|
|
 |
Fiche d'espèce de Copépode |
|
|
Harpacticoida ( Ordre ) |
|
|
|
Thalestroidea ( Superfamille ) |
|
|
|
Miraciidae ( Famille ) |
|
|
|
Macrosetella ( Genre ) |
|
|
| |
Macrosetella gracilis (Dana, 1848) (F,M) | |
| | | | | | | Syn.: | Setella gracilis Dana, 1848; Claus, 1863 (p.136, figs.F); Brady, 1883 (p.108, figs.F,M); Giesbrecht, 1892 (p.559, 775, figs.F,M); Wheeler, 1901 (p.188, figs.F,M); I.C. Thompson, 1903 a (p.33); T. Scott, 1894 b (p.109); Carl, 1907 (p.17); Wolfenden, 1911 (p.364); Mori, 1929 (p.201, figs.F); Farran, 1929 (p.211, 298); Steuer, 1935 (p.393, fig.F); Mori, 1937 (1964) (p.115, figs.F,M); Chen & al., 1974 (p.68, figs.F,M); Guangshan & Honglin, 1984 (p.118, tab.); Shih & Young, 1995 (p.75);
Setella longicauda (M) Dana, 1848;
Microsetella gracilis : Jang M.-C & al., 2012 (p.37, Table 3: lapsus calami, abundance and seasonal distribution);
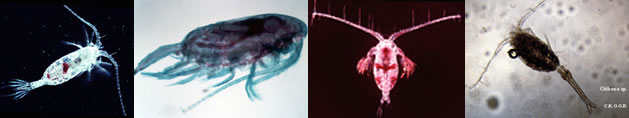
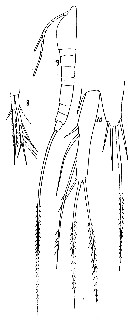
? Macrosetella rosea : Fernandez de Puelles & al., 1996 (p.97, occurrence: p.101, lapsus calami); | | | | Ref.: | | | A. Scott, 1909 (p.230, Rem.); Pesta, 1912 a (p.56, fig.F); 1913 (p.33); Früchtl, 1924 b (p.104); Rose, 1929 (p.53); Wilson, 1932 a (p.281, figs.F,M); Rose, 1933 a (p.288, figs.F,M); Dakin & Colefax, 1933 (p.208); Steuer, 1935 a (p.393, fig.F, carte); Farran, 1936 a (p.123); Dakin & Colefax, 1940 (p.106, figs.F,M); Sewell, 1940 (p.141); Sewell, 1947 (p.290); Lang, 1948 a (p.770, figs.F,M); Moore, 1949 (p.62); Carvalho, 1952 a (p.161, figs.F,M); Krishnaswamy, 1957 (p.14, Rem.); Marques, 1958 a (p.142); Tanaka, 1960 (p.92); Fish, 1962 (p.29); Tanaka, 1964 (p.17); Owre & Foyo, 1967 (p.105, figs.F,M); Björnberg, 1965 a (p.512, figs.N, juv.); Ramirez, 1969 (p.99, fig.F); Corral Estrada, 1970 (p.251); Shih & al., 1971 (p.53); Razouls, 1972 (p.96, Annexe: p.145); Marques, 1973 (p.255); 1975 (p.49); Björnberg & al., 1981 (p.677, figs.F,M); Marques, 1982 (p.768); Zheng Zhong & al., 1984 (1989) (p.269, figs.F, M); Boxshall, 1979 (p.237, figs.F,M); Sazhina, 1985 (p.100, figs.N); Böttger-Schnack, 1989 (p.33, Rem.); Huys & Boxshall, 1991 (p.409); Kim & al., 1993 (p.271); Huys & Böttger-Schnack, 1994 (p.231, figs.F,M, N, Juv.); Chihara & Murano, 1997 (p.956, Pl.208: F,M); Bradford-Grieve & al., 1999 (p.886, 968, figs.F,M, p.876: chart); Al-Yamani & Prusova, 2003 (p.115, figs.F,M); Conway & al., 2003 (p.211, figs.F,M, Rem.); Boxshall & Halsey, 2004 (p.345: figs.F); Ferrari & Dahms, 2007 (p.58, 84, Rem., Table XX, XXI); Avancini & al., 2006 (p.130, Pl. 98, figs.F,M, Rem.); Eberl & al., 2007 (p.33, phylogenetic analysis); Vives & Shmeleva, 2010 (p.111, figs.F,M, Rem.); Al-Yamani & al., 2011 (p.122, figs.F,M) |  issued from : F.C. Ramirez in Contr. Inst. Biol. mar., Buenos Aires, 1969, 98. [p.98, Lam. XX, figs. 170]. Female (from off Mar del Plata): 170, habitus (lateral right side). Scale bar in mm: 0.8.
