|
|
 |
|
Cyclopoida ( Order ) |
|
|
|
Sapphirinidae ( Family ) |
|
|
|
Sapphirina ( Genus ) |
|
|
| |
Sapphirina metallina Dana, 1849 (F,M) | |
| | | | | | | Syn.: | Saphirina metallina : Brady, 1883 (p.128, figs.F,M); T. Scott, 1894 b (p.125, fig.M) | | | | Ref.: | | | Giesbrecht, 1892 (p.620, 644, 775, figs.M); Thompson & Scott, 1903 (p.240); A. Scott, 1909 (p.255, Rem.); Wolfenden, 1911 (p.360); Pesta, 1920 (p.640, fig.M); Farran, 1929 (p.210, 290); Lehnhofer, 1929 (p.284, Rem., figs.F,M); Rose, 1929 (p.61); 1933 a (p.311, figs.F,M); Farran, 1936 a (p.129); Mori, 1937 (1964) (p.124, figs.F); Wilson, 1942 a (p.206, fig.M); Lysholm & al., 1945 (p.43); Sewell, 1947 (p.266); Moore, 1949 (p.64); Pucher, 1952 (p.102); Krishnaswamy, 1953 (p.73, Rem.M); Chiba, 1956 (p.10, figs.F); Chiba & al., 1957 (p.310); 1957 a (p.12); Marques, 1958 a (p.137, figs.M); Fagetti, 1962 (p.47); Crisafi & Mazza, 1966 (p.611, figs.F,M, Rem.); Saraswathy, 1966 (1967) (p.103); Owre & Foyo, 1967 (p.116, figs.F,M); Vilela, 1968 (p.34, figs.F); Ramirez, 1969 (p.95, figs.M, Rem.); Corral Estrada, 1970 (p.229); Björnberg & al., 1981 (p.607, 671, figs.M); Zheng & al., 1982 (p.116, figs.F,M); Baessa de Aguiar, 1986 (1989) (p.62, figs.F,M); Chae & Nishida, 1994 (p.205, 208, fig.M, integumental structure, pattern color); Chihara & Murano, 1997 (p.990, Pl.226: F,M); Boxshall, 1998 (p.230); Bradford-Grieve & al., 1999 (p.887, 972, figs.F,M); Conway & al., 2003 (p.238, figs.F,M, Rem.); Boxshall & Halsey, 2004 (p.655); Vives & Shmeleva, 2010 (p.378, figs.F,M, Rem.); Gur & al., 2015 (p.8408, cuticular structure vs reflectance, figs.1, 2, 3); Gur & al., 2016 (p.1393, figs. 1, 2, 3, 6,color change vs physical mechanism) | 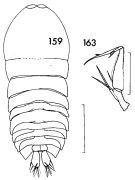 issued from : F.C. Ramirez in Contr. Inst. Biol. mar., Buenos Aires, 1969, 98. [p.94, Lam. XIX, figs. 159, 163]. Male (from off Mar del Plata): 159, habitus (dorsal); 163, A2. Scale bars in mm: 0.6 (159); 0.3 (163).
|
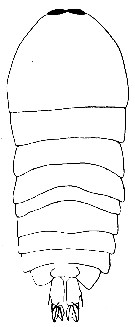 issued from : P. Crisafi & J. Mazza in Atti Soc. pelorit. Sci. fis. mat. nat., 1966, XII (3/4). [p.612, Fig.32]. Male (from Strait of Messina): habitus (dorsal).
|
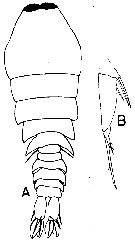 issued from : P. Crisafi & J. Mazza in Atti Soc. pelorit. Sci. fis. mat. nat., 1966, XII (3/4). [p.612, Fig.33]. Female: A, habitus (dorsal); B, A2.
