIX, figs. 9-21]. As Scolecithrix gracilis [as Amallothrix gracilis in text, p.176]. Female: 9-10, habitus (dorsal and lateral, respectively); 11, rostrum; 12, A2; 13, Md; 14, Mx1; 15, distal part of Mx1; 16, Mxp; 17, P1; 18, P2; 19, P3; 20, P4; 21, P5. |
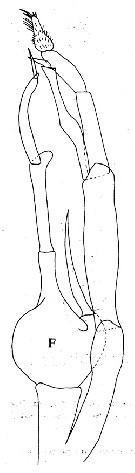 Issued from : J.M Bradford, L. Haakonssen & J.B. Jillett in N. Z. Oceanogr. Inst. , Memoir 90, 1983. [p.84, Fig. 47, F]. Male: F, P5. |
 Issued from : M. Kuriyama & S. Nishida in Crustaceana, 2006, 79 (3). [p.312, Fig. 10 B] Vertical distribution, mean body length of females and abundance of Amallothrix species collected in Sagami Bay (9-14 May 2000). The width of the box denotes the density of 50% of population between 25% and 75% distributional depth. | | | | | (2) Archescolecithrix Vyshkvartzeva, 1989 | |
| | Ref.: | Vyshkvartzeva, 1989 (p.6, 8); 2001 (p.79); Mauchline, 1998 (p.82: M; p.84: F); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.); Vives & Shmeleva, 2007 (p.741); Markhaseva & al., 2013 (p.6, Table 1, 2, 3, 4, Rem.) | | Rem.: | Type: Scolecithrix auropecten Giesbrecht,1892; Total: 1 sp. |  Issued from : N.V. Vyshkvartzeva in Zoosyst. Rossica, 2004 (2005), 13 (2). [p.168, Table 2]. Summary of selected morphological characters in some scolecithrid genera: Archescolecithrix. Apomorphic state is marked by underlining. The most advanced state is marked by underlining and bold type. L: length; W: width; Li: inner lobe; A2 ReI-ReIV: first-fourth ancestral exopodal segments of A2. |
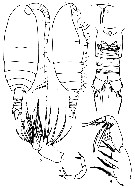 Issued from : N.V. Vyshkvartseva in Explorations of the Fauna of the Seas. USSR Acad. Sci., 1989, 41 (49). [p.10-11, Figs.1-2]. Archescolecithrix auropecten Gisebrecht,1892. Female: 1-2, habitus (dorsal and lateral, respectively); 3, rostrum (ventral view); 4, urosome (ventral); 5, Mx2; 6, Mxp; 7, P5. | | | | | (3) Bradfordiella Andronov, 2007 | |
| | Ref.: | Andronov, 2007 (p.628); Markhaseva & al., 2014 (p.83, Table 1, 2, 3, 4, Rem.: p.83); Laakmann & al., 2019 (p.330, Table 1: considered as incertae sedis genera) | | Rem.: | Type: Bradfordiella kurchatovi. 2 spp.
The assignment of this genus to Scolecitrichidae is questionable; Andronov (2007) showed that the A1, with ancestral segments X-XV fused, the reduced distal part of the Mx1 and the huge Mx2 distinguish this genus from other ''Bradfordians'' genus. Markhaseva, Laakmann & Renz (2003, p.21) consider following characters that do not allow to specify its family placement: 1- Md basis lacking setae (versus 1 to 4 setae in other ''Bradfordians''; 2 - Mx1 setal formula 9, 0, 0, 3 (setae on fused distal basal endite and endopod), 2 and 5 setae; Bradfordiella shares a coxal endite without setae with Heteramalla and Pseudophaenna (versus coxal endite with 1-5 setae in other ''Bradfordians''), a proximal basal endite without setae with Kyphocalanus (Kyphocalanidae) (versus 2-4 setae in other ''Bradfordians''), a distal basal endite fused to the endopod with Rostrocalanidae, Brodskius and some species of Rythabis; 3 - Mx2 enditic lobe of endopod with 1 seta is shared with Kyphocalanus (versus usually 4, rarely 3 setae in other ''Bradfordians''; 4 - Mxp syncoxa in Bradfordiella without setae on praecoxal endites (versus setae present in other ''Bradfordians''); 5 - Mxp, endopodal segment 3 with 1 seta (versus 2-3 setae in other ''Bradfordians'' (Table 3-5 in Markhaseva & al., 2014, p.84) . Until a more detailed description of the genus is prepared, its familly placement remains uncertain. | | Remarques sur les dimensions et le sex-ratio: | | The mean female size is 1.743 mm (n = 3; SD = 0.3000), and in only one male 1.750 mm. The size ratio (Male: Female) is 1.0057. Only one male is known. |  Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014 [p.73, Table 1]. A2 armament (number of seta) in different ''Bradfordian'' genera (females). Bradfordiella: Unresolved family . |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014 [p.75, Table 2]. Md armament (number of seta) in different ''Bradfordian'' genera (females). Bradfordiella: Unresolved family. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014 [p.77, Table 3]. Mx1 armament (number of seta) in different ''Bradfordian'' genera (females). Bradfordiella: Unresolved family. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014 [p.79, Table 4]. Mx2 armament (number of seta) in different ''Bradfordian'' genara (females). Bradfordiella: Unresolved family. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014 [p.81, Table 5]. Mxp setation (number of seta) in different ''Bradfordian'' genera (females). Bradfordiella: Unresolved family. | | | | | (4) Brodskius Markhaseva & Ferrari, 2005 | |
| | Ref.: | Markhaseva & Ferrari, 2005 a (p.114: Def., fig.31, Rem.); Markhaseva & Renz, 2011 (p.67, Rem.); Markhaseva & al., 2014 (p.79, Table 1, 2, 3, 4, Rem.); Renz & Markhaseva, 2015 (p.96, Table 4, Fig.3, biogeography); Laakmann & al., 2019 (p.330, Table 1) transfer this genus in the Tharybidae. | | Rem.: | type: Brodskius benthopelagicus. Markhaseva & Ferrari, 2005. Total: 6 spp. + 1 indet.