|
 issued from : Q.-c Chen & S.-z. Zhang & C.-s. Zhu in Studia Marina Sinica, 1974, 9. [Pl.24, Figs.3-6]. As Setella gracilis. Female (from China Seas): 3, habitus (lateral left side); 4, P5. Male: 5, habitus (lateral left side); 6, P5.
|
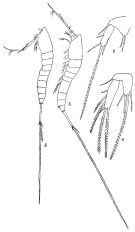
 issued from : F.Y. Al-Yamani & I. Prusova in Common Copepods Northwestern Arabian Gulf : Identification Guide. Kuwait Institute for Scientific Research, 2003. [p.114, Fig.43]. Female: A, habitus (lateral right side); B, P5. Male: C, habitus (lateral right side); D, P5.
|
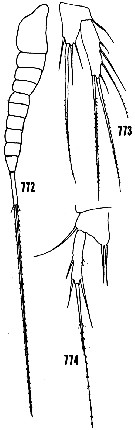 issued from : H.B. Owre & M. Foyo in Fauna Caribaea, 1967, 1 Crustacea, 1: Copepoda. [p106, Figs.772-774]. Female: 772, habitus (lateral); 773, P5. Male: 774, P5.
|
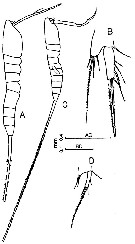
 issued from : F. Giron-Reguer in Thèse Fac. Sc. Paris, 1963. [Fig26]. Female (from Alboran Sea): anterior part of cephalosome.
|
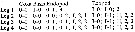 issued from : G.A. Boxshall in Bull. Br. Mus. nat. Hist. (Zool.), 1979, 35 (3). [p.237].Female and Male from 18°N, 25°W. Armature formula of swimming legs P1 to P4. Nota: Cephalosome prolonged anteriorly, without cuticular lenses. Rostrum large,clearly delimited at base and ventrally directed. Caudal rami about 8 times longer than wide. A1 8-segmented in both sexes with aesthetes on segments 4 and 8. A2 with allobasis in female and apparently with separate basis and 2-segmented endopod in male; exopod absent in both sexes. Md and Mx1 both comprising a toothed blade and a single seta representing the palp. Mx2 with 1 (male) and 2 (female) endites. P5 female with 4 setae on basoendopod and 6 setae on exopod. P5 male with 2 setae on basoendopod and 4 setae on exopod.
|
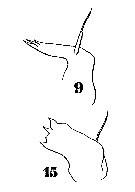
 issued from : T. Mori in Zool. Mag. Tokyo, 1929, 41 (486-487). [Pl. VIII, Figs. 8-10]. As Setella gracilis. Female (from Chosen Strait, Korea-Japan): 8, P4; 9, habitus (lateral); 10, P5.
|
 issued from : T. Mori in The Pelagic copepoda from the neighbouring waters of Japan, 1937 (1964). [Pl. 64, Figs.1-5]. As Setella gracilis. Female: 2, A2; 4, P5; 5, habitus (lateral). Male: 1, habitus (lateral; 3, P5.
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.45, Fig.3]. As Setella gracilis. Female: 3, A1. Aes = aesthetasc.
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.45, Figs.9, 15]. As Setella gracilis. Female: 9, Md: 15, Mx1.
|
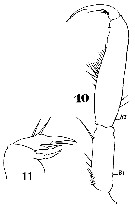 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.45, Figs.10, 11]. As Setella gracilis. Female: 10, Mxp; 11, Mx2.
|
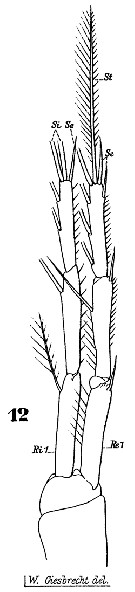 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.45, Fig.12]. As Setella gracilis. Female: 12, P3. Ri1 = endopodal segment 1; Re2 = exopodal segment 1; St = terminal seta; Si = inner seta; Se = outer seta.
|
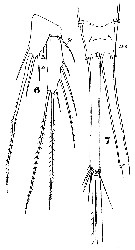 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.45, Figs.6, 7]. As Setella gracilis. Female: 6, P5; 7, anal segment and caudal rami. (dorsal).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.45, Fig.4]. As Setella gracilis. Male: 4, habitus (lateral).