|
 issued from : Z. Zheng, S. Li, S.J. Li & B. Chen in Marine planktonic copepods in Chinese waters. Shanghai Sc. Techn. Press, 1982 [p.116, Fig.71]. Female: a, habitus (dorsal); b, caudal rami (dorsal); c, A2; d, P2; e, endopodal segment 3 of P2; f, P4. Male: g, habitus (dorsal); h, caudal rami (dorsal); i, A2; j, P2; k, endopodal segment 3 of P2; l, P4. Scale bars in mm.
|
 issued from : J. Chae & S. Nishida in Mar. Biol., 1994 (119). [p.208, Table 2, C). Specific color for the male observed with reflected and transmitted light. Nota: Color observed with reflected (R )and transmitted light (T): Metallic gold or silver, strong reflexion, rapidly changing (R); Gold at pigment sites, broad spectrum, red, yellow, blue, magenta and violet where cuticles overlap (T).
|
 issued from : J. Chae & S. Nishida in Mar. Biol., 1994 (119). [p.208, Table 2, E-H). Specific color for the male observed with reflected and transmitted light. E-H, the same individual as in (C), showing color change due to the specimen's slight movement.
|
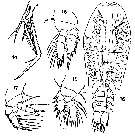 issued from : T. Mori in The Pelagic copepoda from the neighbouring waters of Japan, 1937 (1964). [Pl. 67, Figs.14-18]. Female: 14, A2; 15, P2; 16, habitus (dorsal); 17, P4; 18, P1.
|
 issued from : C. Lehnhofer in Wiss. Ergebn. dt. Tiefsee-Exped. ''Valdivia'', 1929, 22 (5). [p.285, Fig.18]. Female: 3, A2. Male: 1, caudal ramus (dorsal); 2, A1. hy = hyaline margin.
|
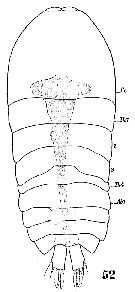 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 19, 1892. Atlas von 54 Tafeln. [Taf.54, Fig.52]. Male: 52, habirus (dorsal).
|
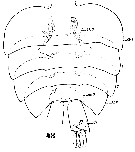 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 19, 1892. Atlas von 54 Tafeln. [Taf.54, Fig.48]. Male: 48, Abdomen (dorsal).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 19, 1892. Atlas von 54 Tafeln. [Taf.54, Fig.47]. Male: 47, A1.
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 19, 1892. Atlas von 54 Tafeln. [Taf.54, Figs.49, 50, 51]. Male: 49, P1 (endopod); 50, P4; 51, P3 (endopod).
|
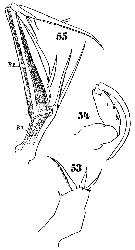 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 19, 1892. Atlas von 54 Tafeln. [Taf.54, Figs.53, 54
, 55]. Male: 53, Mx1; 54, Mxp; 55, A2.
|
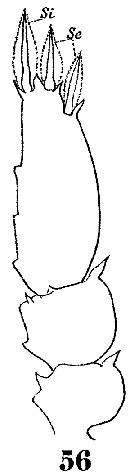 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 19, 1892. Atlas von 54 Tafeln. [Taf.54, Fig.56]. Male: 56, P2 (endopod).
|
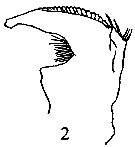 issued from : P.E. Lapernat & C. Razouls in Vie Milieu, 2002, 52 (1). [p.27, Pl. V, fig.2]. Female (from off Malta, Mediterranean Sea): 2, Md.
|
 Issued from : D. Gur, B. Leshem, M. Pierantoni, V. Farstey, D. Oron, S. Weiner & L. Addadi in J. Am. Chem. Soc., 2015, 137. [p.8409, Fig.1] Ultrastructure of Sapphirina metallina. (a) Light microscope image viewed through the dorsal surface showing an array of tightly packed hexagonal crystals. Inset: Fourier transform of the image demonstrating the regular packing. (b, c) Cryo-SEM micrographs of a high-pressure-frozen, freeze-fractured S. metallina copepod: (b) Transverse view showing the guanine crystals aligned perpendicular to the dorsal surface of the animal. The iridophores (Ir) are located just beneath the animal chitin procutile (Pc). Inset: schematic representation of the position of the crystal-containing cells (Ir) relative to the copepod anatomy. The copepod is presented in dorsal view. The right-hand side is a schematic representation of the cross section of the region indicated, which corresponds to the cryo-SEM micrograph. (c) Dorsal view showing the tightly packed perfectly hexagonal crystals and the alternating layers of guanine crystals and cytoplasm beneath the procuticle.
