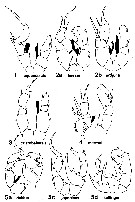|
|
 |
|
Calanoida ( Ordre ) |
|
|
|
Diaptomoidea ( Superfamille ) |
|
|
| |
| | | |
| Pseudodiaptomidae Sars, 1902 ( Diaptomoidea ) | | Ref.: | Sars, 1902 (1903) (p.73); Gurney, 1931 a (p.84); Wright, 1937 (p.160, clé spp.); Nicholls, 1944 (p.8); Brodsky, 1950 (1967) (p.82, 322); Gonzalez & Bowman, 1965 (p.250); Björnberg, 1972 (p.49); Andronov, 1974 a (p.1005); Kiefer, 1978 d (p.55, 57); Madhupratap & Haridas, 1978 (p.257, Rem.); Bowman & Abele, 1982 (p.9); Razouls, 1982 (p.435); Dussart & Defaye, 1983 (p.29); Brodsky & al., 1983 (p.143, 146); Grindley, 1984 (p.217, Rem.: 5 groups, biogeography); Sazhina, 1985 (p.63, 116, N); Zheng Zhong & al.,1984 (1989) (p.245, Rem.); Walter, 1986 (p.129); 1986 a (p.504); Dussart, 1989 (p.6); Grindley, 1990 (p.237, Biogeography); 1990 a (p.237); Huys & Boxshall, 1991 (p.419); Razouls, 1993 (p.308); Chihara & Murano, 1997 (p.893); Bradford-Grieve & al., 1999 (p.884, 902, 904, 952); Bradford-Grieve,1999 b (p.148, Def., Rem.); Ohtsuka & Huys, 2001 (p.461); Boxshall & Halsey, 2004 (p.13; 49; 172: Def.; p.174: Genera key); Mulyadi, 2004 (p.152); Laakmann & al., 2019 (p.330, fig. 2, 3, phylogenetic relationships)
Bradford-Grieve J.M., (2002 onwards). Key to calanoid copepod families. Version 1 : 2 oct 2002. http://www.crustacea.net/crustace/calanoida/index.htm  | | Rem.: | 3 G.: Archidiaptomus, Calanipeda, Pseudodiaptomus. Ces formes saumâtres et dulçaquicoles, parfois littorales ne sont prises que provisoirement en considération (références incomplètes). | 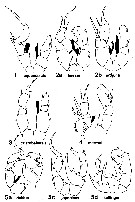 issued from : J.R. Grindley in Crustaceana, 1984, supplt 7. [p.219, Fig.1]. Examples of the five groups of Pseudodiaptomidae distinguished by the arrangement of the endopodites of male P5. Endpodites dermarcated in black in these diagram. Nota : For Grindley (1984, p.218) there are several different groups of species which tend to constitute morphologically and zoogeographically distinct groups. The most important charcters distinguishing the species are the P5, and in particular those of males ; in particular the arrangement of the endopods of the P5 in the males is different for each of several groups and comparable whitin each group. All the species found round the coast of Africa form a group characterized by the possession of both left and right endopods in the P5 of the male (Grindley, 1963). Certain oriental species possess both endopods but they be separated on the possession of a ‘Y’-shaped right endopod in the male P5. Group 1 : Both endopodites present, of which neither is greatly reduced ( Calanipeda, Archidiaptomus ; Mediterranean, Ponto-Caspian region, and Cochin). Group 2 : Both endopodites present of which the right is greatly reduced. (Africa, Asia, Australia). Group 3 : Only the left endopodite present (North and South America). Group 4 : Only the right endopodite present. (Asia and Australia). Group 5 : A long curved medial projection in place of the left endopodite on the second left nasal segment ; right endopodite reduced or absent. (Asia and Australia). Other features appear to agree, for example, the form of the P5 females (used by Sewell, 1924, in his attempt at grouping, fit the same pattern. The simplest and apparently most primitive form of the endopodite appears to be exhibited by Calanipeda aquaedulcis and Archidiaptomus arroorus. In addition to its characteristic endopodites, C. aquaedulcis is unique among Pseudodiaptomidae in having A1 with 25 segments (primitive condition in calanoid copepods). The segmentation of the male geniculate A1 is reduced in Calanipeda, but there are more end segments beyond the articulation than in any other pseudodiaptomid. Archidiaptomus aroorus possesses a number of primitive characters and strong affinities to the family Diaptomidae ; it has been placed in this new genus because of a number of peculiar features including the fully developes left and right endopods in the male P5 ; A1 is 24-segmented which approaches the primitive condition of the Calanoida ; exopod 2-segmented of the female P5 is close to that of the typical Pseudodiaptomidae but it is unique in maintening fully developed endopods of an unusually spinous form (this appears to be a primitive or generalized condition placing this genus very close to the Diaptomidae). |