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.45, Figs.1, 2]. As Setella gracilis. Male: 1, A2; 2, A1.
|
 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.45, Figs.5, 8]. As Setella gracilis. Male: 5, P5; 8, genital segment.
|
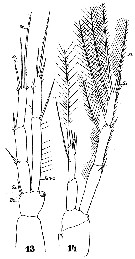 Issued from : W. Giesbrecht in Systematik und Faunistik der Pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. – Fauna Flora Golf. Neapel, 1892, 19 , Atlas von 54 Tafeln. [Taf.45, Fig.4]. As Setella gracilis. Male: 13, P1; 14, P2.
|
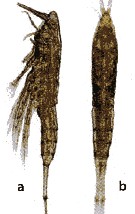 issued from : Y. Al-Yamani, V. Skryabin, A. Gubanova, S. khvorov & I. Prusova in Marine Zooplankton Practical Guide for the Northwestern Arabian Gulf, 2, 2011. [p.123, Fig.273] Female (from Kuwait); a-b, habitus (left lateral and dorsal).
| | | | | Ref. compl.: | | | Wilson, 1932 (p.38); 1942 a (p.193); Sewell, 1948 (p.325, 515); C.B. Wilson, 1950 (p.262); Yamazi, 1958 (p.153, Rem.); Heinrich, 1961 (p.92); Fagetti, 1962 (p.40); Cervigon, 1962 (p.181, tables: abundance distribution); Ganapati & Shanthakumari, 1962 (p.11, 16); Grice & Hart, 1962 (p.287, table 3); Giron-Reguer, 1963 (p.57); V.N. Greze, 1963 a (tabl.2); Grice, 1963 a (p.496); Gaudy, 1963 (p.30, Rem.); Björnberg, 1963 (p.68, Rem.); De Decker, 1964 (p.16, 22, 29, 32); De Decker, 1964 a (p.157); De Decker & Mombeck, 1964 (p.13); El-Maghraby, 1965 (p.58, Appendix); Neto & Paiva, 1966 (p.36, Table III); Furuhashi, 1966 a (p.295, vertical distribution in Kuroshio region, Table 9); Mazza, 1966 (p.73); Pavlova, 1966 (p.45); Ehrhardt, 1967 (p.742, geographic distribution, Rem.); Séguin, 1968 (p.488); Delalo, 1968 (p.138); Deevey, 1971 (p.224); Salah, 1971 (p.320); Bainbridge, 1972 (p.61, Appendix Table I: vertical distribution vs day/night, Table II: %); Apostolopoulou, 1972 (p.329, 376); Corral Estrada & Pereiro Muñoz, 1974 (tab.I); Palet, 1975 (p.660); Vives & al., 1975 (p.54, tab.II, III); Deevey & Brooks, 1977 (p.256, tab.2, Station "S"); Madhu Pratap & al., 1977 (p.138, Table 3: abundance vs. stations); Dessier, 1979 (p.201, 207); Vaissière & Séguin, 1980 (p.23, tab.2); Sreekumaran Nair & al., 1981 (p.493), Vives, 1982 (p.295); Kovalev & Shmeleva, 1982 (p.85); Dessier, 1983 (p.89, Tableau 1, Rem., %); Turner & Dagg, 1983 (p.17, 22); Tremblay & Anderson, 1984 (p.9); Scotto di Carlo & al., 1984 (p.1043); De Decker, 1984 (p.318, 354: chart); 1984 a (p.157); Regner, 1985 (p.11, Rem.: p.40); Binet, 1985 (p.85, tab.3); Sarkar & al., 1986 (p.178); Brinton & al., 1986 (p.228, Table 1); Renon, 1987 (tab.2); Lozano Soldevilla & al., 1988 (p.61); Dessier, 1988 (tabl.1); Böttger-Schnack & Schnack, 1989 (p.27, vertical distribution vs cyanobacteria); Böttger-Schnack, 1989 (p.33); Othman & al., 1990 (p.561, 564, Table 1); Madhupratap & Haridas, 1990 (p.305, Rem: p.317); Hirakawa & al., 1990 (tab.3); Mitra & al., 1990 (fig.3); Böttger-Schnack, 1991 (p.309); Yoo, 1991 (tab.1); McKinnon, 1991 (p.471); O'Neil & Roman, 1994 (p.235); Godhantaraman, 1994 (tab.6, 7); Kouwenberg, 1994 (tab.1); Hwang & Turner, 