Markhaseva & Schulz (2007) incluent ce genre dans la famille des Tharybidae. | | Remarques sur les dimensions et le sex-ratio: | | The mean female size is 1.180 mm (n = 9; SD = 0.198), and in male 1.310 mm (n = 3; SD = 0.1529). The size ratio (Male: Female) computed from only one species (numbered 5) is 1.095. The sex ratio (F: M) is 3. |  Issued from : E.L. Markhaseva & F.D. Ferrari in Invert. Zool., 2005, 2 (2). [p.116, Fig.1, A-D]. Brodskius benthopelagicus Markhaseva & Ferrari, 2005. Type species. Female: A-B, habitus (dorsal and left lateral, respectively); C, forehead with rostrum (ventral view); D, posterior prosome and urosome (left lateral). |
 Issued from : E.L. Markhaseva & F.D. Ferrari in Invert. Zool., 2005, 2 (2). [p.118, 119, Figs.3, 4]. Brodskius benthopelagicus Markhaseva & Ferrari, 2005. Type species. Female: A, Mx2 (posterior setation not shown); B, Mx2 (anterior view); C, Mxp (setae of endopod not drawn); D, Mxp endopod. | | | | | Byrathis Markhaseva & Ferrari, 2005 | | Rem.: | Cf. Diaixidae | | | | (5*) Cenognatha Bradford-Grieve, 2001 | |
| | Ref.: | Bradford-Grieve, 2001 a (p.792, Def., Rem.); Ohtsuka & al., 2003 (p.61, 62: Rem.); Bradford-Grieve, 2004 (p.287); Boxshall & Halsey, 2004 (p.192: F); Vyshkvartzeva, 2005 (p.166, 168, Table 2); Markhaseva & Ferrari, 2005 a (p.111, Fig.31, Rem.); Markhaseva & Schulz, 2010 (p.5, Rem.); Markhaseva & al., 2014 (p.81, Table 1, 2, 3, 4, Rem.) ; Laakmann & al., 2019 (p.330, Table 1: genus transfered in Diaixidae ) . | | Rem.: | au sens large dans cette Famille. type: Neoscolecithrix antarctica. 1 sp. (provisoirement). Bradford-Grieve, 2004 (p.285) inclut dans ce genre les deux espèces Neoscolecithrix caetanoi et farrani. |  Issued from : N.V. Vyshkvartzeva in Zoosyst. Rossica, 2004 (2005), 13 (2). [p.168, Table 2]. Summary of selected morphological characters in some scolecithrid genera: Cenognatha. Apomorphic state is marked by underlining. L: length; W: width; Li: inner lobe; A2 ReI-ReIV: first-fourth ancestral exopodal segments of A2. | | | | | (6) Diaiscolecithrix Markhaseva, Schulz & Renz, 2010 | |
| | Ref.: | Markhaseva & al., 2010 (p.114, Def.); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.); Renz & Markhaseva, 2015 (p.96, Table 4, fig.3, biogeography) | | Rem.: | Total: 1 sp. + 1 indet. | | Remarques sur les dimensions et le sex-ratio: | | The body size for only two females is 1.50 mm and 1.51 mm. |  Issued from : E.L. Markhaseva, K. Schulz & J. Renz in Arthropoda Selecta, 2010, 19 (3). [p.115, Fig.1, E, J]. Diaiscolecithrix andeep female: E, oral cone^like structure, distal part, ventral view); J, cephalosome and oral cone-like structure, anterior lateral view (filaments of rostrum broken). Scale bars: 0.1 mm. This genus appears to be the only genus to possess a well developed oral cone-like structure among clausocalanoidean families that bear sensory setae on Mx2 and Mxp (i.e Diaixidae, Tharybidae, Scolecitrichidae, Parkiidae, Phaennidae, Rostrocalanidae). | | | | | (7*) Falsilandrumius Vyshkvartzeva, 2001 | |
| | Ref.: | Vyshkvartzeva, 2001 (p.79, 93); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Vyshkvartzeva, 2005 (p.16167, 168, Table 2); Markhaseva & Ferrari, 2005 a (p.111, Fig.31, Rem.); Markhaseva & al., 2014 (p.81, Table 1, 2, 3, 4, Rem.); Renz & Markhaseva, 2015 (p.96, Table 4, Fig.3, biogeography) ; Laakmann & al., 2019 (p.330, Table 1) transfer tis genus in Diaixidae. | | Rem.: | au sens large dans cette Famille. Type: Scaphocalanus bogorovi. Total: 3 spp. | | Remarques sur les dimensions et le sex-ratio: | | The mean female size is 4.610 mm. (n = 4, SD = 1.150), and probably from one male: 5.11 mm. The sex ratio (Female: Male) = 3:1 |  Issued from : N.V. Vyshkvartzeva in Zoosyst. Rossica, 2004 (2005), 13 (2). [p.167, 168, Table 2]. Summary of selected morphological characters in some scolecithrid genera: Falsilandrumius. Apomorphic state is marked by underlining. L: length; W: width; Li: inner lobe; A2 ReI-ReIV: first-fourth ancestral exopodal segments of A2. | | | | | (8*) Grievella Ferrari & Markhaseva, 2000 | |
| | Ref.: | Ferrari & Markhaseva, 2000 b (p.1080); Ohtsuka & al., 2003 (p.62: Rem.); Bradford-Grieve, 2004 (p.276, 287: Table 2); Boxshall & Halsey, 2004 (p.190: F); Vyshkvartzeva, 2005 (p.167, 168, Table 2); Markhaseva & Ferrari, 2005a (p.111, Fig.31, Rem.); Markhaseva & al., 2014 (p.81, Table 1, 2, 3, 4, Rem.); Laakmann & al., 2019 (p.330, Table 1) transfer th.is genus in Diaixidae | | Rem.: | Type: Grievella shanki Ferrari & Markhaseva, 2000. Total: 1 sp. |  Issued from : N.V. Vyshkvartzeva in Zoosyst. Rossica, 2004 (2005), 13 (2). [p.168, Table 2]. Summary of selected morphological characters in some scolecithrid genera: Grievella. Apomorphic state is marked by underlining. The most advanced state is marked by underlining and bold type. L: length; W: width; Li: inner lobe; A2 ReI-ReIV: first-fourth ancestral exopodal segments of A2. |
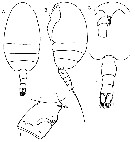 Issued from : F.D. Ferrari & E.L. Markhaseva in Proc. Biol. Soc. Washington, 2000, 113 (4). [p.1081, Fig.1]. Grievella shanki Ferrari & Markhaseva, 2000. Female: A-B, habitus (dorsal and lateral, respectively); C, forehead (ventral view); F, urosome (dorsal view); J, genital complex, left lateral view (arrow to oviducal opening). Note the bumps posteriorly on the genital complex. |
 Issued from : F.D. Ferrari & E.L. Markhaseva in Proc. Biol. Soc. Washington, 2000, 113 (4). [p.1085, Fig.4]. Grievella shanki Ferrari & Markhaseva, 2000. Female: A, Mx2, with setation of praecoxal and coxal lobes (posterior view); B, Mx2, proximal lobe of basis (posterior view); C, Mx2, distal lobe basis plus exopod (posterior view); D, syncoxa and basis of Mxp (anterior view); E, distal lobe of basis and endopod (posterior view). |
 Issued from : F.D. Ferrari & E.L. Markhaseva in Proc. Biol. Soc. Washington, 2000, 113 (4). [p.1082, Fig.2 C; p.1086, Figs. A, B]. Grievella shanki Ferrari & Markhaseva, 2000. Female: A, P1 (anterior view); B, Von Vaupel Klein's organ of P1 (anterior view); C, left A1, articulating segments 22-24. Note an ear-like extension anteriorly near distal margin of articulating segment 22 of A1. | | | | | (9) Heteramalla Sars, 1907 | |
| | Syn.: | Heteremalla : Rose, 1929 (p.26); 1933 a (p.135) | | Ref.: | Sars, 1907 a (p.16); A. Scott, 1909 (p.86, 87); Sars, 1925 (p.142); Sewell, 1929 (p.176); Tanaka, 1960 a (p.115); Vervoort, 1965 (p.29, Rem.); Bradford, 1973 (p.138, 140); Roe, 1975 (p.341: Rem.); Razouls, 1982 (p.309); Bradford & al., 1983 (p.87, Déf.); Mauchline, 1988 (p.737, pores cuticulaires); Razouls, 1993 (p.310); Mauchline, 1998 (p.82: F); Vyshkvartzeva, 2001 (p.79); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Vyshkvartzeva, 2004 (2005) §p.166, 168, Table 2); Vives & Shmeleva, 2007 (p.742); Markhaseva & al., 2014 (p.84, Table 1, 2, 3, 4, Rem.: p.84); Laakmann & al., 2019 (p.330, Table 1: considered as incertae sedis genera) | | Rem.: | type: Heteramalla dubia Sars, 1907. 1 sp. |  Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014 [p.73, Table 1]. A2 armament (number of seta) in different ''Bradfordian'' genera (females). Heteramalla: Unresolved family . |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014 [p.75, Table 2]. Md armament (number of seta) in different ''Bradfordian'' genera (females). Heteramalla: Unresolved family. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014 [p.77, Table 3]. Mx1 armament (number of seta) in different ''Bradfordian'' genera (females). Heteramalla: Unresolved family. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014 [p.79, Table 4]. Mx2 armament (number of seta) in different ''Bradfordian'' genara (females). Hetaramalla: Unresolved family. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014 [p.81, Table 5]. Mxp setation (number of seta) in different ''Bradfordian'' genera (females). Heteramalla: Unresolved family. |