|
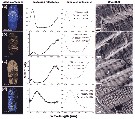 Issued from : D. Gur, B. Leshem, M. Pierantoni, V. Farstey, D. Oron, S. Weiner & L. Addadi in J. Am. Chem. Soc., 2015, 137. [p.8410, Fig.2, a-d]. Reflectance and structural properties of individual copepods: (a-d) Sapphirina metallina Column 1 (left) shows light microscope images of representative sapphitinid male specimens and column 2 their measured reflectances. Column 4 shows representative cryo-SEM images of the crystal-cytoplasm layer arrays and column 3 the simulated reflectance spectra. The simulated reflectance was calculated on the basis of thecytoplasm (Cy) and crystal (Cr) thicknesses measured in cryo-SEM images from many differently colored specimens. The results of these measurements are shown in column 3 for each color. The measured reflectance en each spectrum is normalized to a silver mirror; the measured reflectance of (d) was also normalized to the crystal coverage area because of the lack of uniformity of the specimen. The seeming disagreement between the measured and calculated reflectance spectra at short wavelengths in (c) and (d) is due to the short-wavelength edge of the light source
|
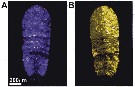 ssued from : D. Gur, B. Leshem, V. Farstey, D. Oron, L. Addadi & S. Weiner in Adv. Funct. Mater., 2016, 26 [p.1394, Fig.1, A, B). Optical response (reflection mode) of sapphirinid male of Sapphirina metallina to either dark or light conditions. A: dark-adapted; B: light-adapted.
|
 Issued from : D. Gur, B. Leshem, V. Farstey, D. Oron, L. Addadi & S. Weiner in Adv. Funct. Mater., 2016, 26. [p.1396, Fig.3, A, B). The reflectance and structural properties that accompany the male sapphirinid color change. C: dark-adapted; D, light-adapted. Column I: light microscope images of the specimens; column II: measured reflectance; column III: simulated reflectance spectrum. The simulated reflectance was calculed based on the cytoplasm (Cy) and the crystal (Cr) thickness measured in cryo-SEM images: Dark-adaptated Cy: 190 ± 8 nm, Cr: 72 ± 4 nm. Light-adaptated Cy: 135 ± 5 nm, Cr: 69 nm ± 5 nm. Column IV: representative cryo-SEM images of the crystal-cytoplasm layer arrays.