1995 (p.207, Table 1, 2, fig. 1, 2, 3: swimming behaviour); Webber & Roff, 1995 (tab.1); Shih & Young, 1995 (p.75); Böttger-Schnack, 1995 (p.93); 1996 (p.1088); Webber & al., 1996 (tab.1); O'Neil & al., 1996 (p.89, ingestion); Böttger-Schnack, 1997 (p.410); Suarez-Morales & Gasca, 1997 (p.1525); Park & Choi, 1997 (Appendix); Sharaf & Al-Ghais, 1997 (tab.1); Go & al., 1997 (tab.1); Noda & al., 1998 (p.55, Table 3, occurrence); Hure & Krsinic, 1998 (p.103); Padmavati & al., 1998 (p.349); Hopcroft & al., 1998 (tab.2); Gilabert & Moreno, 1998 (tab.1,2); Alvarez-Cadena & al., 1998 (t.1,2,3,4); Suarez-Morales & Gasca, 1998 a (p.113); O'Neil, 1998 (p.43, nutrition & behaviour vs Trichodesmium substrat); Harvey & al., 1999 (p.1, 49: Appendix 5, in ballast water vessel); Siokou-Frangou, 1999 (p.478); Lavaniegos & Gonzalez-Navarro, 1999 (p.239, Appx.1); El-Serehy, 1999 (p.172, Table 1, occurrence); Neumann-Leitao & al., 1999 (p.153, tab.2); Razouls & al., 2000 (p.343, Appendix); El-Sherif & Aboul Ezz, 2000 (p.61, Table 3: occurrence); Fernandez-Alamo & al., 2000 (p.1139, Appendix); Lopez-Salgado & al., 2000 (tab.1); Vukanic, 2003 (p.139, tab.1); Hsieh & al., 2004 (p.398, tab.1); Rezai & al., 2004 (p.486, tab.2, 3, abundance, Rem., p.495, tab.8); Chang & Fang, 2004 (p.456, tab.1); Daly Yahia & al., 2004 (p.366, fig.4); Lan & al., 2004 (p.332, tab.1); Gallienne & al., 2004 (p.5, tab.3); Kazmi, 2004 (p.230); Choi & al., 2005 (p.710: Tab.III); Baker & al., 2005 (tabl.); Smith & Madhupratap, 2005 (p.214, tab.4,5); Berasategui & al., 2005 (p.485, tab.1); Berasategui & al., 2006 (p.485: fig.2); Rakhesh & al., 2006 (p.93, Table 2, spatial distribution); Hwang & al., 2006 (p.943, tabl. I); Dias & Araujo, 2006 (p.78, Rem., chart); Khelifi-Touhami & al., 2007 (p.327, Table 1); Hwang & al., 2007 (p.25); Eberl & Carpenter, 2007 (p.205); Jitlang & al., 2008 (p.65, Table 1); Rakhesh & al., 2008 (p.154, abundance vs stations); Tseng L.-C. & al., 2008 (p.153, fig.5, Table 2, occurrence vs geographic distribution, indicator species); Humphrey, 2008 (p.85: Appendix A); Neumann-Leitao & al., 2008 (p.799: Tab.II, fig.6); Morales-Ramirez & Suarez-Morales, 2008 (p.514, 524); Fernandes, 2008 (p.465, Tabl.2); Pagano, 2009 (p.115); C.-Y. Lee & al., 2009 (p.151, Tab.2); Miyashita & al., 2009 (p.815, Tabl.II); Tseng & al., 2009 (p.327, fig.5, feeding); Cornils & al., 2010 (p.2076, Table 3); Hernandez-Trujillo & al., 2010 (p.913, Table 2); Dias & al., 2010 (p.230, Table 1, fig.5 a); Mazzocchi & Di Capua, 2010 (p.430); Medellin-Mora & Navas S., 2010 (p.265, Tab. 2); Fazeli & al., 2010 (p.153, Table 1); W.-B. Chang & al., 2010 (p.735, Table 2, abundance); Hsiao S.H. & al., 2011 (p.475, Appendix I); Tseng L.-C. & al., 2011 (p.239, Table 3, abundance %); Hsiao & al., 2011 (p.317, Table 2, indicator of seasonal change); Kâ & Hwang, 2011 (p.155, Table 3: occurrence %); Andersen N.G. & al., 2011 (p.71, Fig.3: abundance); Tseng L.