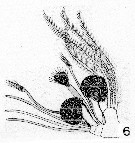 Issued from : A. Scott inSiboga -Expeditie XXIX a, Part I, 1909. [Pl. XXXIII, fig.6]. As Heteramalla dubia ( = Heteramalla sarsi Roe, 1975). Female: 6, Mx2 (distal portion). Nota: The apical lobes of Mx2 are fusrnished with flattened plumose setae. The apex carriçes three types of sensory organs. The 1st type is represented by 3 long and simple appendages which terminate in a blunt point. The 2nd type also consists of 3 appendages, subequal in length, but each one terminates in a small amalliform head. One of the 2nd series is distinctly stouter than the others and the head is more expanded. The 3rd type is represented by 2 very short and stout appendages, each with an enormously developed ''amalla'', resembling the single one in Amallophora typica (= Xanthocalanus typicus . |
 Issued from : N.V. Vyshkvartzeva in Zoosyst. Rossica, 2004 (2005), 13 (2). [p.168, Table 2]. Summary of selected morphological characters in some scolecithrid genera: Heteramalla. Apomorphic state is marked by underlining. The most advanced state is marked by underlining and bold type. L: length; W: width; Li: inner lobe; A2 ReI-ReIV: first-fourth ancestral exopodal segments of A2. | | | | | (10*) Landrumius Park, 1983 | |
| | Syn.: | Lophothrix Giesbrecht,1895 (part.) | | Ref.: | Park, 1983 (p.165,191, Déf.); Razouls, 1993 (p.310); Mauchline, 1998 (p.85: F); Vyshkvartzeva, 2001 (p.79); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Vyshkvartzeva, 2004 (2005) (p.167, 168, Table 2); Markhaseva & Ferrari, 2005a (p.11, fig.31, Rem.); Markhaseva & al., 2014 (p.81, Table 1, 2, 3, 4, Rem.); Laakmann & al., 2019 (p.330, Table 1) transfer tgis genus in Diaixidae. | | Rem.: | au sens large dans cette Famille. Total: 4 spp. | | Remarques sur les dimensions et le sex-ratio: | | The mean female size is 7.644 mm (n = 5; SD = 0.8359) in species numbered 1 to 3, the species 4 excluded because the size very different (3.750 mm). Males unknown. |  Issued from : N.V. Vyshkvartzeva in Zoosyst. Rossica, 2004 (2005), 13 (2). [p.168, Table 2]. Summary of selected morphological characters in some scolecithrid genera: Landrumius. Apomorphic state is marked by underlining. The most advanced state is marked by underlining and bold type. L: length; W: width; Li: inner lobe; A2 ReI-ReIV: first-fourth ancestral exopodal segments of A2. | | | | | (11) Lophothrix Giesbrecht, 1895 | |
| | Syn.: | Scolecithrix (part.) : Giesbrecht & Schmeil, 1898 (p.41) | | Ref.: | Giesbrecht, 1895 c (p.254); Wolfenden, 1904 (p.120); A. Scott, 1909 (p.98); Wolfenden, 1911 (p.267); Sewell, 1929 (p.193); Sars, 1925 (p.162); Rose, 1933 a (p.144, spp. Key); Davis, 1944 (p.40); Brodsky, 1950 (1967) (p.240); Tanaka, 1961 (p.150); Vervoort, 1965 (p.57, Rem.); Owre & Foyo, 1967 (p.66, clé spp.); Bradford, 1973 (p.140,144, Redef.); Razouls, 1982 (p.310); Gardner & Szabo, 1982 (p.277); Park, 1983 (p.177, 178, Redef.); Bradford al., 1983 (p.88, Def.); Mauchline, 1988 (p.737, cuticular pores); Razouls, 1993 (p.310); Chihara & Murano, 1997 (p.899); Mauchline, 1998 (p.82: M; p.85: F); Bradford-Grieve & al., 1999 (p.931, spp. Key); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.192: F); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.); Vives & Shmeleva, 2007 (p.744, spp. Key); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | type : Lophothrix frontalis Giesbrecht,1895. Bradford & al. (1983) dénombre 2 espèces 'sûres' (*), 4 probables (4 étant transférées dans le G. Landrumius) et 1 douteuse. Total provisoire: 7 spp. (dont 1 douteuse). | | Remarques sur les dimensions et le sex-ratio: | | If one accepts the case of L. latipes, The mean female size is 5.573 mm (n = 12; SD = 1.5082), and 4.169 mm in male (n = 4; SD = 1.4024). The size ratio (Male: Female) established in one case is 0.945. If the species numbered 3 is excluded , we have for female the mean 6.092 mm (n = 10; SD = 0.9785), and 5.260 mm in male. The sex ratio (Female: male) is 3. | 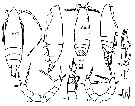 Issued from : K.A. Brodskii in Fauna of the USSR, Zool. Inst. Acad. Sci USSR, 1950 (1967), No 35. [p.245, Fig.154]. Lophothrix frontalis Giesbrecht, 1895. Female and male. NP = habitus; S1 = P1; S5 = P5; R = rostrum. |
 Issued from : T. Park in Biol. Antarct. Seas XIII, Antarct. Res. Ser., 1983, 38 (3). [p.180, Fig.8]. Lophothrix frontalis. Female: b, Mx2; c, Mxp. | | | | | (12) Macandrewella A. Scott, 1909 | |
| | Ref.: | A. Scott, 1909 (p.100); Sewell, 1929 (p.201); Farran, 1936 a (p.104); Tanaka, 1961 a (p.157); Bradford, 1973 (p.140); Gopalakrishnan, 1973 (table spp., p.189: Rem.); Razouls, 1982 (p.313); Bradford & al., 1983 (p.91, Def.); Campaner, 1989 (p.234: Rem.); Razouls, 1993 (p.310); Mauchline, 1998 (p.78, 84: F; 82: M); Vyshkvartzeva, 2001 (p.80); Ohtsuka & al., 2003 (p.62: Rem.); Bradford-Grieve, 2004 (p.287); Ohtsuka & al., 2002 (p.532, Rem.: emend., spp. Key: F,M); Boxshall & Halsey, 2004 (p.190: F); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.); Kuriyama & Nishida, 2006 (p.293, Rem.: p.313); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | Type: Macandrewella joanae A. Scott,1909. Total: 11 spp. (+ 1 ?). | | Remarques sur les dimensions et le sex-ratio: | | La moyenne des longueurs des femelles est de 3,406 mm (n= 8; S= 0,288; Cv= 0,085) et de 3,204 mm pour les mâles (n= 6; S= 0,307; Cv= 0,096). Le rapport des longueurs (M/F) est de 0,953 ou 95,3 % (n= 6; S= 0,056; Cv= 0,059). Le sex-ratio (F/M) est de 1,14. | | | | (13) Mixtocalanus Brodsky, 1950 | |
| | Ref.: | Brodsky, 1950 (1967) (p.237, Def.); Bradford, 1973 (p.140); Razouls, 1982 (p.314); Bradford & al., 1983 (p.91, Def.); Vyshkvartzeva, 1989 (p.18, 23, Rem.); Razouls, 1993 (p.310); Mauchline, 1998 (p.81: M; p.84: F); Vyshkvartzeva, 2001 (p.79-80); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.); Vives & Shmeleva, 2007 (p.814); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | type: Amallophora altera Farran,1929. Total: 3 spp. | | Remarques sur les dimensions et le sex-ratio: | | The mean female size is 2.604 mm. (n = 5, SD = 0.388), and the mean male size is 2.39 mm. (n = 2, SD = 0.127). The size ratio male: female is 0.918. |  Issued from : N.V. Vyshkvartseva in Explorations of the Fauna of the Seas. USSR Acad. Sci., 1989, 41 (49). [p.20-21, Figs.5-6]. Mixtocalanus robustus Brodskii, 1950. Female: 1-2, habitus (dorsal and lateral, respectively); 3, rostrum (frontal view); 4, urosome (ventral); 5, Mx2; 6, distal part of Mx2 with sensory setae; 7, Mxp; 12, P5. | | | | | (14*) Neoscolecithrix Canu, 1896 (part.) | |
| | Syn.: | Oothrix Farran, 1905 (p.42); van Breemen, 1908 a (p.67); Rose, 1933 a (p.140) | | Ref.: | Brodsky, 1950 (1967) (p.225); Fosshagen, 1972 a (Rem.: p.5-6); Bradford, 1973 (p.147, 149, Rem.); Razouls, 1982 (p.369); Bradford & al., 1983 (Def., p.123); Hulsemann, 1985 a (p.55, Rem.: 2 groups); Alvarez, 1985 a (p.197, Rev., 2 groups: 'koehleri', 'farrani' ); Razouls, 1993 (p.311); Mauchline, 1998 (p.78: F,M; p.87: F); Vyshkvartzeva, 1999 (2000) (p.217-218); Bradford-Grieve, 2001 a (p.781, 791, Def.); Bradford-Grieve, 2004 (p.287); Boxshall & Halsey, 2004 (p.192); Markhaseva & Ferrari, 2005 a (p.111, Fig.31, Rem.); Markhaseva & al., 2014 (p.81, Table 1, 2, 3, 4, Rem.); Renz & Markhaseva, 2015 (p.96, Table 4, fig.3, biogeography); Laakmann & al., 2019 (p.330, Table 1) transfer yjis genus in Diaixodae. | | Rem.: | type: Neoscolecithrix koehleri Canu,1896. 8 spp. + 1 indet.