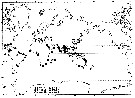 issued from : J.R. Grindley in Crustaceana, 1984, supplt 7. [p.224, Fig.2]. Distribution of the five groups of Pseudodiaptomidae. |
 Issued from : G.A. Boxshall & S.H. Halsey in An Introduction to Copepod Diversity. The Ray Society, 2004, No 166, Part. I. [p.172]. Armature formula of swimming legs P1 to P4. Nota: Spine and setal formula sometimes reduced. Nota : Female P5 biramous with 1-segmented endopods in Archidiaptomus, uniramous in other genera ; uniramous leg typically 5-segmented, symmetrical, primitively joined by distinct intercoxal slerite or with coxae fused ; comprising coxa, basis and 3-segmented exopod. Coxa unarmed. Basis with outer seta. 1st exopodal segment typically elongate ; 1st and 2 nd exopodal segments each with outer spine, 2 nd produced into process at inner distal angle. 3rd exopodal segment small, fused to apical element and with small inner process proximally. - Male P5 asymmetrical, primitively biramous, uniramous due to loss of endopod in some species ; separate coxa and basis present on both sides ; right leg with 3-segmented exopod, endopod 1-segmented, often bifid ; endopod sometimes absent. 3rd exopodal segment typically curved, sometimes claw-like, armed with 1 large seta and 1 or 2 setules on concave margin. Left leg with 2-segmented exopod, 1st segment with outer spine, 2 nd segment irregularly shaped, with distal spines. Endopod typically an elongate , unsegmented lobe. - Eggs contained in paired, multiseriate sacs, or in a single sac according to species. |
 Issued from : G.A. Boxshall & S.H. Halsey in An Introduction to Copepod Diversity. The Ray Society, 2004, No 166, Part. I. [p.173]. A, Pseudodiaptomus ishigakiensis habitus female; B, habitus male; C, female P5; D, male P5. E, Pseudodiaptomus hessei female A2; F, female Md. [Nishida, 1985a: A-D; Grindley, 1963: E-F. |
 Issued from : J.M. Bradford-Grieve in NIWA Biodiversity Memoir 111, 1999. [p.148]. Spine and setae formula in swimming legs P1 to P4. P5 female uniramous or biramous ( Archidiaptomus), may be slightly asymmetrical; coxa and basis separate, basis usually with a posterolateral seta; exopod 3-segmented (terminal 2 segments fiused in Archidiaptomus. Ovisac present. P5 male uniramous or biramous, asymmetrical; exopods 2- or 3-segmented; spines , rxopod al segments, and endopods, if present, variously modified into organs which appear to be adapted for clasping. | | | | | (1) Archidiaptomus Madhupratap & Haridas, 1978 | |
| | Ref.: | Madhupratap & Haridas, 1978 (p.253); Razouls, 1982 (p.445); 1993 (p.308); Dussart & Defaye, 1983 (p.36); Grindley, 1984 (p.217, 218, 220); Walter, 1986 (p.129); Mauchline, 1998 (p.67); Bradford-Grieve,1999 b (p.148, Def.); Boxshall & Halsey, 2004 (p.174) | | Rem.: | Total: 1 sp. | | | | (2) Calanipeda Kritschagin, 1873 | |
| | Syn.: | Poppella Richard, 1888 (p.43); de Guerne & Richard, 1889 (p.149); Giesbrecht & Schmeil, 1898 (p.62) | | Ref.: | Dussart, 1967 a (p.85); Grindley, 1984 (p.217); Razouls, 1982 (p.435); 1993 (p.308); Walter, 1986 (p.129); Dussart, 1989 (p.12); Bradford-Grieve,1999 b (p.148, Déf.); Boxshall & Halsey, 2004 (p.174) | | Rem.: | Total: 1 sp. |  Issued from : B. Dussart in Les Copépodes des eaux continentales. N. Boubée & Cie (edit.), 1967, Tome I. [p.87, Fig. 22]. Calanipeda aquae-dulcis Kritschagin, 187. Female and male (from Camargue, France). Nota: Clorinity 0.3 to 18 g per litre and pH 6.9 to 8.4; temperature from 3 to 30°C. Male body length: 1.00 mm and female: 1.30 to 1.45 mm. | | | | | (3) Pseudodiaptomus Herrick, 1884 | |
| | Syn.: | Weismannella Dahl,1894 c (p.19);
T. Scott, 1894 b (p.39);