-C. & al., 2011 (p.47, Table 2, occurrences vs mesh sizes); Maiphae & Sa-ardrit, 2011 (p.641, Table 2, 3, Rem.); Pepin & al., 2011 (p.273, Table 2, seasonal abundance); Isari & al., 2011 (p.51, Table 2, abundance vs distribution); Selifonova, 2011 a (p.77, Table 1, alien species in Black Sea); Shiganova & al., 2012 (p.61, Table 4); Uysal & Shmeleva, 2012 (p.909, Table I); Salah S. & al., 2012 (p.155, Tableau 1); Jose & al., 2012 (p.20, fig.3 a,b,c: % vs monsoon); DiBacco & al., 2012 (p.483, Table S1, ballast water transport); Zizah & al., 2012 (p.79, Tableau I, Rem.: p.86); Johan & al., 2012 (2013) (p.1, Table 1); Miloslavic & al., 2012 (p.165, Table 2, transect distribution); Tseng & al., 2012 (p.621, Table 3: abundance); Lavaniegos & al., 2012 (p. 11, Appendix); Belmonte & al., 2013 (p.222, Table 2, abundance vs stations); in CalCOFI regional list (MDO, Nov. 2013; M. Ohman, comm. pers.); Melo Jùnior & al., 2013 (p.363, Table 2, egg production); Tseng & al., 2013 (p.507, seasonal abundance); Tseng & al., 2013 a (p.1, Table 3, abundance); Rakhesh & al., 2013 (p.7, Table 1, abundance vs stations); Jagadeesan & al., 2013 (p.27, Table 3, 5, 6, fig.11, seasonal variation); Peijnenburg & Goetze, 2013 (p.2765, genetic data); Lidvanov & al., 2013 (p.290, Table 2, % composition); Hwang & al., 2014 (p.43, Appendix A: seasonal abundance) ; Bonecker & a., 2014 (p.445, Table II: frequency, horizontal & vertical distributions); Zaafa & al., 2014 (p.67, Table I, occurrence); Dias & al., 2015 (p.483, Table 2, abundance, biomass, production, Table 4: % vs. season); Nakajima & al., 2015 (p.19, Table 3: abundance); Rojas-Herrera & al., 2016 (p.40, Table 2: temporal abundance); Zakaria & al., 2016 (p.1, Table 1) ; Benedetti & al., 2016 (p.159, Table I, fig.1, functional characters); Ben Ltaief & al., 2017 (p.1, Table III, Summer relative abundance); Marques-Rojas & Zoppi de Roa, 2017 (p.495, Table 1); Jerez-Guerrero & al., 2017 (p.1046, Table 1: temporal occurrence); El Arraj & al., 2017 (p.272, table 2, seasonal composition); Bonecker C.T. & al., 2017 (p.247, fig.10: abundance day/night vs. vertical types of mass water); Benedetti & al., 2018 (p.1, Fig.2: ecological functional group); Dias & al., 2018 (p.1, Tables 2, 4, 5: vertical distribution, abundance vs. season); Palomares-Garcia & al., 2018 (p.178, Table 1: occurrence); Acha & al., 2020 (p.1, Table 3: occurrence % vs. ecoregions). | | | | NZ: | 21 | | |
|
Carte de distribution de Macrosetella gracilis par zones géographiques
|
| | | | | | | | | | | | | | | 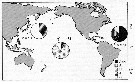 Issued from : R. Eberl, S. Cohen, F. Cipriano & E.J. Carpenter in Aquat. Biol., 2007, 1. [p.37, Fig.2]. Issued from : R. Eberl, S. Cohen, F. Cipriano & E.J. Carpenter in Aquat. Biol., 2007, 1. [p.37, Fig.2].
Global distribution of M. gracilis cytochrome c oxydase subunit I (COI) haplotypes at different sampling sites with haplotypes shared between Atlantic (A) and Hawai (H) and Japan (J) and singleton haplotypes (S) where pie slices with the same shading represent different haplotypes except for singletons.
Number of individuals sampled (n) is indicated for each region.