Une comparaison des caractères chez les (F) conduit Hulsemann (1985, p.60, 61) à définir 2 groupes: N. koehleri , N. magna , N. sp. Bradford 1973 et N. farrani , N. watersae , N. antarctica . Pour Vyshkvartzeva ces trois dernières espèces appartiennent aux Scolecitrichidae.
Ohtsuka, Boxshall & Fosshagen (2003, p.53: Historique du genre) | | Remarques sur les dimensions et le sex-ratio: | | The mean female size is 3.196 mm (n = 13; SD = 0.9578), and the mean male size is 2.804 mm (n = 9; SD = 0.8847). The size ratio (male: female) is ± 1 (n = 5; SD = 0.0480). Species n°2 included in the computation, because it is possible Cenognatha. |  issued from : J.M. Bradford-Grieve in N. Z. J. Mar. Freshw. Res., 2001, 35. [p.788, Fig.4]. Neoscolecithrix ornata Bradford-Grieve, 2001. Female: A-B, habitus (lateral and dorsal, respectively); C-D, posterior part of prosome and urosome (dorsal and lateral, respectively); E, genital double-somite (ventral view); F, rostrum (lateral view); G, rostrum (posterior view). |
 issued from : J.M. Bradford-Grieve in N. Z. J. Mar. Freshw. Res., 2001, 35. [p.789, Fig.5]. Neoscolecithrix ornata Bradford-Grieve, 2001. Female: A, A1; C, Md; E, Mx2; F, Mxp. | | | | | (15) Omorius Markhaseva & Ferrari, 2005 | |
| | Ref.: | Markhaseva & Ferrari, 2005 a (p.111, Def., fig.31, Rem.); Markhaseva & Renz, 2011 (p.68, Rem.); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.); Renz & Markhaseva, 2015 (p.96, Table 4, fig.3, biogeography) | | Rem.: | type: Omorius atypicus. Markhaseva & Ferrari, 2005 Total: 2 spp. | | Remarques sur les dimensions et le sex-ratio: | | The mean female size is 1.715 mm (n = 2). Males unknown. | 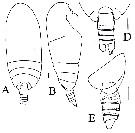 Issued from : E.L. Markhaseva & F.D. Ferrari in Invert. Zool., 2005, 2 (2). [p.145, Fig.20, A-B, D-E]. Omorius atypicus female. Type species. A-B, habitus (dorsal and right lateral, respectively); D-E, posterior prosome and urosome (dorsal and left lateral, respectively). |
 Issued from : E.L. Markhaseva & F.D. Ferrari in Invert. Zool., 2005, 2 (2). [p.147, Fig.22, A, B-C]. Omorius atypicus female. Type species. A, Mx2; B, syncoxa of Mxp; C, basis and endopod of Mxp. | | | | | (16) Parascaphocalanus Brodsky, 1955 (? Scolecitrichidae ) | |
| | Ref.: | Brodsky, 1955 a (p.195); Bradford, 1973 (p.140); Roe, 1975 (p.321, Rem.); Razouls, 1982 (p.314); Brodsky & al., 1983 (p.135); Bradford & al., 1983 (p.91, Rem.); Razouls, 1993 (p.310); Mauchline, 1998 (p.82: M; p.85: F); Vyshkvartzeva, 2001 (p.80, 87, 93); Ohtsuka & al., 2003 (p.61, 62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Markhaseva & Ferrari, 2005 a (p.111, fig.31, Rem.); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | Type: Parascaphocalanus zenkevitchi Brodsky, 1955. Total: 1 sp. | | Remarques sur les dimensions et le sex-ratio: | | Body size from one species: female 2 mm, and male 2mm. | | | | (17) Paraxantharus Schulz, 2006 | |
| | Ref.: | Schulz, 2006 (p.48); Markhaseva, 2010 (p.269, 275, Table 1, Rem.); Markhaseva & al., 2014 (p.81, Table 1, 2, 3, 4, Rem.); Renz & Markhaseva, 2015 (p.96, Table 4, fig.3, biogeography); Laakmann & al., 2019 (p.330, Table 1) transfer this genus in Diaixidae. | | Rem.: | Type: Paraxantharus brittae Schulz,2006. Total: 2 spp. | | Remarques sur les dimensions et le sex-ratio: | | The female size in only one species is 1.560 mm. and the male of another species is 1.445 mm. The size ratio (Male: Female) probably near of 0.93. |  issued from : E.L. Markhaseva & Schulz in Proc. Zool. Inst. RAS, 2010, 314 (1). [p.16, Table.1]. Selected character states of Paraxantharus Females. Data from Markhaseva, 2010. Abbreviations: br = brush-like; sel = sclerotized; w = worm-like sensory setae. |
 Issued from : K. Schulz in Mitt. hamb. zool. Mus. Inst., 2006, 103. [p.49, Fig.1, A, B, C, D, E]. Paraxantharus brittae male (Type species). A-B, habitus (lateral and dorsal views, respectively); C-D, rostrum (anteroventral and lateral views, respectively); E, caudal rami (ventral view). Scale bars: 0.05 mm. |
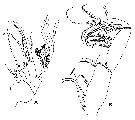 Issued from : K. Schulz in Mitt. hamb. zool. Mus. Inst., 2006, 103. [p.52, Fig.3]. Paraxantharus brittae male (Type species). A, Mx2 (endopod separated; sclerotized seta of endopod arrowed; B, Mxp, tip of brush-like seta (arroxed). | | | | | (18) Parkius Ferrari & Markhaseva, 1996 | |
| | Ref.: | Ferrari & Markhaseva, 1996 (p.266); Bradford-Grieve, 2004 (p.287); Boxshall & Halsey, 2004 (p.186, 188, 190); Vyshkvartzeva, 2004 (p.176); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.); Renz & Markhaseva, 2015 (p.96, Table 4, fig.3, biogeography) | | Rem.: | Boxshall & Halsey, 2004 (p.188; 190: F) considèrent que la famille des Parkiidae doit être incluse dans les Scolecitrichidae faute d'une véritable analyse phylogénétique. Total: 1 sp. + 1 indet. | | Remarques sur les dimensions et le sex-ratio: | | For one female the mean female size is 1.975 mm, and for one male from another species the size is 1.92 mm. Apparently the size ratio (male : female) should be 0.972. | | | | (19) Plesioscolecithrix Markhaseva & Dahms, 2004 | |
| | Ref.: | Markhaseva & Dahms, 2004 (p.328, Def.); Vyshkvartzeva, 2004 (2005) (p.166, 168, Table 2); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.); Markhaseva & al., 2013 (p.22, Table 1, 2, 3, 4, Rem.: p.84); Laakmann & al., 2019 (p.330, Table 1: considered as incertae sedis genera) | | Rem.: | Type: Plesioscolecithrix juhlae Markhaseva & Dams, 2004. Total: 1 sp.