| | | | | Compl. Ref.: | | | Carl, 1907 (p.18); Sewell, 1948 (p.346, 462, 515); C.B. Wilson, 1950 (p.322); King & Hida, 1955 (p.11); Ganapati & Shanthakumari, 1962 (p.10, 16); V.N. Greze, 1963 a (tabl.2); Björnberg, 1963 (p.88, Rem.); De Decker & Mombeck, 1964 (p.13); Mazza, 1966 (p.73); 1967 (p.344); Pavlova, 1966 (p.45); Furuhashi, 1966 a (p.295, vertical distribution in Kuroshio region, Table 9);Vinogradov, 1968 (1970) (p.78); Delalo, 1968 (p.139); Deevey, 1971 (p.224); Binet & al., 1972 (p.69); Björnberg, 1973 (p.370, 389); Corral Estrada & Pereiro Muñoz, 1974 (tab.I); Deevey & Brooks, 1977 (p.156, tab.2, Station "S"); Boxshall, 1977 b (p.557); Tranter, 1977 (p.596, 599); Dessier, 1979 (p.207); Rajaram & Krishnaswamy, 1980 a (p.154); Vives, 1982 (p.296); Kovalev & Shmeleva, 1982 (p.86); Dessier, 1983 (p.89, Tableau 1, Rem., %); Guangshan & Honglin, 1984 (p.118, tab.); Regner, 1985 (p.11, Rem.: p.42); Renon, 1987 (tab.2); Lozano Soldevilla & al., 1988 (p.61); Böttger-Schnack & al., 1989 (p.1089) ; Cervantes-Duarte & Hernandez-Trujillo, 1989 (tab.3); Yoo, 1991 (tab.1); Hernandez-Trujillo, 1991 (1993) (tab.I); Lozano, 1991 (p.173); Baessa De Aguiar, 1991 (1993) (p.108); Böttger-Schnack, 1995 (p.93); Shih & Young, 1995 (p.77); Chae & al., 1996 (p.20); Böttger-Schnack, 1997 (p.409); Noda & al., 1998 (p.55, Table 3, occurrence); Hure & Krsinic, 1998 (p.104); Alvarez-Cadena & al., 1998 (t.1,4); Suarez-Morales & Gasca, 1998 a (p.113); Lapernat, 1999 (p.38, Rem.); 2000 (tabl.3, 4); Razouls & al., 2000 (p.343, tab. 5, Appendix); Lapernat & Razouls, 2001 (tab.1); Fernandez-Alamo & al., 2000 (p.1139, Appendix); Suarez-Morales & al., 2000 (p.751, tab.1); Lopez-Salgado & al., 2000 (tab.1); Vukanic, 2003 (p.139, tab.1); Shimode & Shirayama, 2004 (tab.2); Rezai & al., 2004 (p.490, tab.2); Lan & al., 2004 (p.332, tab.1); Lo & al., 2004 (p.89, tab.1); Zuo & al., 2006 (p.164: tab.1); Jitlang & al., 2008 (p.65, Table 1); Ayon & al., 2008 (p.238, Table 4: Peruvian samples); Fernandes, 2008 (p.465, Tabl.2); C.-Y. Lee & al., 2009 (p.151, Tab.2); Williamson & McGowan, 2010 (p.273, Table III, Pacific central gyres: N & S); Mazzocchi & Di Capua, 2010 (p.429); Medellin-Mora & Navas S., 2010 (p.265, Tab. 2); Hsiao S.H. & al., 2011 (p.475, Appendix I); Maiphae & Sa-ardrit, 2011 (p.641, Table 2, 3, Rem.); Andersen N.G. & al., 2011 (p.71, Fig.3: abundance); Guo & al., 2011 (p.567, table 2, indicator); in CalCOFI regional list (MDO, Nov. 2013; M. Ohman, comm. pers.); Terbiyik Kurt & Polat, 2013 (p.1163, Table 2, seasonal distribution); Gur & al., 2015 (p.8408, cuticular structure vs reflectance, figs.1, 2, 3); Gur & al., 2016 (p.1393, figs. 1, 2, 3, 6, color change vs physical mechanism); Zakaria & al., 2016 (p.1, Table 1); Benedetti & al., 2016 (p.159, Table I, fig.1, functional characters) | | | | NZ: | 19 | | |
|
Distribution map of Sapphirina metallina by geographical zones
|
| | | | | | | | | | | | | | |  issued from : C. Lehnhofer in Wiss. Ergebn. dt. Tiefsee-Exped. ''Valdivia'', 1929, 22 (5). [p.327, Fig.58]. issued from : C. Lehnhofer in Wiss. Ergebn. dt. Tiefsee-Exped. ''Valdivia'', 1929, 22 (5). [p.327, Fig.58].
Zoogeographical distribution of S. metallina. |
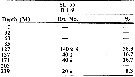 issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1, Crustacea, 1: Copepoda. Copepods of the Florida Current. 1967. [p.116, Table 57]. issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1, Crustacea, 1: Copepoda. Copepods of the Florida Current. 1967. [p.116, Table 57].