Nota: Macrosetella gracilis uses colonies of the nitrogen-fixing cyanobacterium Trichodesmium spp. as a floating substrate and nursery.
The results of phylogenetic analyses COI sequences were in agreement with previous studies on the distribution of M. gracilis based solely on morphological characteristics (Huys & Böttger-Schnack, 1994), abd confirmed the occurrence of genetically similar M. gracilis in both the Atlantic and the Pacific.The hypothesis of a global distribution of M. gracilis was supported by low genetic distances between most COI sequences across the sampling range, and by shared haplotypes between the Atlantic and the Pacific. Surprisingly, the authors did not find shared haplotypes between Hawaiian and Japanese samples. Genetic diversity of COI sequences was hignest in Japanese samples.
Phylogenetic analysis placed samples from the Atlantic and the Pacific within the same clades, suggesting some level of exchange between the two oceans or retention of ancestral polymorphism across a huge geographic area.
The availability of floating substrate as Trichodesmium (Sheridan & al., 2002) has been shown by Thiel & Gutow (2005 a, b) to be important for connectivity between areas. The effect of habitat preference and life history on population structure of copepods has previously been shown by a comparison of drifting and resident calanoid copepod species (Bucklin & al., 2000). Resident Acartia clausii showed significant genetic differences between different fjords, whereas drifting Calanus spp. did not differ significantly.
Genetic distances between M. gracilis in different regions found by the authors were closer to the distances found in studies of some open ocean calanoid copepods within and across ocean basins (0.5 to 10.1 %) (Bucklin & al., 2003; Goetze, 2003). Genetic distances between Macrosetella gracilis and Miracia efferata (28.7 to 31.3 %, K2P) in the author's studies were similar to distances found between different species and genera in calanoid copepods (Bucklin & al., 2000; 2003).
One clade in the authors’s dataset was separated from the rest by 21 mutational steps (up to 8.5 % sequence divergence). This is a high intraspecific divergence for a pelagic copepod (Goetze, 2005). The possibility of cryptic speciation within M. gracilis exists (future studies using multiple loci are needed to address this possibility. Several species of copepods appear to have diverged at the molecular level without becoming morphologically distinct, for example the estuarine copepod Eurytemora affinis (Lee, 2000), and the pelagic copepod Rhincalanus nasutus (Goetze, 2003), all at levels of divergence simolar to those found between M. gracilis clades. Data from other genes are needed to determine whether the observed divergences between M. gracilis COI sequences are signs of cryptic speciation.
The possibility of sympatric speciation exists. Sibling species often have distinct habitat preferences (differences in salinity or depth distribution). The depth distribution of M. gracilis in the Red Sea with a shallow and a deep ‘population’ (Böttger-Dchnack & Schnack, 1989) could suggest the presence of sibling species.
The cyanobacterium Trichodesmium spp. Is capable of producing toxins (Cox & al., 2005). Adaptation and various levels of tolerance to these toxins by the copepod could also lead to speciation in M. gracilis. (O’Neil & al., 1996 ; 1998 ; Enberl & Carpenter, 2007).
The largest genetic diversity was found among Japanese haplotypes. It is possible that this area harbors remnants of the oldest and most diverse population. The sampling area of the authors was north of the presumed ‘Center of Origin’ in the Indo-West Pacific, where the highest levels of diversirty have been observed for many marine species (Briggs, 1999). Briggs suggested that within the Pacific, radiation of diversity occurred eastward. This hypothesis is in accordance with the genetic data of the authors, where levels of diversity are higher in Japan than in Hawaii. If the hypothesis of an older population with higher genetic diversity near Japan holds true, increased sampling should reveal more Japanese haplotypes and perhjaps the presence of common haplotypes A to E in Japanese waters. Increased sampling may also reveal more rare haplotypes that are separated from these common haplotypes by only one mutational step.
Shared haplotypes between oceans, as well as lack of geographical partitioning of haplotypes, suggest gene flow between the Atlantic and the Pacific (either historical or current) or long-term maintenance of widespread ‘ancestral’ haplotypes. Geographical lineage sorting between Atlantic and Pacific populations might be expected if the population of M. gracilis in the different oceans had been separated since the rise of the Isthmus of Panama (3.5 million years ago) and no gene flow exists. Variance in reproductive success also influences the persistence of different haplotypes in a population (Avise, 2000) as is the case for M. gracilis, where reproduction and larval survival are linked to the presence of Trichodesmium spp. Colonies (Björnberg, 1965 a).