Non inclus dans la clé des genres in Boxshall & Halsey (2004, p.188). |  Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014 [p.73, Table 1]. A2 armament (number of seta) in different ''Bradfordian'' genera (females). Plesioscolecithrix: Unresolved family . |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014 [p.75, Table 2]. Md armament (number of seta) in different ''Bradfordian'' genera (females). Plesioscolecithrix: Unresolved family. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014 [p.77, Table 3]. Mx1 armament (number of seta) in different ''Bradfordian'' genera (females). Plesioscolecithrix: Unresolved family. |
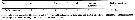 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014 [p.79, Table 4]. Mx2 armament (number of seta) in different ''Bradfordian'' genara (females). Plesioscolecithrix: Unresolved family. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014 [p.81, Table 5]. Mxp setation (number of seta) in different ''Bradfordian'' genera (females). Plesioscolecithrix: Unresolved family. |
 Issued from : N.V. Vyshkvartzeva in Zoosyst. Rossica, 2004 (2005), 13 (2). [p.168, Table 2]. Summary of selected morphological characters in some scolecithrid genera: Plesioscolecithrix. Apomorphic state is marked by underlining. The most advanced state is marked by underlining and bold type. L: length; W: width; Li: inner lobe; A2 ReI-ReIV: first-fourth ancestral exopodal segments of A2. | | | | | (20) Pseudoamallothrix Vyshkvartzeva, 2000 | |
| | Syn.: | Amallothrix : Bradford-Grieve & al., 1999 (part., p.881, 931, spp. Key); Scolecithricella : Bradford-Grieve & al., 1999 (part., p.881, 932) | | Ref.: | Vyshkvartzeva, 1999 (2000) (p.227); 2001 (p.83); 2003 (p.46: Rem.: M); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.192: F); Markhaseva & Ferrari, 2005 a (p.111, fig.31, Rem.); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | type: Amallothrix profunda Brodsky,1950. Total: 15 spp. (dont 1 douteuse). | | Remarques sur les dimensions et le sex-ratio: | | The genus shows two groups of body size:
1st Group: Mean size < 3.5 mm (species numbered 1, 2, 3, 6, 7, 9, 10, 12, 13, 14), the mean female size is 2.491 mm (n = 19; SD = 0.6668), and the mean male size is 2.285 mm (n = 13; SD = 0.6788). The size ratio (Male: Female) is 0.979 (n = 3; SD = 0.1112). The sex ratio (Female: Male) = 1.43.
In the 2nd Group (body size > 3. 5 mm in species numbered 4, 5, 8, 11): The mean female size is 4.551 mm (n = 7; SD = 1.1465), and the mean male size is 3.808 mm (n = 4; SD = 0.2300). The size ratio (Male: Female) is 0.994. The sex ratio (Female: Male) is 2. |  Issued from : N.V. Vyshkvartzeva in Zoosyst. Rossica, 2000, 8 (2). [p.238, Table 2]. Diagnostic features of Pseudoamallothrix. SmP5 : pediger somite of P5; A: 8-12th fused in both A1, also 20-21st in right A1. Nota: 13 (male: 8) species included definitely included in the genus. | | | | | (21) Puchinia Vyshkvartzeva, 1989 | |
| | Ref.: | Vyshkvartzeva, 1989 a (p.29); 2001 (p.79); Mauchline, 1998 (p.82: F); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Vyshkvartzeva, 2004 (2005) (p.166, 168, Table 2); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.); Markhaseva & Schulz, 2010 (p.17, Rem); Markhaseva & al., 2014 (p.44, Table 1, 2, 3, 4, Rem.: p.85); Laakmann & al., 2019 (p.330, Table 1: in incertae sedis genera) | | Rem.: | type: Puchinia obtusa . Les soies sensitives de Mx2 indiquent que ce genre appartient aux Scolecithricidae et présente les caractères les plus évolués dans cette famille. Exop.1 & 2 de A2 à nombreuses petites soies et la Ri de Mx1 à 3 art. révèlent des caractères archaïques. Total: 1 sp. | | Remarques sur les dimensions et le sex-ratio: | | The female size, for only one individual, is about 2.95 mm. |  Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014 [p.73, Table 1]. A2 armament (number of seta) in different ''Bradfordian'' genera (females). Puchinia: Unresolved family . |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014 [p.75, Table 2]. Md armament (number of seta) in different ''Bradfordian'' genera (females). Puchinia: Unresolved family. |
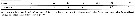 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014 [p.77, Table 3]. Mx1 armament (number of seta) in different ''Bradfordian'' genera (females). Puchinia: Unresolved family. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014 [p.79, Table 4]. Mx2 armament (number of seta) in different ''Bradfordian'' genara (females). Puchinia: Unresolved family. |
 Issued from : E.L. Markhaseva, S. Laakmann & J. Renz in Mar. Biodiv., 2014 [p.81, Table 5]. Mxp setation (number of seta) in different ''Bradfordian'' genera (females). Puchinia: Unresolved family. |
 Issued from : N.V. Vyshkvartzeva in Zoosyst. Rossica, 2004 (2005), 13 (2). [p.168, Table 2]. Summary of selected morphological characters in some scolecithrid genera: Puchinia. Apomorphic state is marked by underlining. L: length; W: width; Li: inner lobe; A2 ReI-ReIV: first-fourth ancestral exopodal segments of A2. | | | | | (22) Racovitzanus Giesbrecht, 1902 | |
| | Ref.: | Giesbrecht, 1902 (p.26); Wolfenden, 1911 (p.259); Brodsky, 1950 (1967) (p.266); Tanaka, 1961 a (p.185); Bradford, 1973 (p.140); Razouls, 1982 (p.314); Gardner & Szabo, 1982 (p.295); Park, 1983 (p.171, Redef.); Bradford & al., 1983 (Def., p.91); Mauchline, 1988 (p.737, pores cuticulaires); Razouls, 1993 (p.310); Chihara & Murano, 1997 (p.899); Mauchline, 1998 (p.80, 85: F; p.82: M); Vyshkvartzeva, 2001 (p.80); Ohtsuka & al., 2003 (p.61, 62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.);Vives & Shmeleva, 2007 (p.816, spp. Key); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | type: Racovitzanus antarcticus Giesbrecht,1902.
Total provisoire: 2 spp. | | Remarques sur les dimensions et le sex-ratio: | | La moyene des longueurs des femelles est de 2,038 mm (n=2; S= 0,124; Cv= 0,061) et de 1,850 mm (n= 2; S= 0,198; Cv= 0,107)pour les mâles. Le rapport des longueurs (M/F) est de 0,936 ou 93,6 % (n= 2; S= 0,042; Cv= 0,046). Le sex-ratio est de 1. |  Issued from : J.M. Bradford, L. Haakonssen & J.B. Jillett inN Z Oceanogr. Inst. memoir 90, 1983. [p.92, Fig.53]. Racovitzanus antarcticus Giesbrecht,1902. Female (from 62°37'S, 169°51'E): A, habitus (lateral); B, rostrum; C, Mx2; E, P5. Male (from Ross Sea): F, P5. | | | | | (23*) Rythabis Schulz, 1995 | |
| | Ref.: | in Schulz & Beckmann, 1995 (p.199); Ohtsuka & al., 2003 (p.61, 62: Rem.); Bradford-Grieve, 2004 (p.287); Boxshall & Halsey, 2004 (p.190: F); Markhaseva & Ferrari, 2005 a (p.11, fig.31, Rem.: p.149, 157, 164); Markhaseva & al., 2014 (p.44, Table 1, 2, 3, 4, Rem.: p.85); Renz & Markhaseva, 2015 (p.96, Table 4, Fig.3, biogeography); Laakmann & al., 2019 (p.330, Table 1: considered as incertae sedis genera) | | Rem.: | Type: Rythabis atlantica Schulz & Beckmann, 1995. Total: 4 spp.