Vertical distribution of Sappjirina metallina at the ''40-Mile Station'' in the Florida Current ( E Miami: ± 25°35'N, 79°27'W; depth 738 m).
SL 55: 21 VII 1958. B: during midnight. |
| | | | Loc: | | | Antarct. (Indian: continent, very rare) (in Wolfenden, 1911), South Africa, Angola, Congo, off St. Helena Is. (N & SE), off Trindade Is., off Ascension Is., G. of Guinea, Ivorian shelf, Dakar, off Cape Verde Is. (S, N & NW), off Mauritania, Canary Is., off Madeira, Lisboa, Rio de Janeiro, Guadeloupe, Caribbean Sea, Caribbean Colombia, Yucatan, Venezuela, G. of Mexico, Cuba, Florida, Sargasso Sea, off Bermuda (Station "S"), off SW Azores, Medit. (Algiers Bay, Ligurian Sea, Napoli, Strait of Messina, off Malta, N & S Adriatic Sea, Ionian Sea, Aegean Sea, W Egyptian coast, Iskenderun Bay, Lebanon Basin), Red Sea, G. of Aden, off Socotra, Arabian Sea, Maldive Is., Madagascar (Nosy Bé), Indian, India (Lawson's Bay), Bay of Bengal, Nicobar Is. (Nankaurie Harbour), W Australia, Straits of Malacca, Indonesia-Malaysia, Ambon Bay, Philippines, G. of Thailand, Viet-Nam, China Seas (Yellow Sea, East China Sea, Taiwan Strait, South China Sea), Taiwan (E, NW, N: Mienhua Canyon), S. Japan (Kuchinoerabu Is.), Japan, off Kamtchatka, Pacif. (W equatorial), Pacific (central gyres: N & S), Australia (Great Barrier), New Zealand, Gilbert Is., Hawaii, off S Hawaii, California, W Baja California, W Mexico , G. of Tehuantepec, Clipperton Is., Galapagos, Peru, N Chile | | | | N: | 130 | | | | Lg.: | | | (34) F: 2,04-1,92; M: 1,98-1,92; (35) F: 2,22; (46) M: 1,9; (91) F: ± 1,2; (135) F: 2,3; (180) F: 2,26-2,07; M: 2,14-2; (254) M: 2,1; (327) F: 2,12; (340) F: 2,1-1,8; M: 2; (449) F: 2,3; M: 1,9; (458) F: 2; 1,87; M: 2,08; 1,96; (530) M: 3; (531) M: 2,8; (618) M: 2,18; (648) F: 2,52-1,68; M: 2,58-1,61; (705) F: 2,112-1,942; M: 2,093-1,867; (788) F: 1,72-1,62; M: 1,7-1,5; (794) F: 2,5-1,3; M: 2,3-2; (805) F: 2,39-1,2; M: 2,39-1,4; (991) F: 1,68-2,52; M: 1,61-2,58; (1023) F: 1,86; M: 1,86; {F: 1,20-2,52; M: 1,40-3,00} | | | | Rem.: | epi-mesopelagic, (off Malta: 2000-3000 m).
Sampling depth (Antarct.) : 1200 m. Overall Depth Range: 0-500 m (Deevey & Brooks, 1977, Station "S"). 0-217 m at Station T-1 (E Tori Is., E middle Japan) from Furuhashi (1966 a).
See in DVP Conway & al., 2003 (version 1) | | | Last update : 09/12/2020 | |
|
|
 Any use of this site for a publication will be mentioned with the following reference : Any use of this site for a publication will be mentioned with the following reference :
Razouls C., Desreumaux N., Kouwenberg J. and de Bovée F., 2005-2025. - Biodiversity of Marine Planktonic Copepods (morphology, geographical distribution and biological data). Sorbonne University, CNRS. Available at http://copepodes.obs-banyuls.fr/en [Accessed December 01, 2025] © copyright 2005-2025 Sorbonne University, CNRS
|
|
 |
 |






