Anthropogenic factors have been implicated in changing the distribution of many marine species, especially dispersal through ballast water exchange (Callton & geller, 1993). In addition to rafting on Trichodesmium spp., dispersal of M. gracilis could be facilitated by shipping activity. M. gracilis was found in ballast water after mid-ocean exhange in the Pacific (Choi & al., 2005). |
 Issued from : A.C.T. Bonecker, A. V. de Araujo, C.O. Dias, M.M.S. Castro, P.F. Carvalho, R.M. Lopes & S.C.L. Bonecker in A.P.C. Falcao & D.L. Moreira (ed.) Ambiente pelagico caracterizaçao ambiental regional de Bacia de Campos, Atlantico Sudoeste. Rio de Janeiro. Elsevier. 2017, v.5, p.247-281. [p.261, Fig.10] Issued from : A.C.T. Bonecker, A. V. de Araujo, C.O. Dias, M.M.S. Castro, P.F. Carvalho, R.M. Lopes & S.C.L. Bonecker in A.P.C. Falcao & D.L. Moreira (ed.) Ambiente pelagico caracterizaçao ambiental regional de Bacia de Campos, Atlantico Sudoeste. Rio de Janeiro. Elsevier. 2017, v.5, p.247-281. [p.261, Fig.10]
Abundance (ind. m-3) sampled during night and day into four water masses in the Campos Basin (Brazil).
AT: Tropical water; ACAS: Central water of South Atlantic; AIA: Intermediate water of Antarctica; ACS: Deep water Circumpolar water superior.
Sampling at station c (22°54'30''S, 40°43'W), depth 1900 m (end of the continental slope).
Nota: On 117 taxa, with 81 species identified, this species is the most abundant (206,32 ind.m-3), following Farranula gracilis and Clausocalanus furcatus. |
 Issued from : J.-S. Hwang & J.T. Turner in P.S.Z.N. 1: Mar. Ecol., 1995, 16 (3). [p.209: Table 1, p.210: Table 2]. Issued from : J.-S. Hwang & J.T. Turner in P.S.Z.N. 1: Mar. Ecol., 1995, 16 (3). [p.209: Table 1, p.210: Table 2].
Table 1: Experimental protocol.
Table 2: Swimming versus non-swimming behaviour, and behavioural transitions per minute. Means (bold type), ranges, and standard deviations (SD). Number of repeated observations = 500.
Copepods collected in surface net from waters near Pitou (NE coast Taiwan: 25°N, 122°E) in daylight in July 1993.
Compare with Oithona nana, O. similis, Oncaea venusta, Temora turbinata and Undinula var. taiwanicus by the same authors. |
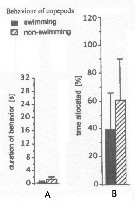 Issued from : J.-S. Hwang & J.T. Turner in P.S.Z.N. 1: Mar. Ecol., 1995, 16 (3). [p.211: Figs.1, 2]. Issued from : J.-S. Hwang & J.T. Turner in P.S.Z.N. 1: Mar. Ecol., 1995, 16 (3). [p.211: Figs.1, 2].
Fig.1: A, Mean durations periods (seconds) of swimming versus non swimming behaviour by Macrosetella gracilis from north-east coast of Taiwan (near Pitou) in daylight in July 1993.
Fig.2: B, mean percentages of time allocated to swimming versus non-swimming behaviour by Macrosetella gracilis.