Le changement de famille est contesté. Pour Markhaseva & Schulz (2007, p.742) l'attribution de ce genre à la famille des Tharybidae ou Scolecitrichidae n'est pas totalement résolue . | | Remarques sur les dimensions et le sex-ratio: | | The mean female size is 1.248 mm (n = 5; SD = 0.1117). Males unknown. |  Issued from : K. Schulz & W. Beckmann in Sarsia, 1995, 80. [p.200, 201, Figs.1 & 2]. Rythabis atlantica Schulz & Beckmann, 1995. Type species. Female: A-B, habitus (dorsal and lateral); C, Md palp; D, forehead with rostral cone (ventral view); E, Mx2; F, Mxp; H, A2. Scale bar = 100 µm. | | | | | (24) Scaphocalanus Sars, 1900 | |
| | Syn.: | Amallophora (part.) Sars, 1902 (1903) (p.50) | | Ref.: | Sars, 1900 (p.35); A. Scott, 1909 (p.95); Sars, 1920 c (p.8); 1925 (p.169); Farran, 1926 (p.257); Sewell, 1929 (p.205); Wilson, 1932 a (p.76, clé spp.); Rose, 1933 a (p.146, clé spp.); Mori, 1937 (1964) (p.49); Rose, 1942 (p.115); Davis, 1949 (p.41); Rose, 1942 (p.115); Brodsky, 1950 (1967) (p.246, spp. Key ); Tanaka, 1961 a (p.157); Vervoort, 1965 (p.65, Rem.); Bradford, 1973 (p.140,143, Redéf.); Séret, 1979 (p.113); Razouls, 1982 (p.316); Gardner & Szabo, 1982 (p.281); Park, 1982 (p.78, Redef., spp. Key, Rem.: p.125); Bradford & al., 1983 (p.93, Def.,); Schulz, 1987 (p.105, Rem.); Mauchline, 1988 (p.737, cuticular pores); Razouls,1993 (p.310); Chihara & Murano, 1997 (p.900); Mauchline, 1998 (p.80, 84, 85: F, figs.198, 232; p.82: M); Bradford-Grieve & al., 1999 (p.931, 932, spp. Key); Vyshkvartzeva, 2001 (p.80); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.192: F); Markhaseva & Ferrari, 2005 a (p.111, fig.31, Rem.); Kuriyama & Nishida, 2009 (p.293, table 7, 10, vertical distribution); Vives & Shmeleva, 2007 (p.750, spp. Key); Markhaseva & Renz, 2011 (p.67, Rem.); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | type: Amallophora magna T. Scott,1894.
Pour Bradford & al (1983) on a 14 espèces 'sûres' (*) et 14 'probables' (**). Total provisoire de 32 spp. (dont 1 douteuse) + 5 indet. | | Remarques sur les dimensions et le sex-ratio: | | La longueur moyenne des femelles est 2, 614 mm (n = 61; SD = 1,2896, et pour les mâles 2, 744 mm (n = 40, SD = 1,3845). Le rapport des tailles males: femelles est 1, 050, ou 0, 976 (n = 19; SD = 0,1040 si l'on ne considère que les espèces présentant les deux sexes. Il apparaît à l'évidence deux groupes de talles parmi l'ensemble des espèces, 9 avec des dimensions supérieures à 3 mm et 28 espèces inférieures à 3 mm. |  Issued from : F. Vives & A.A. Shmeleva in Fauna Iberica, 2004, 29. [p.763, Fig.432]. After Park, 1982. Type species: Female: A, habitus (lateral); B, forehead (lateral); C, same (dorsal); D, last thoracic segment and urosome (lateral); E, distal part of Mx2; F, P4; G, P5. Male: H, P5. |
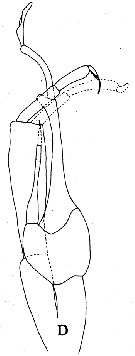 Issued from : J.M. Bradford, L. Haakonssen & J.B. Jillett in N. Z. Oceanogr. Inst. Memoir 90, 1983. [p.100, Fig.59 ]. Male: D, P5. |
 Issued from : M. Kuriyama & S. Nishida in Crustaceana, 2006, 79 (3). [p.312, Fig. 10 C] Vertical distribution, mean body length of females and abundance of Scaphocalanus species collected in Sagami Bay (9-14 May 2000). The width of the box denotes the density of 50% of population between 25% and 75% distributional depth. | | | | | (25) Scolecithricella Sars, 1902 | |
| | Syn.: | Scolecithrix (part.) | | Ref.: | Sars, 1902 (1903) (p.54); A. Scott, 1909 (p.88); Sars, 1925 (p.187); Sewell, 1929 (p.211); Wilson, 1932 a (p.83); Rose, 1933 a (p.156); Mori, 1937 (1964) (p.50); Rose, 1942 (p.149); Davis, 1949 (p.45); Brodsky, 1950 (1967) (p.268, spp. Key); Vervoort, 1951 (p.111-112, Rem.); Tanaka, 1962 (p.38); Vervoort, 1965 (p.65, Rem.); Owre & Foyo, 1967 (p.60); Bradford, 1973 (p.142, Redef.); Park, 1980 (p.26, spp. Key); Razouls, 1982 (p.325); Gardner & Szabo, 1982 (p.299); Bradford & al., 1983 (p.102, Def.); Campaner, 1984 a (p.171, Key of F); Zheng Zhong & al., 1984 (1989) (p.240); Mauchline, 1988 (p.737, cuticular pores); Schulz, 1991 (p.208); Razouls, 1993 (p.310); Chihara & Murano, 1997 (p.900); Mauchline, 1998 (p.81: M, figs.176, 194; p.84: F); Bradford-Grieve & al., 1999 (part., p.881, 931, 932, spp. Key); Vyshkvartzeva, 1999 (2000) (p.233, 238, Redef.); Vyshkvartzeva, 2001 (p.83); Ohtsuka & al., 2003 (p.61, 62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.); Kuriyama & Nishida, 2009 (p.293, table 7, 10, vertical distribution); Vives & Shmeleva, 2007 (p.773, spp. Key); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | Bradford & al. (1983) dénombre 9 espèces 'sûres' (*) dans ce genre et 16 probables, 3 dont le statut générique nécessiterait d'être précisé, enfin 2 espèces congénériques d'un groupe : 'Scolecithrix ctenopus' proche de Scolecithricella . Type: Scolecithrix minor Brady,1883. Total provisoire de 31 spp. + 6 indet. | | Remarques sur les dimensions et le sex-ratio: | | Si l'on ne tient compte que des espèces le plus sûrement dans ce genre (numérotées dans la liste générale comme 1, 4, 6, 9, 10, 12, 14, 16, 17, 18, 19, 20, 24, 28, 29, 30, 32, 33), la moyenne des longueurs des femelles est de 1,669 mm (n = 29; SD = 0,4251), et de 1,486 mm pour les mâles (n = 24; SD = 0,4809). Le rapport des longueurs (M/F) est de 1,085. Si l'on ne prend en considération que le cas des espèces présentant des mesures de males et de femelles on obtient 1,021 (n = 10; SD = 0.0,0634. |  Issued from : N.V. Vyshkvartzeva in Zoosyst. Rossica, 2000, 8 (2). [p.238, Table 2]. Diagnostic features of Scolecithricella. SmP5 : pediger somite of P5. Nota: 11 (male: 9) species included definitely and 5 probably included in the genus. |
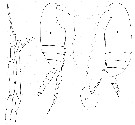 Issued from : J.M. Bradford, L. Haakonssen & J.B. Jillett in N. Z. Oceanogr. Inst. Memoir 90, 1983. [p.108, Fig. 65, A, D, E, F]. Scolecuithricella minor (Brady, 1883). Female (from New Zeland): A, habitus (lateral view); D, P5. Male (from New Zeland): E, habitus (lateral view); F, P5. |