Schmackeria Mrazek,1894; Mazellina Rose, 1957 (p.235); Walter & al., 2006 (p.215-216, Rem.) | | Ref.: | Giesbrecht & Schmeil, 1898 (p.63); A. Scott, 1909 (p.116); Früchtl, 1924 b (p.47); Sewell, 1924 (p.784, Rem.); Gurney, 1931 a (p.20); Sewell, 1932 (p.233, Rem.); Wilson, 1932 a (p.101); Wright, 1937 a (p.160, clé spp.M); Dakin & Colefax, 1940 (p.89); Nicholls, 1944 (p.8); Brodsky, 1950 (1967) (p.322); Carvalho, 1952 a (p.145); Ummerkutty, 1960 (p.184: Rem.); Kasturirangan, 1963 (p.35); Tanaka, 1963 (p.12); Johnson, 1964 (p.33); Gonzalez & Bowman, 1965 (p.250); Wellershaus, 1969 (p.254); Andronov, 1974 a (p.1005); Pillai, 1976 (1980) (p.243, Rem., groups Key, Biogeo); Razouls, 1982 (p.436); Ranga Reddy & Radhakrishna, 1982 (p.255); Dussart & Defaye, 1983 (p.29); Zheng Zhong & al., 1984 (1989) (p.246); Grindley, 1984 (p.217); Walter, 1984 (p.369); 1986 (p.131, 162 : groups & sub-groups Key); 1986 a (p.502); 1987 (p.363, 365: clé spp., Rem., p.366, Biogeo.); Mauchline, 1987 (p.712); Dussart, 1989 (p.6, clé spp.); Walter, 1989 (p.590, spp. Key, Rem.); Jacoby & Greenwood, 1991 (p.405, species coexisting); Razouls, 1993 (p.308); Chihara & Murano, 1997 (p.893); Mauchline, 1998 (p.67); Bradford-Grieve & al., 1999 (p.884, 953: spp. Key); Bradford-Grieve,1999 b (p.149, Déf., Rem.); Bradford-Grieve, 2004 (p.287); Boxshall & Halsey, 2004 (p.174); Mulyadi, 2004 (p.152, spp. Key in Indonesian waters, Rem.: p.162); Walter & al., 2006 (p.203, 212: key of the 14 species known from the Philippines, Rem.: Revised species groups, key of species group and sub-groups) | | Rem.: | Vyshkvartzeva (1980: comm. pers.) attire l'attention sur le fait que considérer le genre Mazellina comme synonyme de Pseudodiaptomus est une opinion qui n'emporte pas la conviction. Pour Vervoort (1965, p.97) il ne paraît pas y avoir de doute dans l'identité des deux genres (M. galleti semble très proche de P. dauglishi). Type: Pseudodiaptomus pelagicus Herrick,1884. Total: 80 spp. + 2 var. + 2 indet. Formes côtières, saumâtres, dulçaquicoles; épibenthiques de jour.
L'examen de ce genre n'est envisagé dans ce travail que succinctement du fait qu'il est assez rare de l'observer en mer, sinon entraîner par les courants provenant des eaux saumâtres littorales ou/et d'estuaires. Ce genre présente néanmoins un intérêt tout particulier du fait que les formes fossiles des copépodes sont rarissimes. De ce fait l'ancienneté de ce groupe de crustacés est en débat (voir in Kabata, 1986) de même que l'arbre phylogénétique. Sewell (1956) a tenté d'expliquer la distribution des copépodes, particulièrement des pseudodiaptomides en considérant la dérive des continent et en postulant que les espèces modernes existaient antérieurement au Crétacé. Grindley (1969) suggère que le Pseudodiaptomidae auraient une origine tardivement au Tertiaire et auraient connu une spéciation et une dispersion au Quaternaire; le même auteur en 1984 pense que les ancêtres des Pseudodiaptomidae plus récents se seraient dispersés à travers la Tethys durant le Crétacé ou au début du Tertiaire.
| | Remarques sur les dimensions et le sex-ratio: | | The mean female size is 1.357 mm (n = 56; SD = 0.2776) , and the mean male size is 1.087 mm (n = 55; SD = 0.2271). The size ratio (male : female) is 0.81 (n = 64; SD = 0.0876). |  issued from : T.C. Walter, S. Ohtuska & L.V. Castillo in Proc. Biol. Soc. Washinton, 2006, 119 (2). [p.314, Table 2]. The species groups and sub-groups are based primarily on the male P5, because female characters are not consistent among and within species-groups. |
 issued from : T.C. Walter, S. Ohtsuka, S. Putchakarn, K. Pinkaew & S. Chullasorn in Proc. Biol. Soc. Washington, 2002, 115 (3). [p.666, Table 3]. |
 issued from : T.C. Walter, S. Ohtsuka, S. Putchakarn, K. Pinkaew & S. Chullasorn in Proc. Biol. Soc. Washington, 2002, 115 (3). [p.663, Table 2]. Number of egg-sac(s) for the species groups of Pseudodiaptomus. |
 Issued from : G.A. Boxshall & S.H. Halsey in The Ray Society, N°166, 2004. (p.173, Fig. 40]. Pseudodiaptomidae. Pseudodiaptomus ishigakiensis: A, female habitus; B, male habitus); C, female P5; D, male P5. Pseudodiaptomus hessei: E, female A2; F, female mandible. Nishida, 1985 a: A-D; Grindley, 1963: E-F. | | | | | | |
|
|
 Toute utilisation de ce site pour une publication sera mentionnée avec la référence suivante : Toute utilisation de ce site pour une publication sera mentionnée avec la référence suivante :
Razouls C., Desreumaux N., Kouwenberg J. et de Bovée F., 2005-2026. - Biodiversité des Copépodes planctoniques marins (morphologie, répartition géographique et données biologiques). Sorbonne Université, CNRS. Disponible sur http://copepodes.obs-banyuls.fr [Accédé le 17 janvier 2026] © copyright 2005-2026 Sorbonne Université, CNRS
|
|
 |
 |