Rem: This harpactocoid averged shorter periods of non-swimming and longer periods of swimming than did the cyclopoids. The swimming of M. gracilis was not as 'jerky' as that of O. venusta, as measured by behavioural transition/minute, being more like that of the two Oithona species. |
| | | | Loc: | | | sub-Antarct. (Indian), South Africa (off Cape of Good Hope, E & W), Namibia, Angola, Baia Farta, Congo, G. of Guinea, Dakar, off E St. Paul Is., off Cape Verde Is., off Mauritania-Morocco, Cap Ghir, Canary Is., off Madeira, off SW Azores, Argentina, off Rio de La Plata (shelfbreak), Brazil (S, Ubatuba, off Rio de Janeiro, Campos Basin, off Vitoria-Cabo de Sao Tomé, off Macaé, off Natal), off Amazon, Barbada Is., Caribbean Sea, Caribbean Colombia, Bahia de Mochima (Venezuela), Cariaco Basin, Yucatan, Jamaica, E Costa Rica, G. of Mexico, Cuba, Florida, Sargasso Sea, off Bermuda (Station "S"), Chesapeake Bay, Long Island, G. of Maine, off Woods Hole, Newfoundland, Iceland, Faroe, North Sea, Ibero-morocco Bay, off W Tangier, Medit. (M'Diq, Alboran Sea, Gulf of Annaba, Castellon, Banyuls, G. of Lion, Ligurian Sea, Tyrrhenian Sea, Messina, Gulf of Taranto, NW Tunisia, G. of Gabes, Adriatic Sea, Ionian Sea, Aegean Sea, Thracian Sea, Black Sea, Lebanon Basin, Egyptian coast, Alexandria), Sharm El-Sheikh, Taba, Safaga, Red Sea, Gulf of Oman, G. of Aden, Arabian Sea, Araby (South coast), Arabian Gulf, UAE coast, off Madagascar S, Mascarene Basin, Mauritius, Rodrigues Is. - Seychelles,Karavatti & Agatti Is. (offside & lagoon), Indian, India (Saurashtra coast, Mangalore coast, S, Lawson's Bay, Mandarmani creek, Hooghly estuary), E India (coastal, Gulf of Mannar, Palk Bay, Godavari region, Kakinada Bay), Bay of Bengal, Straits of Malacca, G. of Thailand, Indonesia-Malaysia, Sarawak: Bintulu coast, Ambon Bay (Baie d'Amboine), Tioman Is., SW Celebes, Philippines, Viet-Nam, China Seas (Yellow Sea, East China Sea, South China Sea), Kurochio Current region, Taiwan Strait, Taiwan (SW, E, S, W, Kaohsiung Harbor, NW, NE, Pitou), Okinawa, S Korea, Korea Strait, S Japan Sea, Japan, Kuchinoerabu Is., Tanabe Bay, Bering Sea, Aleutian Is., Pacif. (W equatorial), Australia (G. of Carpentaria, Great Barrier, New South Wales), N New Zealand, New Caledonia, Is. Fidji, Hawaii, California, W Baja California (Bahia Magdalena), La Paz, Gulf of California, Bahia of los Angeles, Acapulco Bay, (rare) G. of Tehuantepec, W Costa Rica, Clipperton Is., Bahia Cupica (Colombia), off Chile | | | | N: | 251 | | | | Lg.: | | | (34) F: 1,52-1,25; M: 1,2-1,08; (46) F: 1,5-1,4; M: 1,3-1,16; (66) F: 1,57-0,94; M: 1,25-0,94; (91) F: 1,5-1,2; M: 1,3-1,15; (104) F: 1,4; M: 1,1; (109) F: 1,3-1,2; M: 1,2-1; (138) F: 1,5-1,21; M: 1,16-1,13; (180) F: 1,7; (188) F: 1,5-1,4; M: 1,3-1,16; (237) F: 1,8; M: 1,25; (254) F: 1,45*; (332) F: 1,64-1,23; (336) F: 1,64-1,32; M: 1,63-1,48; (618) F: 1,66; (651) F: 1,68-1,33; M: 1,39-1,12; (721) [Red Sea-Indian] F: 1,6-0,88; M: 1,34-0,86; [Okinawa] F: 1,62-1,22; M: 1,22-1,18 ; (786) F: 1,4-1,3; (937) F: 1,22; M: 1,01; (991) F: 0,98-1,62; M: 0,88-1,30; {F: 0,88-1,80; M: 0,86-1,63}
* : without caudal setae, 325 mm with caudal setae. | | | | Rem.: | épipélagique.
Sampling depth (sub-Antarct.) : 400-3000 m.
Observé dans les ballasts des navires à San Francisco.
Voir aussi les remarques en anglais | | | Dernière mise à jour : 25/10/2022 | |
|
|
 Toute utilisation de ce site pour une publication sera mentionnée avec la référence suivante : Toute utilisation de ce site pour une publication sera mentionnée avec la référence suivante :
Razouls C., Desreumaux N., Kouwenberg J. et de Bovée F., 2005-2026. - Biodiversité des Copépodes planctoniques marins (morphologie, répartition géographique et données biologiques). Sorbonne Université, CNRS. Disponible sur http://copepodes.obs-banyuls.fr [Accédé le 29 janvier 2026] © copyright 2005-2026 Sorbonne Université, CNRS
|
|
 |
 |