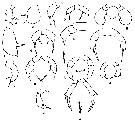 Issued from : F. Vives & A.A. Shmeleva in Fauna Iberica, 2007, 29. [p.775, Fig.441]. Examples of female P5 of Scolecithricella. P5 flattened, leaf-like: A-E; P5 not flattened, leaf-like but with section more or less rounded: K-L. A: S. abyssalis; B: S. dentata; C: S. minor; D: S. orientalis; E: S. ovata; F: S. profundaG: S. vittata; H: S. aspinosa; I: S. canariensis; J: S. longipes; K: S. lobophora; L: S. spinata; M: S. unispinosa; N: S. laminata; O: S. marquesae. Rem: Some species had been transfered in other genus than Scolecithricella: S. laminata, S. ovata, S. canariensis in Pseudoamallothrix; S. aspinosa, S. lobophora in Amallothrix and S. marquesae in Scolecitrichopsis/ |
 Issued from : F. Vives & A.A. Shmeleva in Fauna Iberica, 2007, 29. [p.776, Fig.442]. Examples of male P5 of Scolecithricella. A: S. abyssalis; B: S. minor; C: ; D: S. ovata; E: S. laminata; F: terminal segment S. profunda; G: S. tenuiserrata or S. profunda; H-I: right and left exopod segments, respectively of S. profunda; J: S. dentata; K: S. vittata; L: S. tenuiserrata. Nota: izq = left leg; dcha = right leg. |
 Issued from : M. Kuriyama & S. Nishida in Crustaceana, 2006, 79 (3). [p.312, Fig. 10 A] Vertical distribution, mean body length of females and abundance of Scolecithricella species collected in Sagami Bay (9-14 May 2000). The width of the box denotes the density of 50% of population between 25% and 75% distributional depth. | | | | | (26) Scolecithrix Brady, 1883 | |
| | Ref.: | Brady, 1883 (p.56); Giesbrecht, 1892 (part., p.56, 265); Giesbrecht & Schmeil, 1898 (part., p.41); Sars, 1900 (p.45); Sars, 1902 (1903) (p.49, Rem.); Wolfenden, 1904 (p.120); Esterly, 1905 (p.163, spp. Key); 1906 a (p.64); van Breemen, 1908 a (p.69); A. Scott, 1909 (p.87); Esterly, 1911 (p.327); Wolfenden, 1911 (p.250); Sars, 1925 (p.175, Rem.); Sewell, 1929 (p.209, Rem.); Wilson, 1932 a (p.81); Rose, 1933 a (p.150); Mori, 1937 (1964) (p.53); Rose, 1942 (p.115); Oliveira, 1946 (1949) (p.459); Brodsky, 1950 (1967) (p.273, Rem.); Tanaka, 1962 (p.35); Vervoort, 1965 (p.65, Rem.); Bradford, 1973 (p.140, 141, Déf.); Razouls, 1982 (p.347); Bradford & al., 1983 (p.112, Def.); Park, 1983 (p.166, Redef.); Zheng Zhong & al., 1984 (1989) (p.240, Rem.); Mauchline, 1988 (p.737: pores cuticulaires); Razouls, 1993 (p.310); Chihara & Murano, 1997 (p.902); Mauchline, 1998 (p.78, 84: F; p.81: M); Bradford-Grieve & al., 1999 (p.931, 933, spp. Key); Vyshkvartzeva, 2001 (p.80, 83); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.); Vives & Shmeleva, 2007 (p.799, spp. Key); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | Type: Undina danae Lubbock,1856.
Bradford & al. (1983, p.113) incluent 2 spp. "sûres" (*), plus 1 probable (**) dans ce genre. Transferts probables dans d'autres genres (***).
Total provisoire 13 spp.:
| | Remarques sur les dimensions et le sex-ratio: | | Only two species (numbered 3 and 4 in the genus list) are considered for the measurments. The mean female size is 1.753 mm (n = 4; SD = 0.5955), and 1.663 mm in male (n = 4; SD = 0.5928). The size ratio (Male: Female) is 0.945. The sex ratio (F: M) is 1. | 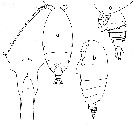 Issued from : T. Park in Biol. Antarct. Seas XIII, Antarct. Res. Ser., 1983, 38 (3). [p.168, Fig.1]. Scolecithrix danae (Lubbock, 186). Type species. Female: a-b, habitus (dorsal and lateral, respectively); c, urosome (lateral); d, rostrum (anterior view). |
 Issued from : T. Park in Biol. Antarct. Seas XIII, Antarct. Res. Ser., 1983, 38 (3). [p.169, Fig.2]. Scolecithrix danae (Lubbock, 186). Type species. Female: b, Mx2; c, Mxp. |
 Issued from : J.M. Bradford, L. Haakonssen & J.B. Jillett in N. Z. Oceanogr. Inst. Memoir 90, 1983. [p.75, Fig.41, F, G]. Undina danae Lubbock, 1856. Type species. Female (from New Zealand): F, Mx1; G, Mx2. |
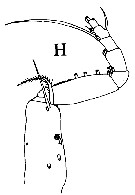 Issued from : J.M. Bradford, L. Haakonssen & J.B. Jillett in N. Z. Oceanogr. Inst. Memoir 90, 1983. [p.75, Fig.41, H]. Undina danae Lubbock, 1856. Type species. Female (from New Zealand): H, Mxp. |
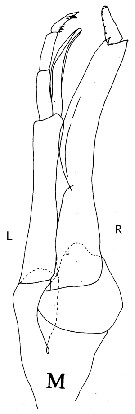 Issued from : J.M. Bradford, L. Haakonssen & J.B. Jillett in N. Z. Oceanogr. Inst. Memoir 90, 1983. [p.76, Fig.42, M]. Undina danae Lubbock, 1856. Type species. Male (from New Zealand): M. P5. | | | | | (27) Scolecitrichopsis Vyshkvartzeva, 2000 | |
| | Syn.: | Scolecithricella : Bradford-Grieve & al., 1999 (p.932) | | Ref.: | Vyshkvartzeva, 1999 (2000) (p.219); 2001 (p.83); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.192: F); Schulz, 2005 (Rem.: p.68-69); Markhaseva & Ferrari, 2005 a (p.111, fig.31, Rem.); Blanco-Bercial & al., 2011 (p.103, Table 1, Fig.2, 3, 4, molecular biology, phylogeny); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.); Renz & Markhaseva, 2015 (p.96, Table 4, Fig.3, sex ratio, biogeography) | | Rem.: | type: Scolecithrix ctenopus Giesbrecht,1888. Total: 7 spp. [+ 3 probables]. | | Remarques sur les dimensions et le sex-ratio: | | The average lengths of the females is 1.873 mm (n = 11, SD = 0.5186), and 2.035 mm for males (n = 10; SD = 0.5489). The length ratio (Male : Female) is 1.087. The males belonging to this genus seem to be slightly longer than females. The sex ratio is 1.2. |  Issued from : N.V. Vyshkvartzeva in Zoosyst. Rossica, 2000, 8 (2). [p.238, Table 2]. Diagnostic features of Scolecitrichopsis. SmP5 : pediger somite of P5; A: 8-12th fused in both A1, also 20-21st in right A1. Nota: 6 (male: 4) species included definitely and 3 probably included in the genus. | | | | | (28) Scolecocalanus Farran, 1936 | |
| | Ref.: | Farran, 1936 a (p.102); Tanaka, 1961 a (p.156); Bradford, 1973 (p.140); Razouls, 1982 (p.361); Bradford & al., 1983 (p.113, Def.); Campaner, 1989 (p.234: Rem.); Vervoort, 1990 (p.177, 182, Rem.); Razouls, 1993 (p.310); Mauchline, 1998 (p.80: F; p.81: M); Vyshkvartzeva, 2001 (p.80); Ohtsuka & al., 2002 (p.534); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Kuriyama & Nishida, 2006 (p.293, fig.7: vertical distribution, Rem.: p.313); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | Total: 5 spp. | | Remarques sur les dimensions et le sex-ratio: | | The average lengths of the females is 4.227 mm (n = 6, SD = 0.4399), and 4.150 mm for males (n = 3; SD = 0.1732). The length ratio (Male: Female) is 0.99. | | | | (29) Scopalatum Roe, 1975 | |
| | Syn.: | 'Amallophora altera' Group: Bradford, 1973 (part., p.144, 145) | | Ref.: | Roe, 1975 (p.335); Razouls, 1982 (p.299); Bradford & al., 1983 (p.114, Déf.); Razouls, 1993 (p.311); Mauchline, 1998 (p.81: M; p.82: F); Vyshkvartzeva, 2001 (p.79); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.190: F; p.189: fig.F); Markhaseva & Ferrari, 2005a (p.111, fig.31, Rem.); Vives & Shmeleva, 2007 (p.804, spp. Key); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | type: Scopalatum gibbera Roe,1975. Total: 5 spp. + 1 indet. | | Remarques sur les dimensions et le sex-ratio: | | La moyenne des longueurs des femelles est de 2,403 mm (n= 4; S= 0,781; Cv= 0,325). | 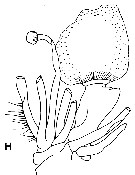 Issued from : G.A. Boxshall & S.H. Halsey in The Ray Society, 2004, N° 166. [p.189, Fig.45, H]. Scopalatum gibbera female: H, terminal part of Mx2. |
 Issued from : J.M. Bradford, L. Haakonssen & J.B. Jillett in N. Z. Oceanogr. Inst. Memoir 90, 1983. [p.115, Fig.71, B]; Scopalatum sp. (from East New Zealand). Female: B, Mx2. | | | | | (30) Scottocalanus Sars, 1905 | |
| | Syn.: | Scottcalanus : Tanaka, 1961 a (p.140) | | Ref.: | Sars, 1905 c (p.7); A. Scott, 1909 (p.103); Sars, 1925 (p.157); Sewell, 1929 (p.183); Wilson, 1932 a (p.80); Rose, 1933 a (p.143); Mori, 1937 (1964) (p.48); Brodsky, 1950 (1967) (p.241); Vervoort, 1965 (p.35, Rem.); Owre & Foyo, 1967 (p.63, clé spp.); Morris, 1970 (p.2309: Rem.); Bradford, 1973 (p.140); Razouls, 1982 (p.342); Gardner & Szabo, 1982 (p.273); Bradford & al., 1983 (p.115, Def.); Park, 1983 (p.197, Redef., Key of F); Mauchline, 1988 (p.737, cuticular pores); Campaner, 1989 (p.234: Rem.); Ferrari, 1992 (p.392, tab.3); Razouls, 1993 (p.311); Chihara & Murano, 1997 (p.902); Mauchline, 1998 (p.82: M; p.85: F); Vyshkvartzeva, 2001 (p.80: Def.); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Mulyadi, 2004 (p.25); Markhaseva & Ferrari, 2005 a (p.111, fig.31, Rem.); Kuriyama & Nishida, 2006 (p.293, fig.7: vertical distribution, Rem.: p.313); Vives & Shmeleva, 2007 (p.807, spp. Key); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | Type: Scolecithrix securifrons T. Scott,1894. 15 spp. (dont 1 douteuse) + 1 indet. | | Remarques sur les dimensions et le sex-ratio: | | La moyenne des longueurs des femelles est de 4,309 mm (n = 26; SD = 0,9324), et de 4,531 mm pour les mâles (n = 19; SD = 0,7674). Le rapport des longueurs (M/F) est de 1,052. Le rapport des sexes (F/M) est de 1,27. | 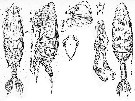 Issued from : G.O. Sars in Résult. Camp. scient. Prince Albert I, 69, 1924. [Pl.XLV, 1-8]. Female: 1-2, habitus (dorsal and lateral views, respectively); 3, forehead; 4, rostrum (frontal view); 5, P5. Male: 7, habitus (dorsal); 8, P5. | | | | | (31) Undinothrix Tanaka, 1961 | |
| | Ref.: | Tanaka, 1961 a (p.153); Bradford, 1973 (p.140); Razouls, 1982 (p.362); Bradford & al., 1983 (p.122); Razouls, 1993 (p.311); Mauchline, 1998 (p.84: F); Vyshkvartzeva, 2001 (p.80); Ohtsuka & al., 2003 (p.62: Rem.); Boxshall & Halsey, 2004 (p.190: F); Markhaseva & al., 2014 (p.68, Table 1, 2, 3, 4, Rem.) | | Rem.: | Total: 1 sp. | 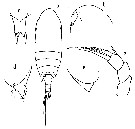 issued from : O. Tzanaka in Publ. Seto Mar. Biol. Lab., 1961, IX (1). [p.155, Fig.112, a-e, o]. Undinothrix spinosa. Type species. Female: a, habitus (dorsal aview); b, forehead (lateral view); c, rostrum (frontal view); d, posterolateral expansion of the last thoracic segment and genital segment (lateral left side); e, same (lateral right side); 0, P5. | | | | | (32*) Xantharus Andronov, 1981 | |
| | Ref.: | Andronov, 1981 (p.1719); Bradford & al., 1983 (p.60, 104, Rem.); Mauchline, 1998 (p.81: M; p.84: F); Schulz, 1998 (p.42: Rem.: Scolecithrichidae); Vyshkvartzeva, 1999 (2000) (p.217: Rem: Scolecitrichidae); 2001 (p.79); Ohtsuka & al., 2003 (p.61: Rem.); Bradford-Grieve, 2004 (p.287); Boxshall & Halsey, 2004 (Rem.: p.160; p. 190: F); Vyshkvartzeva, 2004 (2005) (p.167, 168, Table 2); Markhaseva & Ferrari, 2005 a (p.111, Fig.31, Rem.); Schulz, 2006 (p.55: emend.); Markhaseva & Schulz, 2010 (p.13, Table 1, Rem); Markhaseva & al., 2013 (p.20, Table 1, 2, 3, 4, Rem.); Laakmann & al., 2019 (p.330, Table 1) transfer this genus in Diaixidae. | | Rem.: | Total: 4 spp.
Pour Markhaseva & al. (2013, p.20) ce genre doit être transféré dans la famille des Diaixidae. | | Remarques sur les dimensions et le sex-ratio: | | The mean female size is 1.183 mm (n = 6; SD = 0.2282), and in males 0.930 mm (n = 4; SD = 0.2894). The size ratio (Male: Female) is 0.79.
The sex ratio (Female: Male) is 2. |  issued from : E.L. Markhaseva in Proc. Zool. Inst. RAS, 2010, 314 (1). [p.16, Table.1]. Selected character states of Xantharus Females from Andronov, 1981, Schulz, 1998, Schulz and Kwasniewski, 2004 and Bradford-Grieve, 2005. . Abbreviations: br = brush-like; sel = sclerotized; w = worm-like sensory setae. |
 Issued from : N.V. Vyshkvartzeva in Zoosyst. Rossica, 2004 (2005), 13 (2). [p.168, Table 2]. Summary of selected morphological characters in some scolecithrid genera: Xantharus. Apomorphic state is marked by underlining. The most advanced state is marked by underlining and bold type. L: length; W: width; Li: inner lobe; A2 ReI-ReIV: first-fourth ancestral exopodal segments of A2. |
 Issued from : K. Schulz in Mitt. hamb. zool. Mus. Inst., 2006, 103. [p.56, Fig.5]. Xantharus renatehaassae male. A-B, habitus (dorsal and lateral, respectively); C-D, rostrum (anteroventral and lateral views, respectively; E, pediger 5 and urosome (lateral view); F, caudal rami (ventral view). Scale bars: 0.05 mm.) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
|
 Toute utilisation de ce site pour une publication sera mentionnée avec la référence suivante : Toute utilisation de ce site pour une publication sera mentionnée avec la référence suivante :
Razouls C., Desreumaux N., Kouwenberg J. et de Bovée F., 2005-2026. - Biodiversité des Copépodes planctoniques marins (morphologie, répartition géographique et données biologiques). Sorbonne Université, CNRS. Disponible sur http://copepodes.obs-banyuls.fr [Accédé le 06 mars 2026] © copyright 2005-2026 Sorbonne Université, CNRS
|
|
 |
 |